Abstract
Background:
Some studies have suggested that statins, which have cholesterol-lowering and anti-inflammatory properties, may have antitumor effects. Effects of statins on inflammatory breast cancer (IBC) have never been studied.
Methods:
We reviewed 723 patients diagnosed with primary IBC in 1995–2011 and treated at The University of Texas MD Anderson Cancer Center. Statin users were defined as being on statins at the initial evaluation. Based on Ahern et al's statin classification (JNCI, 2011), clinical outcomes were compared by statin use and type (weakly lipophilic to hydrophilic (H-statin) vs lipophilic statins (L-statin)). We used the Kaplan–Meier method to estimate the median progression-free survival (PFS), overall survival (OS) and disease-specific survival (DSS), and a Cox proportional hazards regression model to test the statistical significance of potential prognostic factors.
Results:
In the multivariable Cox model, H-statins were associated with significantly improved PFS compared with no statin (hazard ratio=0.49; 95% confidence interval=0.28–0.84; P<0.01); OS and DSS P-values were 0.80 and 0.85, respectively. For L-statins vs no statin, P-values for PFS, DSS, and OS were 0.81, 0.4, and 0.74, respectively.
Conclusion:
H-statins were associated with significantly improved PFS. A prospective randomised study evaluating the survival benefits of statins in primary IBC is warranted.
Keywords: inflammatory breast cancer, statin, hydrophilic, lipophilic, progression-free survival, overall survival
Inflammatory breast cancer (IBC) is rare, comprising only 2–5% of breast cancer incidences, but is the most lethal type of breast cancer, representing 8–10% of breast cancer mortality (Keeneth et al, 2005). Clinically, IBC presents with apparent inflammatory changes of the breast, characterised by diffuse erythema and oedema of the skin with or without an underlying palpable mass. The diagnosis is pathologically confirmed by recognising invasive carcinoma in a core biopsy specimen (Dawood et al, 2011). Although patients with primary IBC have no evidence of distant metastases at the time of diagnosis, the disease tends to have rapid progression from onset, higher risk of recurrence, and shortened survival compared with locally advanced non-IBC (Cristofanilli et al, 2007). Despite advances in breast cancer treatment and a multidisciplinary approach (introduced at MD Anderson Cancer Center in 1974), patients with IBC continue to have poor prognosis (Gonzalez-Angulo et al, 2007). The biological mechanism responsible for the aggressive nature of primary IBC is not yet fully understood. Although calling this type of breast cancer ‘inflammatory' implies the existence of an underlying inflammatory mechanism, the term is a clinical description reflecting redness of the skin and does not denote the presence of an obvious pathologic inflammatory infiltrate in these tumours.
Statins, 3-hydroxyl-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, are cholesterol-lowering agents that significantly reduce cardiovascular events and overall mortality; they are also recognised as powerful anti-inflammatory agents Lefer (2002). Statin use substantially increased between the periods of 1988 and 1994 and 2005 and 2008, with a 10-fold increase in use by people aged 45 years and older (Ma et al, 2005; National Center for Health Statistics (US), 2010). Interestingly, multiple preclinical studies have demonstrated that statins possess tumour-suppressive effects and reduce metastatic potentials in breast cancer animal models and cell lines (Rao et al, 1998; Shibata et al, 2004; Ghosh-Choudhury et al, 2010; Mandal et al, 2011). HMG-CoA reductase has a crucial role in the regulation of the mevalonate pathway, which has been shown to contribute to tumour aetiology. Dysregulation of the mevalonate pathway was found to upregulate ectopic HMG-CoA reductase expression and to promote colony formation and tumour growth in breast cancer cells in vivo (Clendening et al, 2010). Increased mevalonate synthesis promoted tumour proliferation in a mouse breast cancer model (Duncan et al, 2004). Furthermore, in a meta-analysis of 865 breast cancer patients from six microarray data sets, high HMG-CoA reductase mRNA levels were found correlated with poor survival outcomes (Clendening et al, 2010). A review by Konstantinopoulos et al (2007) mentions, among several possible antitumor mechanisms of statins, the importance of post-translational modifications. Statins inhibit production of isoprenoids, which are one of the important products of the mevalonate pathway and substrates for geranylgeranylation, a type of post-translational modification that adds the geranylgeranyl isoprene unit to the target protein, such as RHO proteins, thus inhibiting carcinogenesis (Konstantinopoulos et al, 2007).
Some evidence from the bedside supports these proposed mechanisms. One study showed that post-diagnostic statin use decreased the risk of breast cancer recurrence (Kwan et al, 2008). A large Danish study showed that use of simvastatin, a highly lipophilic statin, reduced recurrence risk by 10 fewer cases per 100 women over 10 years among Danish women with Stage I—III breast cancer (Ahern et al, 2011). In the same study, Ahern et al (2011) showed the simvastatin group had 30% reduction in recurrence over 10 years (hazard ratio (HR)=0.70; 95% confidence interval (CI)=0.57–0.8). Nielsen et al (2012) reported that statin use was associated with reduced cancer-related death in a large Danish study that included over 295 000 cancer patients. Interestingly, pravastatin, a hydrophilic statin, was associated with a statistically significant increase in breast cancer incidence in the CARE trial (Sacks et al, 1996). Additionally, a large prospective study by Cauley et al (2006) involving 156 351 healthy women showed that lipophilic statins (simvastatin, lovastatin, and fluvastatin) were associated with lower incidence of invasive breast cancer, suggesting that certain statins may contribute to the primary prevention of breast cancer.
Overall results of multiple epidemiologic studies on the effects of statin use on breast cancer risks, however, are inconclusive with the majority finding no clear association (Bonovas et al, 2005). As noted by Prowell et al (2006), many of the observational studies, which have analysed findings for all statin users as a single group, may have failed to reveal protective effects of certain types of statins. Studies comparing outcomes for different types of statins could reveal antitumor effects of a subset.
