Abstract
Background:
Multiple early gastric cancers (EGCs) may develop in 6–14% of patients even after achieving curative endoscopic submucosal dissection (ESD); however, a useful biomarker for predicting recurrence is not available. The present study investigated whether the expression of CD44 variant 9 (CD44v9), a functional cancer stem cell marker, in the primary gastric cancer tissue represents an indicator of recurrence.
Methods:
Eighty-eight patients who underwent ESD for EGC from 2008 to 2010 were enrolled and monitored for recurrence for 3 years. The expression levels of CD44v9 in the tissue of initial EGCs were evaluated by immunohistochemistry, and the recurrence rate was compared between CD44v9-positive and CD44v9-negative groups. The mucin phenotype and expression of microRNA-21 (miR-21) and programmed cell death protein 4 (PDCD4) were also analysed.
Results:
The recurrence rate of EGC was significantly higher in the CD44v9-positive group than in the CD44v9-negative group (hazard ratio (HR), 21.8; 95% confidence interval (CI), 5.71–83.1). However, mucin phenotypes and the expression of miR-21 and PDCD4 did not predict recurrence after ESD. Meanwhile, grade of gastric atrophy was also identified as a significant marker of multiple recurrence (HR, 4.95; 95% CI, 1.30–18.8).
Conclusion:
CD44 variant 9 expression represents a potential predictive marker for recurrence in EGC.
Keywords: gastric cancer, CD44 variant 9, cancer stem cell, recurrence, biomarker, endoscopic procedure
Endoscopic submucosal dissection (ESD) is a widely used treatment for early gastric cancer (EGC) and is associated with good prognosis (Imaeda et al, 2006; Gotoda, 2007). However, multiple EGCs may develop even after achieving curative resection, with previous reports showing that multiple primary gastric carcinomas developed in 6–14% of patients with gastric carcinoma (Nomura et al, 1991; Parsonnet et al, 1991; Huang et al, 1998). Therefore, the optimal follow-up strategy to identify multiple recurrences of EGC after ESD is controversial because of the lack of a suitable biomarker for predicting recurrence. Helicobacter pylori (H. pylori) eradication has been found to reduce the recurrence rate of multiple EGC after ESD to one-third (Fukase et al, 2008), suggesting that concomitant H. pylori infection or inflammation potentially influences recurrence (Suzuki et al, 2009). In other words, one-third of the recurrent tumours cannot be prevented after H. pylori eradication. In addition, because a consensus has not been reached regarding how often endoscopy follow-up should be performed after ESD, there is an important need for predictive markers to identify patients with an increased risk of multiple recurrences of EGC.
Recently, cancer stem cells (CSCs) that exhibit stem cell-like characteristics such as multilineage potential and self-renewal potential have been identified in cancer tissue (Reya et al, 2001). Cancer stem cells are resistant to therapy because they have enhanced protection against reactive oxygen species (ROS; Diehn et al, 2009), suggesting that a link exists between CSCs and tumour recurrence and metastasis. CD44, a major adhesion molecule for the extracellular matrix, is implicated in a wide variety of physiological processes, including tumour cell invasion and metastasis (Nagano et al, 2004). Moreover, CD44 has been identified as one of the cell surface markers associated with cancer stem-like cells in various solid tumours (Collins et al, 2005; Dalerba et al, 2007). We recently reported that CD44 variant 9 (CD44v9) interacts with and stabilises xCT, a glutamate-cystine transporter, resulting in increased intracellular levels of reduced glutathione (GSH). CD44v9-positive cells demonstrate an enhanced ability to suppress the production of ROS, resulting in subsequent therapeutic resistance, recurrence, and metastasis of tumours (Ishimoto et al, 2011; Tsugawa et al, 2012; Yae et al, 2012). These findings suggest that CD44v9 has a specific function in the regulation of ROS defence and tumour growth. Therefore, if the primary EGC tissue is CD44v9 positive with the potential of maintaining such an oxidative stress defense mechanism, multifocal recurrence may be more probable, even after curative resection is achieved by ESD.
