Abstract
Purpose
The aim of the study was to examine TOP2A gene copy number changes as a means to identify groups of breast cancer patients with superior benefit from treatment with anthracyclines.
Materials and methods
Tumour tissue was retrospectively collected and successfully analysed for TOP2A in 773 of 980 Danish patients randomly assigned to receive intravenous CMF (cyclophosphamide, methotrexate, and fluorouracil) or CEF (cyclophosphamide, epirubicin, and fluorouracil) in DBCG trial 89D. Subgroup analyses on this material published by Knoop et al [11] and updated by Nielsen et al [21], demonstrated that superiority of CEF over CMF is limited to patients with TOP2A aberrations, defined as patients whose tumours have TOP2A ratio below 0.8 or above 2.0. The Subpopulation Treatment Effect Pattern Plot (STEPP) technique was applied to these data to explore the pattern of treatment effect relative to TOP2A and to compare that pattern to the ranges previously used to define “aberrations.”
Results
The pattern of treatment effect illustrated by the STEPP analysis confirmed that the superiority of CEF over CMF is indeed limited to patients whose tumours have high or low TOP2A ratios. The hypothesis of no treatment effect-covariate interaction was rejected (p=0.02). Furthermore, results indicated that the interval of TOP2A ratios hitherto denoted as “normal” could be narrower than previously assumed.
Conclusion
A more optimal separation of TOP2A subgroups could be obtained by altering cut-points currently used to define TOP2A amplified and TOP2A deleted tumours by narrowing the TOP2A normal interval, and consequently enlarging the population with TOP2A aberrated tumours.
Keywords: Breast cancer, TOP2A, treatment, anthracycline, STEPP
Introduction
The TOP2A gene encodes topoisomerase IIα (topo IIα), an enzyme catalysing the breakage and reunion of double-stranded DNA leading to relaxation of DNA supercoils; this enzyme is the primary target of anthracyclines [1].
A beneficial effect from substituting methotrexate with anthracyclines (epirubicin or doxorubicin) has been demonstrated in six symmetrically designed trials comparing CEF [2-4] or CAF [5-7] with CMF after radical surgery for early breast cancer, and this has been confirmed in an EBCTCG meta analysis [8]. Superiority has also been shown for epirubicin followed by CMF compared to CMF [9], while four cycles of AC failed to demonstrate superiority to eight cycles of CMF [10].
Translational research has suggested that the modest benefit obtained on average, originates mostly from a large benefit obtained by the small fraction of patients whose tumours have TOP2A aberrations [11]. In fact, while TOP2A aberrations are associated with worse prognosis in non-anthracycline treated cohorts [18, 21], recent reports show little influence of TOP2A on outcome for anthracycline treated cohorts [12, 13], providing indirect evidence that anthracyclines improve results for the TOP2A aberrant subgroup.
Copy number changes of the TOP2A gene may be classified as amplification or deletion using the FISH ratio between signals of TOP2A and centromere 17 gene probes. However, there is no particular biological cut-point to adhere to and no consistent definition of cut-points exists, complicating comparisons among different studies. In the absence of a biologically based and well-defined cut-point, 1.5 was suggested in a pivotal study [14]. Later, a more conservative cut-point of 2.0 was developed for HER2 and it was argued that the same cut-point should be applied for TOP2A [15]. In retrospective studies evaluating the importance of TOP2A copy number changes for adjuvant anthracycline responsiveness in randomised trials both 1.5 [16, 17] and 2.0 [11, 18] were applied as cut-points to define TOP2A amplification.
The present study was undertaken to analyse further the ranges previously used to define TOP2A aberrations and to explore other cut-points to improve classification of groups of patients whose disease responds better to treatment with anthracyclines. To that end we will apply the Subpopulation Treatment Effect Pattern Plot (STEPP) technique to the data from Knoop et al [11].
Materials
The data investigated arise from a clinical trial and a corresponding sub-study, both of which have been previously described in detail [2,11].