To the best of our knowledge, no study has examined the association between statin use and survival outcomes in primary IBC. We hypothesised that statins, particularly lipophilic statins, reduce the recurrence potential of primary IBC. The main purpose of this observational study was to determine whether statin use affected the recurrence rate and survival in patients with primary IBC who were treated at The University of Texas MD Anderson Cancer Center from 1995 to 2011. We also examined other known prognostic variables and the types of statins used to determine their effects on clinical outcomes.
Patients and methods
Patients
We searched the Inflammatory Breast Cancer Database compiled by the Breast Cancer Management System at MD Anderson Cancer Center, which contains 1177 entries of patients diagnosed with all stages of IBC between 24 February 1970 and 27 January 2011. The following exclusion criteria were applied: IBC diagnosed before 1995, stage IV disease, secondary IBC, suspected non-IBC breast cancer, unknown clinical stage, statin use after the initial evaluation at MD Anderson, unclear statin type, and notation of ‘restricted information' in MD Anderson's electronic medical record (EMR) system. Patients with unknown timing of statin use and who were not apparent statin users according to the History and Physical section of the EMR were excluded to avoid the confounding effect of statin and adjuvant treatment. We defined secondary IBC as any IBC that occurred after presentation with a non-IBC breast cancer or IBC noted as ‘secondary IBC' in the EMR. After 454 entries were eliminated under these criteria, a total of 723 patients were included in our study cohort.
Data for age, menopausal status, body mass index (BMI), race, clinical stage, nuclear grade and oestrogen receptor (ER), and progesterone (PR) status were directly extracted from the database. ER status and PR status had been determined with immunohistochemistry (IHC) with a cutoff of 10% for positivity. HER2 status was considered negative if (a) IHC results were 0 to +1 without FISH results or (b) FISH results were negative. HER2 status was considered positive if (a) IHC results were +3 without FISH results or (b) FISH results were positive (regardless of IHC results). Lymphatic/vascular invasion was determined to be present if both lymphatic and vascular invasions were recorded as positive in the database.
From the History and Physical section of the EMR, we collected information on statin use, types of statins used, comorbidities (namely hypertension and diabetes mellitus), and medications other than statins, which could affect survival and relapse outcomes (namely insulin, metformin, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), and bisphosphonates).
Statin users were defined as those whose records indicated statin use at the time of the initial evaluation at MD Anderson Cancer Center, documented in the History and Physical section of the EMR. Patients who were exclusively prescribed lipophilic statins (simvastatin, fluvastatin, and lovastatin) were classified as the lipophilic statin (ℒ-statin) group, whereas those prescribed weakly lipophilic to hydrophilic statins (atorvastatin, pravastatin, and rosuvastatin) were classified as the hydrophilic statin (H-statin) group. The hydrophobicity classification of statins, shown in Table 1, was extracted from the large Danish study by Ahern et al (2011).
Table 1. Statin classification based on the log partition coefficient, adopted from a Danish nationwide prospective cohort study conducted by Ahern et al (2011).
| Solubility classification | Drug name | Anatomical therapeutic chemical code | Log partition coefficient (octanol:water)a |
|---|---|---|---|
| Lipophilic |
Simvastatin |
C10AA01 |
4.7 |
| |
Lovastatin |
C10AA02 |
4.3 |
| |
Fluvastatin |
C10AA04 |
3.5 |
| |
Cerivastatin |
C10AA06 |
3.6 |
| Hydrophilic |
Atorvastatin |
C10AA05 |
1.5 |
| |
Pravastatin |
C10AA03 |
−0.47 |
| Rosuvastatin | C10AA07 | 1.6 |
Reproduced with kind permission of Oxford University Press from Ahern et al (2011).
Partition coefficient for atorvastatin and pravastatin were reported by Kubota et al (2004). Remaining partition coefficients were ascertained from the National Center for Biotechnology Information Pub Chem database (http://pubchem.ncbi.nlm.hih.gov/).
In the analysis based on statin types, patients who used both drug categories or without information on statin types were excluded. We further extracted patients on either simvastatin or atorvastatin and each group was compared with non-statin users. Sixteen patients using other types of statins were excluded from the data. Twenty-nine ℒ-statin users and 44 H-statin users were included in the sub-analyses.
The MDA Institutional Review Board approved the protocol for this study and granted a waiver of informed consent based on the observational nature of the study.
Outcomes
The primary outcome of this study was progression-free survival (PFS), the time in years between the diagnosis date and recurrence, progression or death. Secondary outcomes included overall survival (OS), the time interval in years from the diagnosis date to the date of death due to all causes or the last follow-up date, and disease-specific survival (DSS), the time in years from the diagnosis date to date of death due to breast cancer. For the DSS calculation, only patients whose outcomes were recorded as ‘dead with disease' were considered to have had positive events and others were censored at the time of their death or at their last follow-up dates.