A previous report showed that mucin phenotype subclassification is a predictive recurrence marker after surgical resection for advanced gastric cancer, defined as gastric cancer in which the depth of invasion extends beyond the submucosa. Each tumour was phenotypically classified as an intestinal phenotype, a mixed phenotype, a gastric phenotype on the basis of the sum of their mucin phenotype immunohistochemistry (IHC) scores for gastric markers (MUC5AC and MUC6) and intestinal markers (MUC2 and CD10; Tsukashita et al, 2001). The number of tumours classified as gastric phenotype was significantly higher in tumours with peritoneal recurrence than in tumours without recurrence (Tajima et al, 2004). On the other hand, microRNAs (miRNAs) are small non-coding RNAs that can function as endogenous silencers of target genes and have critical roles in human malignancies (Saito et al, 2006; Saito et al, 2009). MicroRNA-21 (miR-21) is an oncogenic miRNA that is overexpressed in various human malignancies (Chan et al, 2005; Seike et al, 2009) and predominantly targets tumour-suppressor genes such as programmed cell death protein 4 (PDCD4; Fassan et al, 2011). A previous report showed that miR-21 was significantly overexpressed in human gastric cancer tissues and cell lines (Zhang et al, 2008). In addition, the expression level of miR-21 was associated with progression-related factors, such as depth of tumour invasion (Ueda et al, 2010). In addition, PDCD4 is strongly expressed in the nuclei of normal gastric mucosal cells, whereas it is expressed at lower levels in the gastric adenocarcinoma (Kakimoto et al, 2011). Low PDCD4 expression was significantly correlated with poor clinical prognosis (Motoyama et al, 2010). These findings suggest that miR-21 and PDCD4 expression are biomarkers for gastric cancer recurrence (Wu et al, 2010).
The aim of this prospective study was to identify a new biomarker for predicting multiple recurrence of EGC after ESD.
Materials and methods
Additional details are included in the Supplementary Materials.
Patients and specimens
This prospective study included patients who underwent ESD for EGC at the Keio University Hospital (Tokyo, Japan) between February 2008 and March 2010. Early gastric cancer was defined as an adenocarcinoma confined to the mucosa or submucosa of the stomach. Patients who had a history of ESD and in whom multiple ESDs were performed were excluded. Tissue samples for RNA extraction were obtained by endoscopic forceps biopsy. Following forceps biopsy, ESD was performed and resected specimens were obtained for IHC. The follow-up period was defined as the period from the time of initial ESD to the time of the last oesophagogastroduodenoscopy (EGD). Follow-up EGDs were performed at ∼2, 6, 12, 18, 24, and 36 months after initial ESD (median follow-up period 32 months).
Information on the clinical features of the patients such as gender, age, body mass index (BMI), H. pylori infection status at the time of initial ESD, smoking history, tumour location, tumour differentiation, and grade of gastric atrophy were obtained from medical records. H. pylori infection status was determined using the 13C-urea breath test, serological examination, and/or microaerobic bacterial cultivation. The grade of gastric atrophy was evaluated according to the endoscopic-atrophy-border scale described by Kimura and Takemoto (1969), which correlates with the results of histological evaluation (Ito et al, 1996; Satoh et al, 1996). On the basis of this scale, the grade of atrophy was divided into two types: closed-type/mildly extended atrophy and open-type/severely extended atrophy (Kimura and Takemoto, 1969; Ito et al, 1996; Satoh et al, 1996; Suzuki et al, 2004).
The study protocol was approved by the ethics committees of Keio University School of Medicine (Number 19-68-5), and written informed consent was obtained before subject enrolment. The UMIN Clinical Trials Registry number for this study is UMIN000001057 (http://www.umin.ac.jp/ctr/). The study was performed in accordance with the principles of the declaration of Helsinki.