Between 1990 and 1998 the Danish Breast Cancer Cooperative Group recruited 980 Danish high risk patients with primary breast cancer for the trial DBCG 89D. The patients were randomised to 9 cycles of adjuvant chemotherapy with either i.v. CMF (cyclophosphamide, methotrexate and 5-fluorouracil) or i.v. CEF (cyclophosphamide, epirubicin and 5-fluorouracil) repeated every three weeks.
Archival paraffin embedded tissue blocks were obtained from 806 Danish patients enrolled in the trial, corresponding to 82% of the patients enrolled, between September 2001 and August 2002 and analysed centrally for HER2 and TOP2A status. Copy number aberrations of TOP2A were identified using fluorescence-in-situ hybridisation (FISH): The TOP2A ratio for each sample was defined as the ratio of TOP2A gene to centromere 17 signals.
Methods
The Subpopulation Treatment Effect Pattern Plot (STEPP) is an exploratory statistical method developed to illustrate the pattern of treatment effect with respect to a covariate. The technique, introduced in 2000 by Bonetti and Gelber [19], is based on analysing a hypothesised treatment effect in a number of subgroups, which are made to overlap, in order to both uphold the number of subgroups and their size, and thereby the precision of the estimates from each group [19,20]. The resulting correlation between the groups is then taken into account when an overall test for interaction between covariate and treatment effect is carried out.
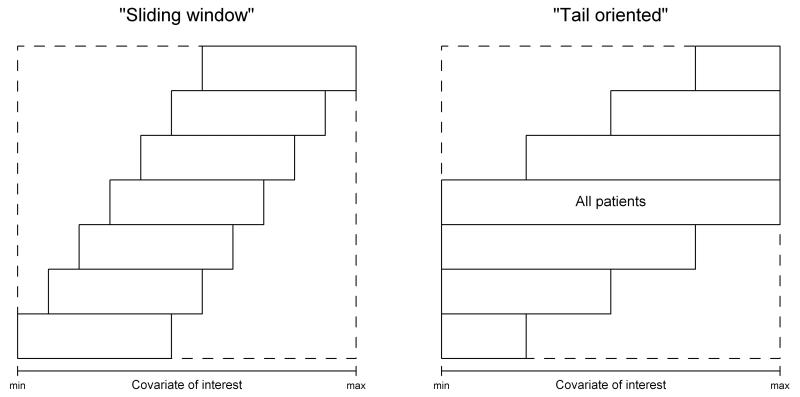
Two different strategies for dividing the data into overlapping subgroups have been proposed. With the “sliding window” strategy, a certain number of subgroups is generated, so that the number of patients in each group, and the number of patients exchanged between groups remains roughly constant. The “tail oriented” strategy produces one subgroup that encompasses all the patients, while the remaining subgroups decrease in size at a nearly constant rate as the covariate value tends to the extremes. By generating the subgroups using the “tail oriented” strategy, the focus is directed at the tail of the covariate distribution.
The two strategies are illustrated in Error! Reference source not found.Figure 1.
Fig. 1.
The principle of two strategies for generating subgroups (Source: Bonetti and Gelber, 2000 [19])
Having generated subgroups according to the chosen strategy, it is then necessary to define some measure of treatment effect, and compute its value in each subgroup. Such a measure can for example be the difference in 10 year disease-free survival between two treatment arms.
A STEPP plot then illustrates how the treatment effect changes with the covariate by depicting the estimated treatment effects against the median covariate values in the corresponding subgroups.
The technique makes a point of defining overlapping subgroups so that the estimated treatment effects are correlated; this correlation should be taken into consideration when interpreting patterns illustrated in STEPP plots. Having estimated the (joint asymptotic) distribution of the subpopulation-specific treatment effects, it is possible to obtain the p-value of a test for the absence of treatment effect-covariate interaction and a confidence band, taking the correlation between groups into account [20].