Statistical analysis
The variables of interest include age, race, BMI, diabetes, and its treatments (insulin and metformin), hypertension and its treatments (ACEIs, ARBs, and beta blockers), bisphosphonate use, clinical stage, menopausal status, nuclear grade, lymphatic invasion, vascular invasion, neoadjuvant therapy, adjuvant therapy, radiation therapy, and definitive surgery within 1 year. Data were first summarised using standard descriptive statistics and frequency tabulation. Associations between categorical variables were assessed via cross-tabulation and the χ2-test or Fisher's exact test, whichever was appropriate. Survival distributions for PFS, OS, and DSS were estimated using the Kaplan–Meier method (Kaplan and Meier, 1958). Both univariate and multicovariate Cox proportional hazard models were applied to assess the effect of covariates of interest on PFS and OS (Cox, 1958). We obtained the final multivariate models by keeping the covariates that were significant in the univariate models first and using a backward selection approach, removing the least significant covariate from the full model one at a time. A P-value of <0.05 was used as the limit for inclusion. Radiation therapy, HER2 status and ER/PR status were treated as stratification factors in the multivariate Cox modes because they did not satisfy the proportional hazards assumption. Age, either continuous or dichotomous, was not statistically significant to predict the survival outcomes and thus was not included in the multivariate models. All computations were carried out using SAS 9.3 (SAS Institute Inc., Cary, NC, USA) and S-PLUS 8.2 software (Tibco Software Inc., Palo Alto, CA, USA).
Results
Patient demographics and clinical characteristics
We analysed 723 women with stage III disease (primary IBC) diagnosed between 12 January 1995 and 27 January 2011. Among these, 73 were statin users at the time of the initial evaluation and 650 were not. Given that the types of statin may influence survival outcomes, the statin users were further divided into lipophilic (L-statin, n=29) and weakly lipophilic to hydrophilic (H-statin, n=44) statin groups according to the classification used by Ahern et al (2011) (Table 1). Table 2 shows the number and percentage of statin users of our cohort by statin type.
Table 2. Frequency and percentage of statin users by statin type.
| Groups | Statin name | N (%) |
|---|---|---|
| L-statin |
Fluvastatin |
1 (3.4%) |
| |
Lovastatin |
1 (3.4%) |
| |
Simvastatin |
27 (93.1%) |
| H-statin |
Atorvastatin |
29 (65.9%) |
| |
Pravastatin |
9 (20.5%) |
| |
Pravastatin and rosuvastatin |
1 (2.3%) |
| Rosuvastatin | 5 (11.4%) |
Abbreviations: L-statin=lipophilic statin; H-statin=hydrophilic to weakly lipophilic statin.
The classification of statins is from Ahern et al (2011), as shown in Table 1.
The mean age of statin users tended to be older (H-statin users 58.8 years, range 39.1 to 75.4; L-statin users 58.9 years, range 27.6–75.4 years; nonusers 48.9 years, range 22.7–87.7 years; P<0.01), and thus a higher proportion of statin users had postmenopausal status (P<0.01). Furthermore, statin users had more comorbidities, including hypertension (P<0.01) and diabetes (P<0.01). Insulin, metformin, beta-blocker, and ACEI/ARB use was more frequent among statin users (P<0.01). The L-statin group was less likely to receive adjuvant chemotherapy than H-statin users (P<0.01). Other patient characteristics, namely ER status, PR status, HER2 status, triple-negative status, nuclear grade, lymphatic/vascular invasion, race, bisphosphonate use, and receipt of neoadjuvant chemotherapy and radiation therapy were well balanced between the three groups. Table 3 summarises the baseline patient characteristics of our cohort.
Table 3. Patient characteristics by statin type.
| Covariate | Levels | No statin | L-statin | H-statin | P-value |
|---|---|---|---|---|---|
| Age |
<50 |
359 (55.2%) |
5 (17.2%) |
6 (13.6%) |
<0.01 |
| |
⩾50 |
291 (44.8%) |
24 (82.8%) |
38 (86.4%) |
|
| Race |
White |
562 (86.5%) |
26 (89.7%) |
41 (93.2%) |
0.71 |
| |
Black |
63 (9.7%) |
3 (10.3%) |
3 (6.8%) |
|
| |
Other |
25 (3.8%) |
0 |
0 |
|
| BMI |
<25 |
162 (26.5%) |
6 (25%) |
4 (10%) |
0.07 |
| |
⩾25 and <30 |
199 (32.5%) |
5 (20.8%) |
13 (32.5%) |
|
| |
⩾30 |
251 (41%) |
13 (54.2%) |
23 (57.5%) |
|
| Menopausal status |
Post |
313 (48.6%) |
24 (82.8%) |
38 (88.4%) |
<0.01 |
| |
Pre |
331 (51.4%) |
5 (17.2%) |
5 (11.6%) |
|
| ER |