Immunohistochemistry
Following deparaffinisation and rehydration, antigens for anti-CD44v9 and anti-phospho-p38MAPK antibodies were retrieved by heating samples in citrate buffer (10 mℳ, pH 6.0) for 10 min at 105 °C. Antigens for anti-MUC2, anti-MUC5AC, and anti-MUC6 antibodies were retrieved by heating samples in citrate buffer (10 mℳ, pH 6.0) for 5 min at 121 °C. Antigens for anti-CD10 antibodies were retrieved by heating samples in citrate buffer (10 mℳ, pH 9.0) for 5 min at 121 °C. Antigens for anti-PDCD4 antibodies were retrieved by heating samples in citrate buffer (10 mℳ, pH 9.0) for 10 min at 121 °C. After antigen retrieval, endogenous nonspecific peroxidases were quenched with 0.3% hydrogen peroxide for 5 min. Nonspecific binding was blocked using Protein block Serum-free (DAKO Japan, Kyoto, Japan), and each section was incubated overnight at 4 °C with the primary antibodies. CD44 variant 9 was detected with a rat monoclonal antibody (1 : 200; Ishimoto et al, 2011). Phospho-p38MAPK was detected using a rabbit monoclonal antibody (Number 9211, Cell Signaling Technology, Beverly, MA, USA; 1 : 100). Monoclonal mouse antibodies were used to detect MUC2 (NCL-MUC-2, Novocastra Laboratories, Newcastle-upon-Tyne, UK; 1 : 100), MUC5AC (NCL-MUC-5AC, Novocastra Laboratories; 1 : 50), MUC6 (NCL-MUC-6, Novocastra Laboratories; 1 : 50), and CD10 (NCL-CD10-270, Novocastra Laboratories; 1 : 50). Programmed cell death protein 4 was detected using a rabbit monoclonal antibody (600-401-965, Rockland, Gilbertsville, PA, USA; 1 : 100). Primary antibody binding was detected using a Vectastain Elite Kit (Vector Laboratories, Burlingame, CA, USA) and 3,3′-diaminobenzidine tetrahydrochloride solution (DAKO Japan). Counterstaining was performed using Gill's haematoxylin (DAKO Japan). The CD44v9 IHC score was defined as the proportion of the tumour area that showed CD44v9-positive staining. The CD44v9 IHC score was calculated by using ImageJ software (US National Institutes of Health, Bethesda, MD, USA) and setting the threshold (0–120) after the red-green-blue stack. The PDCD4 IHC score was defined as the average of the percentage of positive nuclei in each of three high-power fields of areas containing tumour cells.
Statistical analysis
Clinical factors were compared between the recurrence group and the non-recurrence group using the Student's t-test for continuous variables (i.e., age, BMI, serum CEA, serum CA19-9, and follow-up period) and the χ2-test for discrete variables (i.e., gender, H. pylori infection status, smoking history, tumour location, tumour differentiation, and the type of gastric atrophy). The optimal cut-off value for CD44v9 IHC scores, miR-21 expression levels, and PDCD4 IHC scores were determined using the receiver operating characteristic curve. Clinical factors were compared between the CD44v9-positive and CD44v9-negative groups, miR-21-higher and miR-21-lower groups, and PDCD4-higher and PDCD4-lower groups using the Student's t-test (continuous variables) or the χ2-test (discrete variables). The differences in clinical factors among mucin phenotype subclassifications were analysed using one-way analysis of variance (ANOVA) for continuous variables and the χ2-test for discrete variables. The Student's t-test, χ2-test, and one-way ANOVA were applied after normality had been established using the Shapiro–Wilk W-test. The associations of CD44v9 and the type of gastric atrophy with miR-21 and PDCD4 were evaluated using a linear regression model. Recurrence-free curves were calculated using the Kaplan–Meier method and were compared using the log rank test. Univariate and multivariate Cox proportional hazard model analyses were performed to calculate hazard ratios (HRs). Statistical significance was defined as a P-value of <0.05. The data are expressed as mean±s.d.. All statistical analyses were performed using PASW Statistics 18 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
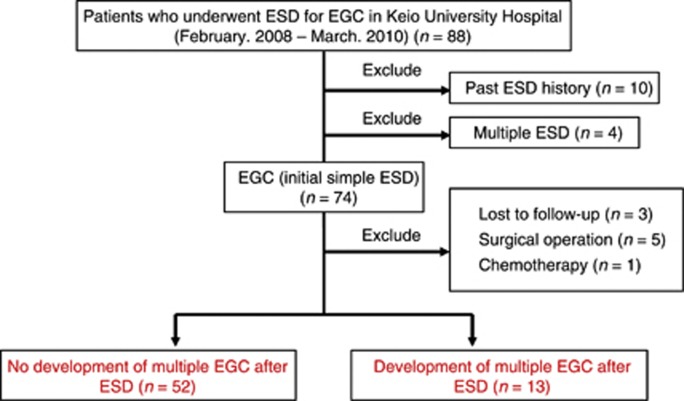
A total of 88 patients were enrolled (Figure 1). Samples from 10 patients who had a history of ESD and from 4 patients in whom multiple ESDs were performed were excluded. Among the 74 initial simple EGC patients, 3 who had never undergone endoscopic examination after initial ESD, 5 who underwent gastrectomy after ESD because of a positive margin, and 1 who was treated with chemotherapy for oesophageal cancer that developed after ESD were also excluded. Thirteen of the 65 remaining patients developed multiple recurrences during the follow-up period, whereas recurrence was not identified in 52 patients. Clinically relevant background factors in the non-recurrence and recurrence group are shown in Table 1. A significant difference in the grade of gastric atrophy was identified between these groups (P=0.046).