Results
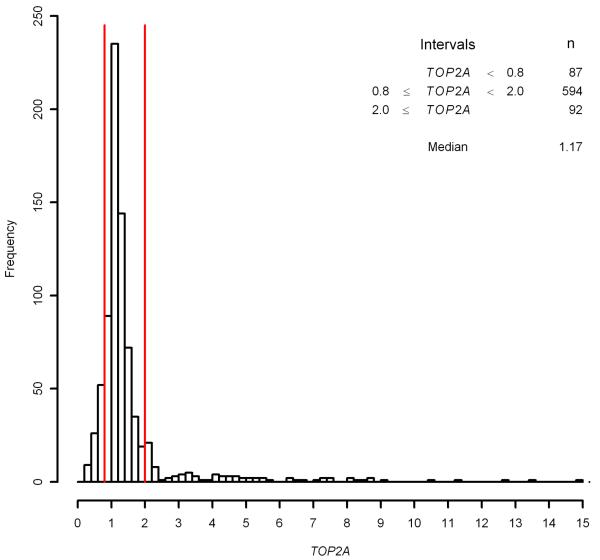
Figure 2 shows the distribution of TOP2A ratios for the 773 patients who received CEF or CMF, and for whom TOP2A assessment was achieved. The cut-points usually applied are indicated with red lines; tumours were prospectively classified as being TOP2A deleted if the ratio was below 0.8 and TOP2A amplified if the ratio was greater than or equal to 2.0, with ratios between 0.8 and 2.0 denoted as TOP2A normal. The corresponding group sizes are also denoted in Figure 2. The relationship between TOP2A status and benefit of treatment with CEF over CMF was investigated by Knoop et al [11] in a retrospective study elaborating on DBCG trial 89D, and the findings are corroborated by an update, published by Nielsen et al [21].
Fig. 2.
Histogram of TOP2A
Ejlertsen et al [2], reported an adjusted relative risk with respect to disease-free survival on treatment with CEF compared with CMF of 0.84 (95%-CI 0.71-0.99). Nielsen et al [21] conducted subgroup analyses on a subset of the same data consisting of the 767 Danish patients eligible for multivariate analysis. They found that looking at the three TOP2A groups separately, a significant improvement in treatment effect was found in disease-free survival on treatment with CEF versus CMF for patients with TOP2A amplified tumours; the adjusted relative risk was 0.39 (N=92, 95%-CI 0.22-0.70), and a similar non-significant improvement for patients with TOP2A deleted tumours; the adjusted relative risk was 0.61 (N=86, 95%-CI 0.35-1.07). In contrast, little difference was seen in disease-free survival on treatment with CEF versus CMF for patients with TOP2A normal tumours; the adjusted relative risk for that group was 0.94 (N=589, 95%-CI 0.73-1.20). The observed interaction between TOP2A status and benefit from treatment with CEF over CMF was significant (p=0.02).
In the present study, the 773 patients were included in a STEPP analysis, where treatment effect was defined as the difference in 10 year disease-free survival on treatment with CEF versus CMF with disease-free survival referring to the time from randomisation to recurrence, other malignancy, death or completion of 10 years of regular follow-up, whichever came first.