Negative |
334 (56.1%) |
17 (60.7%) |
20 (50%) |
0.66 |
| |
Positive |
261 (43.9%) |
11 (39.3%) |
20 (50%) |
|
| PR |
Negative |
394 (67.4%) |
22 (81.5%) |
26 (65%) |
0.28 |
| |
Positive |
191 (32.6%) |
5 (18.5%) |
14 (35%) |
|
| HR |
Negative |
304 (51.2%) |
16 (57.1%) |
16 (40%) |
0.31 |
| |
Positive |
290 (48.8%) |
12 (42.9%) |
24 (60%) |
|
| HER2 |
Negative |
316 (61.4%) |
19 (79.2%) |
27 (71.1%) |
0.12 |
| |
Positive |
199 (38.6%) |
5 (20.8%) |
11 (28.9%) |
|
| TNBC |
Non-TNBC |
420 (75.3%) |
16 (64%) |
28 (71.8%) |
0.41 |
| |
TNBC |
138 (24.7%) |
9 (36%) |
11 (28.2%) |
|
| Nuclear grade |
I |
6 (1%) |
0 |
0 |
0.18 |
| |
II |
102 (17.4%) |
8 (29.6%) |
12 (29.3%) |
|
| |
III |
478 (81.6%) |
19 (70.4%) |
29 (70.7%) |
|
| Lymphatic invasion |
Negative |
209 (35.9%) |
11 (42.3%) |
19 (46.3%) |
0.34 |
| |
Positive |
373 (64.1%) |
15 (57.7%) |
22 (53.7%) |
|
| Vascular invasion |
Negative |
268 (46.2%) |
11 (42.3%) |
20 (50%) |
0.82 |
| |
Positive |
312 (53.8%) |
15 (57.7%) |
20 (50%) |
|
| Lymphatic/vascular invasion |
Either negative |
277 (47.8%) |
11 (42.3%) |
20 (50%) |
0.82 |
| |
Positive/positive |
302 (52.2%) |
15 (57.7%) |
20 (50%) |
|
| Neoadjuvant chemotherapy |
No |
52 (8%) |
0 |
3 (6.8%) |
0.32 |
| |
Yes |
598 (92%) |
29 (100%) |
41 (93.2%) |
|
| Neoadjuvant hormonal therapy |
No |
636 (97.8%) |
29 (100%) |
44 (100%) |
1.00 |
| |
Yes |
14 (2.2%) |
0 |
0 |
|
| Adjuvant chemotherapy |
No |
343 (52.8%) |
25 (86.2%) |
25 (56.8%) |
<0.01 |
| |
Yes |
307 (47.2%) |
4 (13.8%) |
19 (43.2%) |
|
| Adjuvant hormonal therapy |
No |
449 (69.1%) |
21 (72.4%) |
25 (56.8%) |
0.21 |
| |
Yes |
201 (30.9%) |
8 (27.6%) |
19 (43.2%) |
|
| Radiation therapy |
No |
165 (25.4%) |
6 (20.7%) |
11 (25%) |
0.85 |
| |
Yes |
485 (74.6%) |
23 (79.3%) |
33 (75%) |
|
| Diabetes mellitus |
No |
601 (92.5%) |
20 (69%) |
29 (65.9%) |
<0.01 |
| |
Yes |
49 (7.5%) |
9 (31%) |
15 (34.1%) |
|
| Insulin |
No |
637 (98%) |
25 (86.2%) |
40 (90.9%) |
<0.01 |
| |
Yes |
13 (2%) |
4 (13.8%) |
4 (9.1%) |
|
| Metformin |
No |
627 (96.5%) |
24 (82.8%) |
36 (81.8%) |
<0.01 |
| |
Yes |
23 (3.5%) |
5 (17.2%) |
8 (18.2%) |
|
| Hypertension |
No |
520 (80%) |
9 (31%) |
19 (43.2%) |
<0.01 |
| |
Yes |
130 (20%) |
20 (69%) |
25 (56.8%) |
|
| ACEI or ARB |
No |
562(86.5%) |
16 (55.2%) |
23 (52.3%) |
<0.01 |
| |
Yes |
88 (13.5%) |
13 (44.8%) |
21 (47.7%) |
|
| Beta-blocker |
No |
568 (87.4%) |
17 (58.6%) |
26 (59.1%) |
<0.01 |
| |
Yes |
82 (12.6%) |
12 (41.4%) |
18 (40.9%) |
|
| Bisphosphonate |
No |
606 (93.2%) |
24 (82.8%) |
42 (95.5%) |
0.09 |
| |
Yes |
44 (6.8%) |
5 (17.2%) |
2 (4.5%) |
|
| Surgery within 1 year |
No |
77 (11.8%) |
2 (6.9%) |
5 (11.4%) |
0.84 |
| Yes | 573 (88.2%) | 27 (93.1%) | 39 (88.6%) |
Abbreviations: ACEI=angiotensin-converting enzyme inhibitor; ARB=angiotensin receptor blocker; BMI=body mass index; ER=oestrogen receptor; HER2=human epidermal growth factor receptor 2; HR=hazard ratio; PR=progesterone receptor; TNBC=triple-negative breast cancer.
Survival estimates
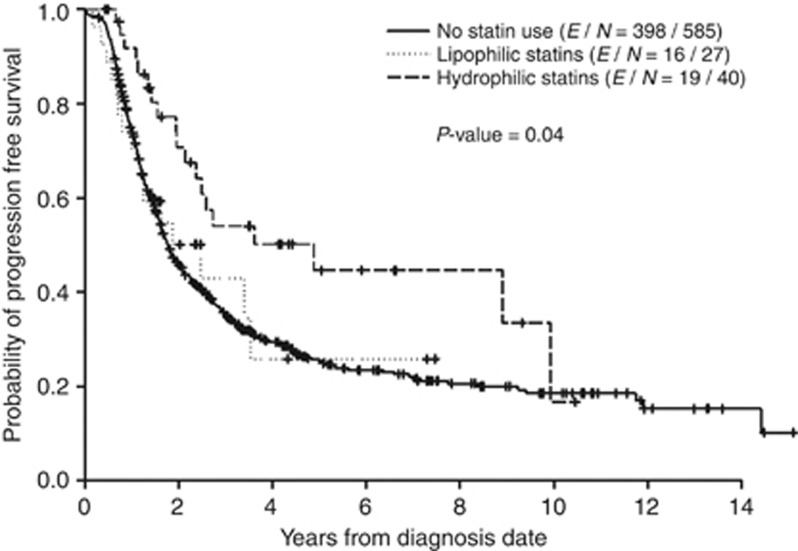
With a median follow-up of 2.9 years, 366 patients died, of which 338 died of breast cancer. Of 652 stage III IBC patients who had follow-up data for disease progression, 433 had progressive disease or died (PD/death). The median PFS time was 1.8 years (95% CI=1.7–2.1). The 2-, 5-, and 10-year PFS rates (95% CI) were 47.4% (43.5–51.6%), 26.8% (23.1–31.1%), and 18.6% (14.7–23.7%), respectively. The factors that were statistically significant in predicting longer time to progression/death in the univariate analysis were higher BMI, statin use, lower nuclear grade, negative lymphatic, and vascular involvement, positive ER status, positive PR status, negative triple-negative status, bisphosphonate use, adjuvant hormonal therapy, and radiotherapy. The group taking any type of statin had a significantly lower recurrence risk (HR (95% CI)=0.63 (0.42–0.96), P=<0.01) than did the group taking no statins. In this analysis, the HRs for OS and DSS were 1.00 (P=0.99) and 0.95 (P=0.83), respectively (Supplementary table). The median PFS times were significantly different among the statin user groups: 4.9 years, 2.5 years, and 1.8 years (P=0.04) for patients who took H-statins, L-statins, and no statin, respectively. The median OS times were 5.1 years, 3.8 years, and 4.3 years (P=0.35) and the median DSS times 5.1 years, 3.8 years, and 4.5 years (P=0.37) for the H-statin, L-statin, and non-statin groups, respectively.