Figure 1.
Study design. This study included 88 patients who underwent ESD for the treatment of EGCs at the Keio University Hospital between February 2008 and March 2010. Of a total of 88 ESD samples, 10 from patients who had a history of ESD and 4 from patients in whom multiple ESDs were performed simultaneously were excluded. Of the 74 initial samples in which ESD was performed, 3 from patients who had never undergone endoscopic examination after their initial ESD, 5 from patients who had gastrectomy because of a positive margin, and 1 from a patient who was treated with chemotherapy for oesophageal cancer that developed after ESD for EGC were excluded. During the follow-up period after ESD, development of multiple recurrence of EGCs were identified in 13 cases, and no recurrences were detected in the remaining 52 cases. Abbreviations: EGC=early gastric cancer; ESD=endoscopic submucosal dissection.
Table 1. Patients' characteristics.
| Non-recurrence (n=52) | Recurrence (n=13) | P-value | |
|---|---|---|---|
| Median follow-up period |
32 months |
36 months |
|
|
Gender, number | |||
| Men (%) | 46 (88.4) | 10 (76.9) | 0.28 |
| Women (%) |
6 (11.5) |
3 (23.1) |
|
|
Age, years | |||
| Mean±s.d. (range) |
66.7±9.8 (46–90) |
71.1±8.3 (53–80) |
0.096 |
|
BMI (kg m−2), % | |||
| mean±SD (range) |
23.0±2.9 (18.6–30.8) |
24.0±4.2 (18.4–33.5) |
0.45 |
|
H. pylori, number | |||
| Positive (%) | 31 (59.6) | 8 (61.5) | 0.83 |
| Negative | |||
| Non-eradication history (%) | 5 (9.6) | 2 (15.4) | |
| Post-eradication history (%) | 14 (26.9) | 3 (23.1) | |
| Unknown (%) |
2 (3.8) |
0 (0.0) |
|
|
Smoking history, number | |||
| Smoker (%) | 11 (21.2) | 3 (23.1) | 0.62 |
| Ex-smoker (%) | 12 (23.1) | 3 (23.1) | |
| Non-smoker (%) | 17 (32.7) | 6 (46.2) | |
| Unknown (%) |
12 (23.1) |
1 (7.7) |
|
|
Location, number | |||
| Upper (%) | 1 (1.9) | 0 (0.0) | 0.88 |
| Middle (%) | 31 (59.6) | 8 (61.5) | |
| Lower (%) |
20 (38.5) |
5 (38.5) |
|
|
Differentiation, number | |||
| Tub1 | 46 (88.5) | 11 (76.9) | 0.71 |
| Tub2 |
6 (11.5) |
2 (15.4) |
|
|
Atrophy, number | |||
| Closed-type (%) | 32 (61.5) | 4 (30.8) | 0.046 |
| Open-type (%) | 20 (38.5) | 9 (69.2) | |
Abbreviation: BMI=body mass index. Bold value indicates a significant difference.