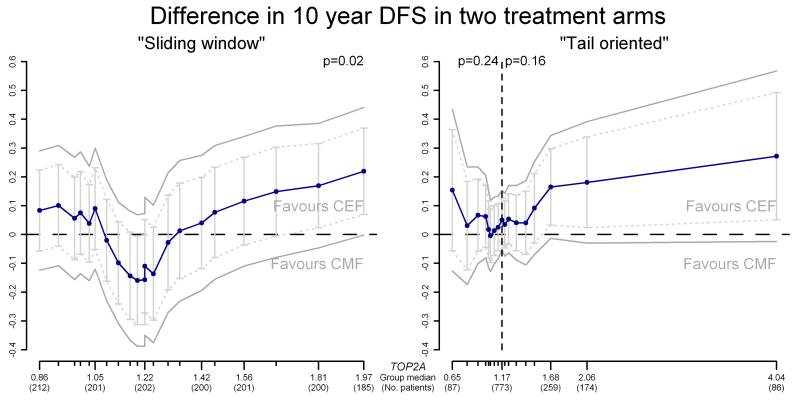
The results are illustrated in Figure 3. The median TOP2A ratio within each subgroup is shown on the horizontal axis and the number of patients in the corresponding subgroup is shown in parentheses (note: values are only provided for some subgroups). The vertical axis shows the difference in 10 year disease-free survival, with positive values favouring CEF and negative values favouring CMF. The treatment effect in each subgroup is plotted against the median TOP2A ratio in that group and the points are connected to show the overall pattern of treatment effect with respect to TOP2A. Furthermore, 95% pointwise confidence intervals are shown as bars on the plot and 95% confidence bands, where the correlation between subgroups is taken into account, are indicated. Finally, overall departure from the null hypothesis of no treatment effect-covariate interaction is indicated by a p-value.
Fig. 3.
Example of STEPP results, using both the “Sliding window” and “Tail oriented” strategies for subgroup division
In the sliding window analysis, subgroups were generated so that their size was approximately 200 patients and the adjacent subgroups had around 180 patients in common. The resulting number of subgroups was 21, corresponding to the number of points on the plot.
In the tail oriented analysis, eight subgroups were generated in each tail, with 17 subgroups in total. The smallest subgroups at the extremes had less than 90 patients and the largest subgroup in the middle contained all 773 patients. In general, the shape of the sliding window pattern shown in Error! Reference source not found.Figure 3 corroborates previous findings that patients with TOP2A normal tumours on average demonstrate similar outcomes for CEF and CMF, while patients whose tumours have low or high TOP2A ratios demonstrate greater benefit of treatment with CEF compared with CMF. Furthermore, substantial fluctuation is noticeable in the CEF versus CMF comparison for TOP2A ratios in the range between 0.8 and 2.0, with cohorts at the extremes of this range benefiting more from CEF and ratios in the middle favouring CMF. This observation may indicate that the lack of superiority of CEF over CMF might be limited to an interval narrower than the TOP2A ratio range from 0.8 to 2.0 used in the publications by Knoop et al [11] and Nielsen et al [21].
The tail oriented pattern in Figure 3 illustrates that as TOP2A values increase or decrease above or below the average value for all patients, the benefit of CEF over CMF increases.
Sensitivity analysis and small sample properties
A sensitivity analysis (results not shown) varying the choice of subgroup parameters indicated that while the p-values for the test for interaction differ, patterns similar to those seen in Figure 3 were observed.
The small sample performance of STEPP was also explored. In particular, we carried out a simulation study in order to establish the coverage of the confidence bands and the false positive rate of the overall test for no interaction between treatment effect and covariate value in a simulated setting.
Survival times were simulated in three different settings, all with no interaction between TOP2A and treatment effect, by randomly generating observations from the exponential distribution and choosing parameters appropriately according to the original dataset. The generated survival times were then combined with TOP2A ratios from the original dataset, thus producing, in each of the three settings, 300 datasets of the correct dimension.
Each dataset was then analysed by means of the STEPP technique, using the “sliding window” approach to generate subgroups.
The recovery of the Type I error for the test of no covariate-treatment effect interaction was verified by noting the number of datasets in each setting where the test showed significance. The level of recovery was satisfactory in all settings; a significant covariate-treatment interaction was found in between 15 and 21 of the 300 datasets, corresponding to a false positive rate of between 5.0 and 7.0%. Similarly, the coverage of the 95% confidence band was assessed by counting the number of cases in each setting where the known “true” value of the treatment effect was not encompassed by the confidence band for at least one subgroup. The confidence band excluded the true value in 8-9% of cases, suggesting that the confidence bands are slightly narrower than they should be.
This is likely to be due to the use of approximate asymptotic results in the construction of the band. Note that while here the small sample properties of the methods appear to be satisfactory, this might not always be the case. In such cases an alternative inferential procedure has recently become available [22].