Table 4 shows the final multicovariate Cox model for PFS, OS, and DSS, comparing the L-statin vs non-statin group, as well as the H-statin vs non-statin group. This multicovariate analysis included HR status and HER2 status as the stratification factors and was adjusted for lymphatic/vascular invasion for PFS and lymphatic/vascular invasion, nuclear grade, and surgery within 1 year for OS and DSS. Compared with the non-statin group, the H-statin group was associated with a lower risk of disease progression or death (HR (95% CI)=0.49 (0.28–0.84), P<0.01). The H-statin group had a trend towards lower HRs for OS and DSS compared with the non-statin group; however, P-values were 0.49 and 0.59, respectively. Lack of either lymphatic or vascular invasion was significantly associated with better PFS compared with the presence of both lymphatic and vascular invasion (HR (95% CI) =0.59 (0.47–0.75), P=<0.01). The Kaplan–Meier curves for PFS of the three groups are shown in Figure 1.
Table 4. Univariate and multicovariate cox model for PFS, OS, and DSS for H-statin and L-statin when compared with non-statin users.
| |
Univariate cox model |
Multicovariate cox modela |
||
|---|---|---|---|---|
| Covariates | HR (95% CI) | P-value | HR (95% CI) | P-value |
|
PFS | ||||
| Statin L vs N | 0.94 (0.57–1.55) | 0.81 | 0.76 (0.41–1.41) | 0.38 |
| H vs N |
0.55 (0.35–0.87) |
0.01 |
0.49 (0.28–0.84) |
<0.01 |
|
OS | ||||
| Statin L vs N | 1.23 (0.71–2.15) | 0.46 | 1.46 (0.73–2.90) | 0.28 |
| H vs N |
0.75 (0.46–1.20) |
0.23 |
0.80 (0.43–1.49) |
0.49 |
|
DSS | ||||
| Statin L vs N | 1.11 (0.61–2.03) | 0.74 | 1.18 (0.54–2.55) | 0.68 |
| H vs N | 0.71 (0.43–1.17) | 0.18 | 0.85 (0.46–1.57) | 0.59 |
Abbreviations: CI=confidence interval; DSS=disease-specific survival; L=lipophilic statin users; H=weakly lipophilic and hydrophilic statin users; N=non-statin users; OS=overall survival; PFS=progression-free survival.
Multicovariate models included radiation therapy, hormonal receptor status and HER2 status as the stratification factors and adjusted for lymphatic/vascular invasion for PFS and lymphatic/vascular invasion, nuclear grade and surgery within 1 year (Yes vs No) for OS and DSS.
Figure 1.
Kaplan–Meier curve for PFS comparing H-statin users, L-statin users, and non-statin users.
Effects of different statins were further investigated in our sub-analysis, in which we compared atorvastatin users, simvastatin users, and non-statin users. Table 5 shows the results of the multicovariate analysis on the primary outcome. Compared with non-statin user, atorvastatin use was associated with statistically significant advantages for PFS (HR (95% CI) =0.48 (0.25–0.95), P=0.03). The observed PFS benefit was similar to that in the analysis comparing H-statin users vs non-statin users, because 66% of patients in the H-statin group used atorvastatin. In a similar vein, 93% of patients in L-statin group used simvastatin. Therefore, the observed HRs for L-statin vs non-statin and H-statin vs non-statin were similar to those for simvastatin vs non-statin and atorvastatin vs non-statin. As the Kaplan–Meier curve for this sub-analysis is practically the same as Figure 1 (H-statin and L-statin vs non-statin), it is not included in this report.
Table 5. Multicovariate Cox model for PFS, OS, and DSS comparing atorvastatin and simvastatin users vs non-statin users.
| |
Multicovariate cox model |
|
|---|---|---|
| Covariates | HR (95% CI) | P-value |
|
PFS | ||
| Statin Atorva vs N |
0.48 (0.25–0.95) |
0.03 |
| Simva vs N |
0.72 (0.38–1.36) |
0.30 |
|
OS | ||
| Statin Atorva vs N |
1.00 (0.49–2.05) |
0.99 |
| Simva vs N |
1.38 (0.67–2.85) |
0.38 |
|
DSS | ||
| Statin Atorva vs N |
1.05 (0.51–2.17) |
0.89 |
| Simva vs N | 1.07 (0.47–2.46) | 0.87 |
Abbreviations: CI=confidence interval; DSS=disease-specific survival; N=non-statin users; OS=overall survival, PFS=progression-free survival.
Discussion
H-statins, not L-statins, were associated with significantly improved PFS in our cohort, which supported our hypothesis that certain statins reduce the recurrence potential of primary IBC. However, the results were not consistent with our thought that lipophilic statins would be more effective.