Expression of CD44v9 in EGCs
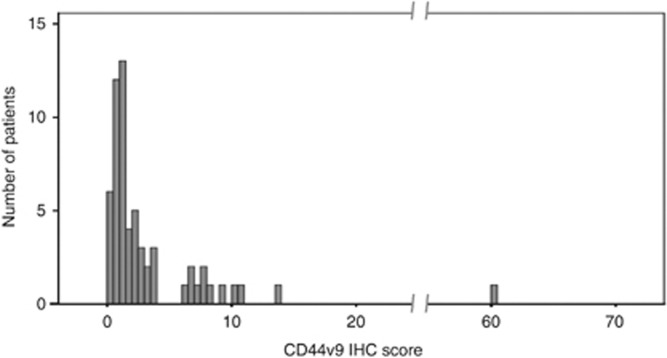
The CD44v9 IHC scores of all 65 EGCs are illustrated in Figure 2. The average CD44v IHC score was 3.58±7.74%. Interestingly, the case that showed an extraordinarily high score (60.3%) was the only case that showed recurrence twice during the follow-up period (22 and 40 months after the initial ESD). Subsequently, patients were allocated into the CD44v9-positive group (n=13) or the CD44v9-negative group (n=52) based on a cut-off level of 3.55% (sensitivity, 76.9% specificity, 93.8%, Supplementary Table 1).
Figure 2.
Histogram of CD44v9 IHC scores for all 65 EGCs. The x axis shows the CD44v9 IHC score (%) and the y axis shows the number of patients. Abbreviations: CD44v9=CD44 variant 9; EGC=early gastric cancer; IHC=immunohistochemistry.
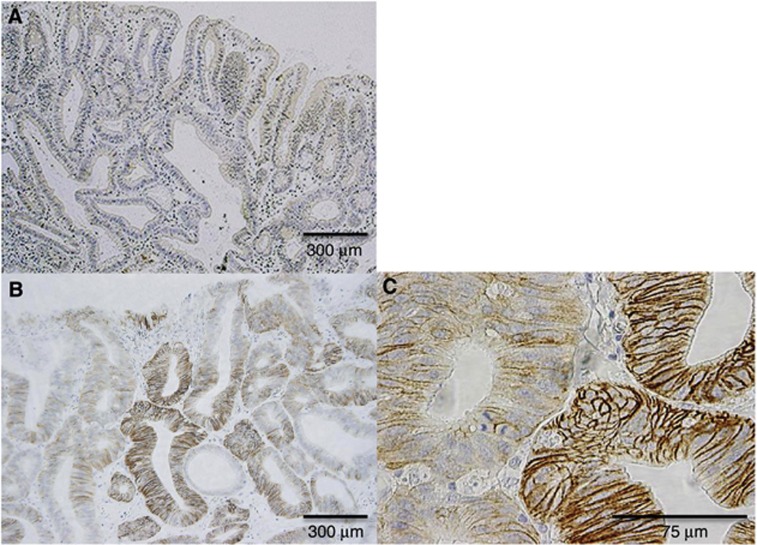
Representative CD44v9 immunohistochemical staining is shown in Figure 3. Although CD44v9 staining was barely detectable in the CD44v9-negative group (Figure 3A), CD44v9 was expressed in the epithelium of tumour glands with a heterogeneous expression pattern in the CD44v9-positive group (Figure 3B). In the high-power field, CD44v9 expression was identified along the cell membrane (Figure 3C). Significant correlations were not identified between CD44v9 positivity and any of the clinical background factors (Supplementary Table 2).
Figure 3.
Representative results of CD44v9 IHC in EGC. (A) CD44v9-negative EGC. (B and C) CD44v9-positive EGC. Inverse correlation between CD44v9 expression and phospho-p38MAPK detection in EGC. Abbreviations: CD44v9=CD44 variant 9; EGC=early gastric cancer; IHC=immunohistochemistry.
We examined the activation of p38MAPK, a major target of ROS, in cancer tissues to assess the role of CD44v9 in the regulation of intracellular oxidative stress. Immunostains for CD44v9 and phospho-p38MAPK, the active form of p38MAPK, are shown in Supplementary Figures 1A and B, respectively. Phospho-p38MAPK staining was barely detectable in the CD44v9-positive area but was apparent in the CD44v9-negative area. The immunostaining patterns of CD44v9 and phospho-p38MAPK were inversely correlated in the tumourous glands of EGC, indicating that CD44v9 expression reduces oxidative stress in cancer cells.