Discussion
Subgroup studies using ad-hoc cut off points to categorise patients into groups according to the TOP2A ratio of the tumour have established TOP2A as an indicator for responsiveness to treatment with anthracyclines in breast cancer patients [11]. In contrast to earlier studies, a recent subset analysis of the NEAT/BR9601 study gave conflicting results [23]. In univariate analysis amplification of HER2 and deletion of TOP2A were associated with a poorer prognosis while copy number of chromosome 17 centromere (CC17) was not. In a multivariate analysis CC17, however, was associated with incremental benefit from epirubicin while HER2 and TOP2A were not [23]. Different cut-offs were used for HER2, TOP2A and CC17. Biological or historical evidence that substantiates particular cut-off points between groups is lacking, and it is therefore of interest to discover whether a more optimal separation of patient subgroups could be obtained by applying different cut-points. A unified definition of cut-points would furthermore facilitate comparison between different studies on the subject.
The STEPP analysis suggests the presence of subgroups of patients who, contrary to the overall conclusion of Ejlertsen et al [2], do not benefit from treatment with CEF. The sliding window strategy for subgroup division was given the highest importance in the STEPP analysis, since the tail oriented strategy focuses on the extremes, where the superiority of CEF seems irrefutable, at least for patients with TOP2A amplified tumours.
Although the DBCG 89D study was not prospectively designed for that purpose, the availability of tumour tissue samples relating to patients enrolled in the study rendered it ideal as the basis for a subsequent biomarker sub-study investigating the relationship between TOP2A and the benefit of treatment with anthracyclines. Furthermore, treatment allocation in DBCG 89D was randomised, thus minimising the risk of obtaining biased results.
The STEPP results indicate that an improved separation of subgroups might be obtained by altering the cut-points currently being applied to define the TOP2A normal cohort. In particular, the results suggest that the cut-point for defining TOP2A deletions could be higher than 0.8, and that the cut-point for defining TOP2A amplifications could be less than 2.0. If this were the case, the TOP2A normal cohort would be smaller than it is currently believed to be, and the groups of patients with TOP2A deleted or TOP2A amplified tumours would be correspondingly larger.
For example, setting the cut-point for defining TOP2A deletions at 1.1, and the cut-point for defining TOP2A amplifications at 1.3 could be considered. These newly identified TOP2A cut-points differ considerably from cut-points previously used as an indicator for responsiveness to treatment with anthracyclines. For instance, the identified cut-point for amplifications of 1.3 is considerably lower than the 2.0 threshold applied by Knoop et al [11], Tanner et al [24] and Nielsen et al [21] and nearer the cut-point of 1.5 applied by Järvinen et al [14] and Di Leo et al [16]. The distribution of TOP2A ratios is denser around the suggested new cut-points compared to 0.8 and 2.0, and the higher the concentration of observations around the cut-points leads to a numerically larger group of patients that could potentially be misclassified.
Using the original cut-points 0.8 and 2.0, only 87 patients are classified as having TOP2A deleted tumours and 92 as having TOP2A amplified tumours, while 594 patients are considered TOP2A normal. These imbalanced group sizes can diminish the statistical power of subgroup analyses aiming to detect a putative treatment benefit within each TOP2A group. Shifting the cut-points, as we have suggested, to 1.1 and 1.3 the groups are more similar in size: There are 308 patients with tumours that have a TOP2A ratio < 1.1; 269 patients have tumours with TOP2A ratio ≥ 1.3; the remaining group has 196 patients. The individual TOP2A subgroups being larger and more equal increases the statistical power with which conclusions about differences in treatment benefit within these groups can be drawn. Indeed this is also likely to apply in those studies where no predictive value of TOP2A has been detected, as lack of power due to the relatively low numbers of TOP2A aberrated tumours [23] will have affected the power to detect the TOP2A-treatment interaction.
In any case, further validation is required to define TOP2A aberration cut-points for use in the clinic.