The inverse association between H-statin use and recurrence risk observed in our study is in agreement with other reports showing that statin use is associated with improved PFS times (Kwan et al, 2008; Ahern et al, 2011). Furthermore, our study is the first to provide clinical evidence potentially linking H-statin use to significantly improved PFS in primary IBC. H-statin users, however, had marginally improved OS and DSS compared with non-statin users. Failure to demonstrate statistically significant results in OS and DSS may have been due to a small cohort size, and/or because statins in general might not be effective in controlling or stopping progression once the disease becomes metastatic. The lack of association of L-statin use with improvement in PFS, DSS, and OS is not consistent with some studies that have shown lipophilic statins being associated with improved outcomes.
In light of improved PFS in H-statin users but not in L-statin users, one can speculate that certain types of statin may block any step involved in initiation of metastasis, including invasion, extravasation, epithelial–mesenchymal transition and angiogenesis, and may block steps involving cancer stem cells. One recent prospective laboratory study, which supports our observational findings is a non-randomised, phase II clinical trial assessing effects of atorvastatin on breast cancer in the neoadjuvant setting, conducted by Bjarnadottir et al (2013). Their study showed that 2 weeks of high-dose atorvastatin reduced the proliferation index of Ki-67 in tumour cells positive for HMG-CoA reductase. It is hypothesised that hydrophilic statins only inhibit HMG-CoA reductase within the liver and, as part of positive feedback, promote upregulation of HMG-CoA reductase in extrahepatic tissue, including the breast. Lipophilic statins such as simvastatin, on the other hand, inhibits both intrahepatic and extrahepatic HMG-CoA reductase activity and thus might be associated with decrease incidence or recurrence (Duncan et al, 2005). In our study, simvastatin, a highly lipophilic statin, demonstrated less protective effects than atorvastatin, a lesser lipophilic statin. Pharmacologically speaking, atorvastatin is much more potent than simvastatin, with stronger binding capacity to HMG-CoA inhibitors (da Costa et al, 2012), which may explain the better outcomes in our H-statin group, in which the majority of patients were using atorvastatin.
We acknowledge that controversy may arise regarding the classification of statins used in this study. We are aware that, depending on the investigators, the hydrophobicity of certain statins can be classified differently. Atorvastatin is often referred to as lipophilic statin, especially when statins are classified based on tissue selectivity and Log D classification at pH=7.4 (McTaggart et al, 2001; White, 2002). When statins are classified based on an octal:water log partition coefficient (Log P), which was adopted by Ahern et al, 2011, the relative lipophobicity of statins at pH=7.4 is indeed a ‘spectrum' and not as clear cut as ‘hydrophilic' vs ‘lipophilic'. Joshi et al (1999) showed the relative lipophobicity of different statins with the most hydrophilic pravastatin as the reference drug (rating of 1), followed by lovastatin (rating of 71), atorvastatin (76), fluvastatin (105), cerivastatin (219) and simvastatin (310) (Joshi et al, 1999). The study by Ahern et al (2011) (JNCI 2011) is one of the most recent works examining association between statin use and breast cancer recurrence risk, which showed promising results with a large cohort of 18 769 women with breast cancer. Our initial hypothesis was that use of a statin, especially a lipophilic statin such as simvastatin, may also improve clinical outcomes in an IBC population. To evaluate the outcomes in a setting that paralleled the design of Ahern et al (2011) study, the largest breast cancer cohort study addressing statin use to date, we used their classification. We are not in a position to disprove their classification. Alterations to their classification system in the future may necessitate reevaluation of our data.
A caveat to our findings is the potential for confounding by indications for statin use. Interestingly, one study showed that hypercholesterolaemia lowers the risk of metastasis in breast cancer, and it was hypothesised that high-serum cholesterol levels impair angiogenesis by suppressing tumoral and endothelial basic fibroblast growth factor and vascular endothelial growth factor (Ozdemir et al, 2004). That study by Ozdemir et al (2004), however, was not adjusted for any medication uses other than chemotherapy and hormonal therapy. None of the women studied were on statin before, during and after breast cancer treatment. Even in light of potential confounding factors, such as those described above, statins are known to have pleiotropic effects, including impeding tumour cell growth and antioxidant properties. Furthermore, if the formation of atherosclerotic vessels in hypercholesterolaemia inhibits recurrence and metastasis, statins, which stabilise endothelial function through their anti-atherosclerotic function, would be expected to favour recurrence or metastasis via the hematogenous route. However, our observations were discordant with this reasoning.
This study is limited by its observational nature. The treatment of statins was not randomly assigned but was determined at the initial evaluation of primary IBC. Medication compliance could not be confirmed and underreporting of statin use could be a potential source of error. Also the duration of statin use could not be assessed. Besides the indication for statin use, other possible confounding factors include other medications not included in this study, which may influence relapse, as well as lifestyle differences such as diet, exercise, alcohol and tobacco intake, socioeconomic status, and educational levels. Those who took statins may represent a population with better access to health care, a more health-conscious lifestyle and higher educational levels, thus potentially favouring a longer PFS time.
One of the strengths of the study is that the MD Anderson Breast Cancer Management System is prospectively and systematically maintained and survival information is updated every 9 months. Furthermore, in this single-institution study, the therapy given was more homogeneous than it might have been at multiple centres. The majority of patients included in our study (99.3%) received neoadjuvant chemotherapy, which is the standard of care for primary IBC. Although most published studies have evaluated all types of breast cancer collectively, we focused on primary IBC, resulting in a study cohort that is more biologically homogeneous and thus provides more useful clinical information. Interestingly, unlike recent publications on non-IBC breast cancer populations, beta-blocker use was not associated with better outcomes in our cohort in the univariate analysis (Barron et al, 2011; Melhem-Bertrandt et al, 2011). Furthermore, ACEI/ARB use and bisphosphonate use were not found to be independent prognostic factors when analysed in the multivariable fashion, although some studies suggest these medications may have survival benefits in breast cancer (Gnant et al, 2009; Eidtmann et al, 2010; Chae et al, 2011). We did not exclude adjuvant use of drugs other than statins; this might explain the discrepancy between our study and others showing efficacy of drugs other than statins on clinical outcomes in breast cancer.