Expression of epithelial mucins in EGCs
All 65 EGCs were classified into three mucin phenotypes based on immunohistochemical staining against CD10, MUC2, MUC5AC, and MUC6 (Supplementary Figure 2): the gastric phenotype, 17 (26.2%) patients; the mixed phenotype, 23 (35.4%) patients; and the intestinal phenotype, 25 (38.5%) patients. A significant association between mucin phenotypes and EGC location (P=0.022) was identified (Supplementary Table 2).
Expression of miR-21 and PDCD4 in EGCs
The average miR-21 expression and PDCD4 IHC scores were 5.70%±5.45% and 20.2%±11.4%, respectively. Sufficient quantities of RNA could not be obtained for four samples, and these were excluded from the miR-21 evaluation. Patients were allocated into the miR-21-lower group (n=27) or the miR-21-higher group (n=34) based on a cut-off level of 3.58 (sensitivity, 53.8% specificity, 43.8%), and the PDCD4-lower group (n=27) and the PDCD4-higher group (n=38) based on a cut-off level of 17.7% (sensitivity, 53.8% specificity, 43.8% Supplementary Table 1). Significant correlations were not identified between the expression of miR-21 and clinical factors, but age was significantly associated with PDCD4 expression (Supplementary Table 2). In addition, significant associations were not identified between CD44v9 expression or gastric atrophy grade and miR-21 or PDCD4 (CD44v9-miR-21: R=0.162, P=0.236; CD44v9-PDCD4: R=0.035, P=0.791; atrophy-miR-21: R=0.136, P=0.297; atrophy-PDCD4: R=0.103, P=0.413).
CD44v9 expression is a potential marker for the development of multiple cancers
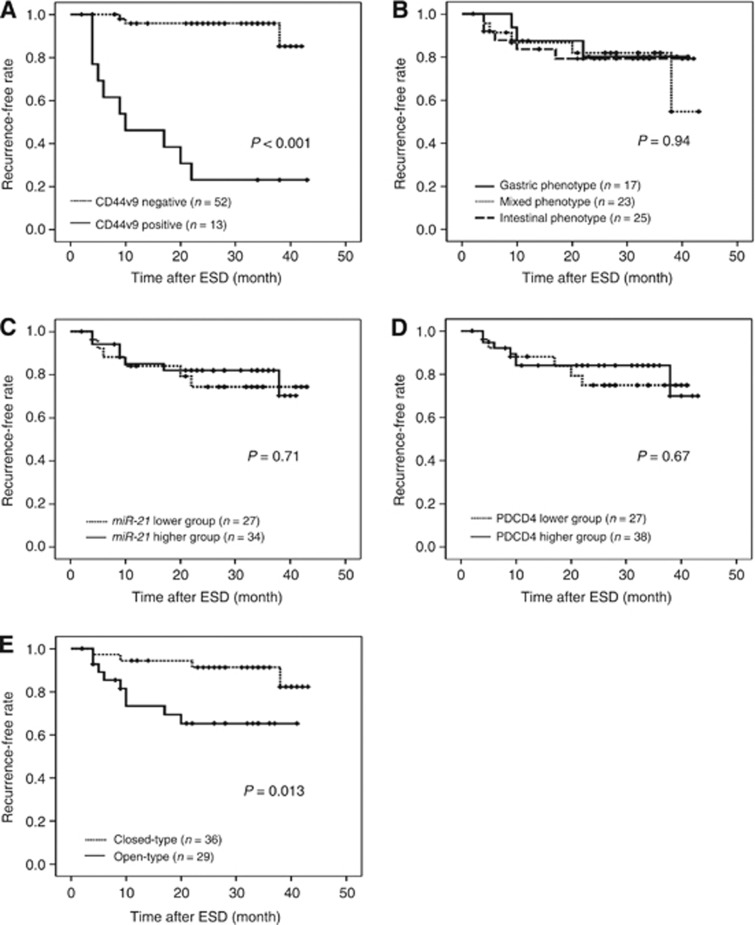
Kaplan–Meier curves for the development of EGC after ESD are shown in Figure 4. During the follow-up period, the development of EGC after ESD was identified in 10 cases (76.9%) in the CD44v9-positive group and in 3 cases (5.8%) in the CD44v9-negative group. When the recurrence rate was analysed using the log rank test, the recurrence rate was significantly higher in the CD44v9-positive group than in the CD44v9-negative group (P<0.001, Figure 4A). On the other hand, the recurrence rate was not significantly different when patients were divided based on the subclassification of tumours into mucin phenotypes (P=0.94, Figure 4B), miR-21 expression (P=0.71, Figure 4C), or PDCD4 expression (P=0.67, Figure 4D). Moreover, the recurrence rate was significantly higher for open-type gastric atrophy than that for closed-type gastric atrophy (P=0.013, Figure 4E). The HRs are shown in Table 2. CD44 