Traditional subgroup analyses can be misleading, partly because repeated testing on the same data increases the risk of finding false positive results. In addition, studies are designed to have, as a whole, the power to detect a certain treatment effect; the power might not be sufficient to detect a treatment difference in subsets, thus possibly leading to false negative results. While this is also true for a STEPP analysis, the STEPP methodology has the advantage that the precision of estimates is increased due to the overlapping subgroups, while the number of analysed subgroups is still sufficiently large for the pattern of treatment effects to be illustrated in a clinically useful way. A further advantage is that the method allows the treatment effect to be defined using traditional statistics, such as fixed time survival estimates.
In this paper we have focused on STEPP analyses with the difference in 10 year disease free survival as a measure of treatment effect. This is a univariate measure, but in fact the STEPP technique could also be applied in a multivariate setting, for example defining treatment effect in terms of adjusted relative risk. Interpreting such results would be more difficult, but a test for the absence of interaction between treatment effect and covariate could still be carried out, to amend the STEPP results. When the data arise from a randomised study, as in the present case, adjusted risk estimates are less imperative; however, in this case a number of patients have been excluded from the original randomised population because availability of tissue samples.
The suggested new cut-off values should not be treated as definitive, and they could indeed be too close to each other: STEPP is an exploratory method designed to illustrate variations in treatment effects, and it is not designed to define specific cut-points for subgroups, which we identified without taking into account their statistical variability. In order to verify that a modification of the cut-points is indeed appropriate, we suggest that this possibility be examined on additional data material.
Acknowledgements
Supported in part by Grant No. CA-75362 from the United States National Cancer Institute.
References
- 1.Gudkov AV, Zelnick CR, Kazarov AR, Thimmapaya R, Suttle DP, Beck WT, Roninson IB. Isolation of genetic suppressor elements, inducing resistance to topoisomerase II-interactive cytotoxic drugs, from human topoisomerase II cDNA. Proc Natl Acad Sci U S A. 1993;90:3231–3235. doi: 10.1073/pnas.90.8.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ejlertsen B, Mouridsen HT, Jensen MB, Andersen J, Cold S, Edlund P, Ewertz M, Jensen BB, Kamby C, Nordenskjold B, Bergh J. Improved outcome from substituting methotrexate with epirubicin: Results from a randomised comparison of CMF versus CEF in patients with primary breast cancer. Eur J Cancer. 2007;43:877–884. doi: 10.1016/j.ejca.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Coombes RC, Bliss JM, Wils J, Morvan F, Espie M, Amadori D, Gambrosier P, Richards M, Aapro M, Villar-Grimalt A, McArdle C, Perez-Lopez FR, Vassilopoulos P, Ferreira EP, Chilvers CE, Coombes G, Woods EM, Marty M. Adjuvant cyclophosphamide, methotrexate, and fluorouracil versus fluorouracil, epirubicin, and cyclophosphamide chemotherapy in premenopausal women with axillary node-positive operable breast cancer: results of a randomized trial. The International Collaborative Cancer Group. J Clin Oncol. 1996;14:35–45. doi: 10.1200/JCO.1996.14.1.35. [DOI] [PubMed] [Google Scholar]