Owing to the aggressive nature of primary IBC and its high early-relapse rate, discovery of more effective therapies for this unique population is desired. Thus, it is crucial to design extensive preclinical studies to elucidate the molecular and biological mechanisms of IBC and its tumorigenesis and metastasis. Data presented in our study are of great interest and are also hypothesis generating. Thus, prospective evaluation of statins in IBC, including double-blinded prospective randomised controlled studies, is warranted to validate these preliminary results.
Acknowledgments
We thank Limin Hsu, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, for his assistance in data management and data clarification; Jie Willey, Department of Breast Medical Oncology, the University of Texas MD Anderson Cancer Center, for assistance with protocol issues; and Sunita Patterson, Department of Scientific Publications, The University of Texas MD Anderson Cancer Center, for editorial assistance. This work was supported by National Institute of Health grants [R01 CA123318] to Naoto Ueno and MD Anderson's Cancer Center Support Grant [CA016672], the Morgan Welch Inflammatory Breast Cancer Research Program and Clinic, and the State of Texas Rare and Aggressive Breast Cancer Research Program Grant to Naoto Ueno.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
San Antonio Breast Cancer Symposium, San Antonio, TX, 4–8 December 2012 (poster discussion).
Supplementary Material
References
- Ahern TP, Pedersen L, Tarp M, Cronin-Fenton DP, Garne JP, Silliman RA, Sørensen HT, Lash TL. Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J Natl Cancer Inst. 2011;103 (19:1461–1468. doi: 10.1093/jnci/djr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol. 2011;29 (19:3635–2644. doi: 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- Bjarnadottir O, Romero Q, Bendahl PO, Jirström K, Rydén L, Loman N, Uhlén M, Johannesson H, Rose C, Grabau D, Borgquist S. Targeting HMG-CoA reductase with statins in a window-of-opportunity breast cancer trial. Breast Cancer Res Treat. 2013;138 (2:499–508. doi: 10.1007/s10549-013-2473-6. [DOI] [PubMed] [Google Scholar]
- Bonovas S, Filioussi K, Tsavaris N, Sitaras NM. Use of statins and breast cancer: a meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol. 2005;23 (34:8606–8612. doi: 10.1200/JCO.2005.02.7045. [DOI] [PubMed] [Google Scholar]
- Cauley JA, McTiernan A, Rodabough RJ. Statin use and breast cancer: prospective results from the Women's Health Initiative. J Natl Cancer Inst 17. 2006;98 (10:700–707. doi: 10.1093/jnci/djj188. [DOI] [PubMed] [Google Scholar]
- Chae YK, Valsecchi ME, Kim J, Bianchi AL, Khemasuwan D, Desai A, Tester W. Reduced risk of breast cancer recurrence in patients using ACE inhibitors, ARBs, and/or statins. Cancer Invest. 2011;29:585–593. doi: 10.3109/07357907.2011.616252. [DOI] [PubMed] [Google Scholar]
- Clendening JW, Pandyra A, Boutros PC. Dysregulation of the mevalonate pathway promotes transformation. PNAS. 2010;107 (34:15051–15056. doi: 10.1073/pnas.0910258107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DR. Regression models and life tables (with discussion) J Roy Statist Soc B. 1958;34:187–220. [Google Scholar]
- Cristofanilli M, Valero V, Buzdar AU, Kau SW, Broglio KR, Gonzalez-Angulo AM, Sneige N, Islam R, Ueno NT, Buchholz TA, Singletary SE, Hortobagyi GN. Inflammatory breast cancer (IBC) and patterns of recurrence: understanding the biology of a unique disease. Cancer. 2007;110 (7:1436–1444. doi: 10.1002/cncr.22927. [DOI] [PubMed] [Google Scholar]
- Dawood S, Merajver SD, Viens P, Vermeulen PB, Swain SM, Buchholz TA, Dirix LY, Levine PH, Lucci A, Krishnamurthy S, Robertson FM, Woodward WA, Yang WT, Ueno NT, Cristofanilli M. International expert panel on inflammatory breast cancer; consensus statement for standardized diagnosis and treatment. Ann Oncol. 2011;22 (3:515–523. doi: 10.1093/annonc/mdq345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa RF, Freire VN, Bezerra EM, Cavada BS, Caetano EW, de Lima Filho JL, Albuquerque EL. Explaining statin inhibition effectiveness of HMG-CoA reductase by quantum biochemistry computations Phys. Chem Chem Phys. 2012;14:1389–1398. doi: 10.1039/c1cp22824b. [DOI] [PubMed] [Google Scholar]
- Dunkan RE, El-Sohemy A, Archer MC. Mevalonate promotes the growth of tumors derived from human cancer cells in vivo and stimulates proliferation in vitro with enhanced cyclin-dependent kinase-2 activity. J Biol Chem 6. 2004;279 (32:33079–33084. doi: 10.1074/jbc.M400732200. [DOI] [PubMed] [Google Scholar]
- Dunkan ER, El-Sohemy A, Archer MC. Statins and cancer development. Cancer Epidemiol Biomarkers Prev. 2005;14 (8:1897–1898. doi: 10.1158/1055-9965.EPI-05-0027. [DOI] [PubMed] [Google Scholar]
- Eidtmann H, Boer R, Bundred N. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST study. Ann Oncol. 2010;21:2188–2194. doi: 10.1093/annonc/mdq217. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Angulo AM, Hennessy BT, Broglio K, Meric-Bernstam F, Cristofanilli M, Giordano SH, Buchholz TA, Sahin A, Singletary SE, Buzdar AU, Hortobágyi GN. Trends for inflammatory breast cancer: is survival improving. Oncologist. 2007;12 (8:904–912. doi: 10.1634/theoncologist.12-8-904. [DOI] [PubMed] [Google Scholar]