variant 9 positivity significantly correlated with multiple recurrences on using the univariate Cox proportional hazards model (P<0.001; HR, 19.7; 95% confidence interval (CI), 5.37–72.4) and the multivariate Cox proportional hazards model (P<0.001; HR, 21.8; 95% CI, 5.71–83.1). The type of gastric atrophy also significantly correlated with multiple recurrences using the univariate Cox proportional hazards model (P=0.022; HR, 4.17; 95% CI, 1.23–14.1) and the multivariate Cox proportional hazards model (P=0.019; HR, 4.95; 95% CI, 1.30–18.8). However, no significant correlations were identified with regard to mucin phenotypes, miR-21 expression, or PDCD4 expression using the univariate Cox proportional hazard model.
Figure 4.
Kaplan–Meier curves. (A–E) Kaplan–Meier curves for recurrence-free rate of EGC.(A) CD44v9-negative (dotted line, n=52) and CD44v9-positive (solid line, n=13) groups; (B) mucin phenotype: gastric phenotype (solid line, n=17), mixed phenotype (dotted line, n=23), and intestinal phenotype (dashed line, n=25) groups; (C) miR-21 expression: miR-21-lower (dotted line, n=27) and miR-21-higher (solid line, n=34) groups; (D) PDCD4 expression: PDCD4-lower (dotted line, n=27) and PDCD4-higher (solid line, n=38) groups; (E) type of gastric atrophy: closed-type (dotted line, n=36) and open-type (solid line, n=29) groups. Abbreviations: CD44v9=CD44 variant 9; EGC=early gastric cancer; ESD=endoscopic submucosal dissection; miR-21=microRNA-21; PDCD4=programmed cell death protein 4.
Table 2. HRs using univariate and multivariate Cox proportional hazard model.
| |
|
Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|---|
| Factor | Class | HR | 95% CI | P-value | HR | 95% CI | P-value |
| CD44v9 |
Positive |
19.7 |
5.37–72.4 |
<0.001 |
21.8 |
5.71–83.1 |
<0.001 |
| |
Negative |
Reference |
|
|
Reference |
|
|
| Mucin phenotype |
Gastric phenotype |
0.87 |
0.21–3.64 |
0.85 |
|
|
|
| |
Mixed phenotype |
1.09 |
0.32–3.76 |
0.89 |
|
|
|
| |
Intestinal phenotype |
Reference |
|
|
|
|
|
|
miR-21 |
Higher |
0.82 |
0.27–2.43 |
0.72 |
|
|
|
| |
Lower |
Reference |
|
|
|
|
|
| PDCD4 |
Higher |
0.79 |
0.27–2.35 |
0.67 |
|
|
|
| |
Lower |
Reference |
|
|
|
|
|
| Type of gastric atrophy |
Open-type |
4.17 |
1.23–14.1 |
0.022 |
4.95 |
1.30–18.8 |
0.019 |
| Closed-type | Reference | Reference | |||||
Abbreviations: CD44v9=CD44 variant 9; CI=confidence interval; HR=hazard ratio; miR-21=microRNA-21; PDCD4=programmed cell death protein 4. Bold values indicate significant differences.