- 4.Levine MN, Bramwell VH, Pritchard KI, Norris BD, Shepherd LE, Abu-Zahra H, Findlay B, Warr D, Bowman D, Myles J, Arnold A, Vandenberg T, MacKenzie R, Robert J, Ottaway J, Burnell M, Williams CK, Tu D. Randomized trial of intensive cyclophosphamide, epirubicin, and fluorouracil chemotherapy compared with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1998;16:2651–2658. doi: 10.1200/JCO.1998.16.8.2651. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter JT, Velez-Garcia E, Aron BS, et al. Five year results of a randomized comparison of cyclophosphamide, doxorubicin and fluorouracil (CAF) versus cyclophosphamide, methotrexate and fluorouracil (CMF) for node positive breast cancer: a Southeastern Cancer Group Study. Proc Am Soc Clin Oncol. 1991;13:66. (abstr 68) [Google Scholar]
- 6.Martin M, Villar A, Sole-Calvo A, Gonzalez R, Massuti B, Lizon J, Camps C, Carrato A, Casado A, Candel MT, Albanell J, Aranda J, Munarriz B, Campbell J, Diaz-Rubio E. Doxorubicin in combination with fluorouracil and cyclophosphamide (i.v. FAC regimen, day 1, 21) versus methotrexate in combination with fluorouracil and cyclophosphamide (i.v. CMF regimen, day 1, 21) as adjuvant chemotherapy for operable breast cancer: a study by the GEICAM group. Ann Oncol. 2003;14:833–42. doi: 10.1093/annonc/mdg260. [DOI] [PubMed] [Google Scholar]
- 7.Hutchins LF, Green SJ, Ravdin PM, Lew D, Martino S, Abeloff M, Lyss AP, Allred C, Rivkin SE, Osborne CK. Randomized, controlled trial of cyclophosphamide, methotrexate, and fluorouracil versus cyclophosphamide, doxorubicin, and fluorouracil with and without tamoxifen for high-risk, node-negative breast cancer: treatment results of Intergroup Protocol INT-0102. J Clin Oncol. 2005;23:8313–21. doi: 10.1200/JCO.2005.08.071. [DOI] [PubMed] [Google Scholar]
- 8.Early Breast Cancer Trialists’ Collaborative Group Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 9.Poole CJ, Earl HM, Hiller L, et al. Epirubicin and Cyclophosphamide, Methotrexate, and Fluorouracil as Adjuvant Therapy for Early Breast Cancer. N Engl J Med. 2006;355:1851–1862. doi: 10.1056/NEJMoa052084. [DOI] [PubMed] [Google Scholar]
- 10.Fisher B, Brown AM, Dimitrov NV, et al. Two Months of Doxorubicin-Cyclophosphamide With and Without Interval Reinduction Therapy Compared With 6 Months of Cyclophosphamide, Methotrexate, and Fluorouracil in Positive-Node Breast Cancer Patients With Tamoxifen-Nonresponsive Tumors: Results From the National Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol. 1990;8:1483–1496. doi: 10.1200/JCO.1990.8.9.1483. [DOI] [PubMed] [Google Scholar]
- 11.Knoop AS, Knudsen H, Balslev E, Rasmussen BB, Overgaard J, Nielsen KV, Schonau A, Gunnarsdóttir KA, Olsen KE, Mouridsen H, Ejlertsen B. Retrospective Analysis of Topoisomerase IIα Amplifications and Deletions As Predictive Markers in Primary Breast Cancer Patients Randomly Assigned to Cyclophosphamide, Epirubicin and Fluorouracil: Danish Breast Cancer Cooperative Group. J Clin Oncol. 2005;23:7483–7490. doi: 10.1200/JCO.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Harris LN, Broadwater G, Abu-Khalaf M, et al. Topoisomerase IIα Amplification Does Not Predict Benefit From Dose Intense Cyclophosphamide, Doxorubicin, and Fluorouracil Therapy in HER2-Amplified Early Breast Cancer: Results of CALGB 8541/150013. J Clin Oncol. 2009;27:3430–3436. doi: 10.1200/JCO.2008.18.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tubbs RR, Barlow WE, Budd GT, Swain E, et al. Outcome of Patients With Early-Stage Breast Cancer Treated with Doxorubicin-Based Adjuvant chemotherapy As a Function of HER2 and TOP2A Status. J Clin Oncol. 