- Ghosh-Choudhury N, Mandal CC, Ghosh-Choudhury N, Ghosh-Choudhury G. Simvastatin induces depression of PTEN expression via NFkappaB to inhibit breast cancer growth. Cell Signal. 2010;22 (5:749–758. doi: 10.1016/j.cellsig.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Pöstlberger S, Menzel C, Jakesz R, Seifert M, Hubalek M, Bjelic-Radisic V, Samonigg H, Tausch C, Eidtmann H, Steger G, Kwasny W, Dubsky P, Fridrik M, Fitzal F, Stierer M, Rücklinger E, Greil R, ABCSG-12 Trial Investigators. Marth C. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360 (7:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- Joshi HN, Fakes MG, Serajuddin ATM. Differentiation of 3-hydroxyl-3-methylglutaryl-coenzyme A reductase inhibityors by their relative lipophilicity. Pharm Pharmacol Commun. 1999;5:269–271. [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assoc. 1958;53:457–481. [Google Scholar]
- Kenneth HW, William AF, Devesa SS, Young HA, Levine PH. Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. J Natl Cancer Inst. 2005;97 (13:966–975. doi: 10.1093/jnci/dji172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, Fujisaki K, Itoh Y, Yano T, Sendo T, Oishi R. Apoptotic injury in cultured human hepatocytes induced by HMG-CoA reductase inhibitors. Biochem Pharmacol. 2004;67 (12:2175–2186. doi: 10.1016/j.bcp.2004.02.037. [DOI] [PubMed] [Google Scholar]
- Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG. Post-translational modifications and regulation of the RAS superfamily of GTPases as anticancer targets. Nat Rev Drug Discovery. 2007;6 (7:541–555. doi: 10.1038/nrd2221. [DOI] [PubMed] [Google Scholar]
- Kwan ML, Habel LA, Flick ED, Quesenberry CP, Caan B. Post-diagnosis statin use and breast cancer recurrence in a prospective cohort study of early stage breast cancer survivors. Breast Cancer Res Treat. 2008;109 (3:573–579. doi: 10.1007/s10549-007-9683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefer DJ. Statins as potent anti-inflammatory drugs. Circulation. 2002;106 (16:2041–2042. doi: 10.1161/01.cir.0000033635.42612.88. [DOI] [PubMed] [Google Scholar]
- Ma J, Sehgal NL, Ayanian JZ, Stafford RS. National trends in statin use by coronary heart disease risk category. PLoS Med. 2005;2 (5:e123. doi: 10.1371/journal.pmed.0020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal CC, Ghosh-Choudhury N, Yoneda T, Choudhury GG, Ghosh-Choudhury N. Simvastatin prevents skeletal metastasis of breast cancer by an antagonistic interplay between p53 and CD44. J Biol Chem. 2011;286 (13:11314–11327. doi: 10.1074/jbc.M110.193714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTaggart F, Buckett L, Davidson R, Holdgate G, McCormick A, Schneck D, Smith G, Warwick M. Preclinical and clinical pharmacology of Rosuvastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Am J Cardiol Mar. 2001;87 (5A:28B–32B. doi: 10.1016/s0002-9149(01)01454-0. [DOI] [PubMed] [Google Scholar]
- Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F, Sood AK, Conzen SD, Hortobagyi GN, Gonzalez-Angulo AM. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011;29 (19:2645–2652. doi: 10.1200/JCO.2010.33.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics (US) (2010) Health, united states (2010. with special feature on death and dying. U.S. http://www.cdc.gov/nchs/data/hus/hus10.pdf. [PubMed]
- Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Eng J Med. 2012;367:1792–1802. doi: 10.1056/NEJMoa1201735. [DOI] [PubMed] [Google Scholar]
- Ozdemir BH, Akcali Z, Haberal M. Hypercholesterolemia impairs angiogenesis in patients with breast carcinoma and, therefore, lowers the risk of metastases. Am J Clin Pathol. 2004;122 (5:696–703. doi: 10.1309/HW2M-YB5T-VF4A-M0Y4. [DOI] [PubMed] [Google Scholar]
- Prowell TM, Stearns V, Trock B. Lipophilic statins merit additional study for breast cancer chemoprevention. J Clin Oncol. 2006;24 (13:2128–2129. doi: 10.1200/JCO.2005.05.1649. [DOI] [PubMed] [Google Scholar]
- Rao S, Lowe M, Herliczek TW, Keyomarsi K. Lovastatin mediated G1 arrest in normal and tumor breast cells is through inhibition of CDK2 activity and redistribution of p21 and p27, independent of p53. Oncogene. 1998;17 (18:2393–2402. doi: 10.1038/sj.onc.1202322. [DOI] [PubMed] [Google Scholar]
- Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Eng J Med. 1996;335 (14:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- Shibata MA, Ito Y, Morimoto J, Otsuki Y. Lovastatin inhibits tumor growth and lung metastasis in mouse mammary cardinoma model: p53-inependent mitochondrial-mediated apoptotic mechanism. Carcinogenesis. 2004;25 (10:1887–1898. doi: 10.1093/carcin/bgh201. [DOI] [PubMed] [Google Scholar]
- White CM. A review of the pharmacologic and pharmacokinetic aspects of rosuvastatin. J Clin Pharmacol. 2002;42 (9:963–970. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.