2009;27:3881–3886. doi: 10.1200/JCO.2008.20.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Järvinen TAH, Tanner M, Rantanen V, Bärlund M, Borg Å , Grénman S, Isola J. Amplification and Deletion of Topoisomerase IIα Associate with ErbB-2 Amplification and Affect Sensitivity to Topoisomerase II Inhibitor Doxorubicin in Breast Cancer. Am J Pathol. 2000;156:839–847. doi: 10.1016/s0002-9440(10)64952-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olsen KE, Knudsen H, Rasmussen BB, Balslev E, Knoop A, Ejlertsen B, Nielsen KV, Schönau A, Overgaard J. Amplification of HER2 and TOP2A and Deletion of TOP2A Genes in Breast Cancer Investigated by New FISH Probes. Acta Oncol. 2004;43:35–42. doi: 10.1080/02841860310019007. [DOI] [PubMed] [Google Scholar]
- 16.Di Leo A, Larsimont D, Gancberg D, Tanner M, Jarvinen T, Rouas G, Dolci S, Leroy J, Paesmans M, Isola J, Piccart M. HER-2 Amplification and Topoisomerase IIα Gene Aberrations as Predictive Markers in Node-positive Breast Cancer Patients Randomly Treated Either with an Anthracycline-based Therapy or with Cyclophosphamide, Methotrexate and 5-Fluorouracil. Clin Cancer Res. 2002;8:1107–1116. [PubMed] [Google Scholar]
- 17.Bartlett JMS, Munro A, Cameron DA, Thomas J, Prescott R, Twelves C. Type 1 Receptor Tyrosine Kinase Profiles Identify Patients With Enhanced Benefit From Anthracyclines in the BR9601 Adjuvant Breast Cancer Chemotherapy Trial. J Clin Oncol. 2008;26:5027–5035. doi: 10.1200/JCO.2007.14.6597. [DOI] [PubMed] [Google Scholar]
- 18.O’Malley FP, Chia S, Tu D, Shepherd LE, Levine MN, Bramwell VH, Andrulis IL, Pritchard KI. Topoisomerase II Alpha and Responsiveness of Breast Cancer to Adjuvant Chemotherapy. J Natl Cancer Inst. 2009;101:644–650. doi: 10.1093/jnci/djp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonetti M, Gelber R. A graphical method to assess treatment-covariate interactions using the Cox model on subsets of the data. Stat Med. 2000;19:2595–2609. doi: 10.1002/1097-0258(20001015)19:19<2595::aid-sim562>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 20.Bonetti M, Gelber R. Patterns of treatment effects in subsets of patients in clinical trials. Biostatistics. 2004;5:465–481. doi: 10.1093/biostatistics/5.3.465. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen KV, Ejlertsen B, Møller S, Jørgensen JT, Knoop A, Knudsen H, Mouridsen HT. The value of TOP2A gene copy number variation as a biomarker in breast cancer: Update of DBCG trial 89D. Acta Oncol. 2008;47:725–734. doi: 10.1080/02841860801995396. [DOI] [PubMed] [Google Scholar]
- 22.Bonetti M, Zahrieh D, Cole BF, Gelber RD. A small sample study of the STEPP approach to assessing treatment-covariate interactions in survival data. Stat Med. 2009;28:1255–1268. doi: 10.1002/sim.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartlett JMS, Munro AF, Dunn JA, McConkey C, et al. Predictive markers of anthracycline benefit: a prospectively planned analysis of the UK National Epirubicin Adjuvant Trial (NEAT/BR9601) Lancet Oncol. 2010;11:266–274. doi: 10.1016/S1470-2045(10)70006-1. [DOI] [PubMed] [Google Scholar]
- 24.Tanner M, Isola J, Wiklund T, Erikstein B, Kellokumpu-Lehtinen P, Malmström P, Wilking N, Nilsson J, Bergh J. Topoisomerase IIα Gene Amplification Predicts Favourable Treatment Response to Tailored and Dose-Escalated Anthracycline Based Adjuvant Chemotherapy in HER-2/neu-Amplified Breast Cancer: Scandinavian Breast Group Trial 9401. J Clin Oncol. 2006;24:2428–2436. doi: 10.1200/JCO.2005.02.9264. [DOI] [PubMed] [Google Scholar]