Discussion
This study represents the first report that CD44v9 expression, a marker characteristic of cancer stem-like cells, in primary EGCs can clearly predict multiple recurrence of EGC after ESD. Evaluation of CD44v9 expression represents a clinically ideal system, because it can be performed non-invasively on resected specimens when monitoring the clinical course after endoscopic treatment for gastric cancer. The HR for multiple recurrences was 21.8 in patients with CD44v9-positive tumours in our study, suggesting that the follow-up after ESD should be performed more frequently in patients with CD44v9-positive tumours than in patients with CD44v9-negative tumours. The present results support prophylactic CD44v9 monitoring following ESD that would ultimately lead to reduced medical expenditure and mortality associated with EGC recurrence.
Previously, we showed that CD44v9 expression maintains a high level of GSH, which resulted in the suppression of ROS-p38MAPK signaling and subsequent resistance to intracellular ROS in gastric cancer (Ishimoto et al, 2011). In the present study, as well as in our previous study, a reduced level of phospho-p38MAPK was identified in CD44v9-positive cells in EGC. Moreover, we have shown that CD44v9 expression was associated with the lung metastasis in the mouse breast cancer cells (Yae et al, 2012). Furthermore, Chen et al. (2013) recently reported the existence of CD44+ cells within the tumours of gastric cancer patients that are endowed with stem cells properties (Chen et al, 2013). These findings suggest that CD44v9-positive cells have CSC-like potential, and tumours with high CD44v9 levels have an increased risk of multiple recurrences. On the other hand, CD44+ cells were detectable in human primary gastric cancer, although they did not express stem cell-like properties or exhibit tumour-initiating properties in xenograft transplantation experiments (Rocco et al, 2012). However, the CD44v9 cell surface marker has the potential to identify CSCs in primary gastric cancer, whereas the general CD44 cell surface marker does not always identify gastric CSCs. It is also possible that CD44v9-positive cells may not necessarily represent true CSCs.
In 1953, Slaughter et al. introduced the concept of the field effect in cancer (also known as field defect or field cancerisation). The field effect of cancer refers to histologically abnormal epithelium adjacent to tumour tissue within the aerodigestive region and was proposed to explain the occurrence of multiple primary tumours and locally recurrent cancer out of a ‘neoplastic tendency involving many cells at once' (Slaughter et al, 1953). It is possible that the background gastric mucosa of CD44v9-positive cancer has a neoplastic tendency, as has been observed in the case of multiple hepatocellular carcinoma development from hepatic cirrhosis caused by hepatitis virus infection. A few studies have classified multiple recurrence as synchronous (<12 months) and metachronous (>12 months; Nasu et al, 2005; Nakajima et al, 2006). However, it would not be appropriate to simply classify gastric cancer according to the time-to-recurrence in the present study because of the field cancerisation concept. In present study, EGC was completely resected in all patients included in the study, and we excluded patients whose endoscopic resected specimens had positive margins (cases of incomplete resection). According to the criteria used in the studies by Nakajima et al. (2006) and Nasu et al. (2005), nine patients developed synchronous recurrence and four patients developed metachronous cancer in the present study. The development of metachronous multiple cancers was identified in 1 of 45 cases (2.2%) in the CD44v9-negative group and in 3 of 6 cases (50.0%) in the CD44v9-positive group (P<0.001; Supplementary Figure 3).
In a previous report, the severity of gastric atrophy was shown to be a predictive factor for multiple gastric cancer (Shiotani et al, 2008; Hanaoka et al, 2010). The present study showed that the recurrence rate was higher in CD44v9-positive tumours than in CD44v9-negative tumours, even when applying the multivariate Cox proportional hazard model adjusted for the type of gastric atrophy. Therefore, CD44v9 expression is an independent predictive factor, irrespective of the severity of gastric atrophy. In addition, the recurrence rate was significantly higher for samples with open-type gastric atrophy than for samples with closed-type gastric atrophy, and a significant difference was identified in the multivariate Cox proportional hazard model adjusted for CD44v9 positivity. The HR for CD44v9 positivity was greater than that for the type of gastric atrophy, suggesting that CD44v9 expression may represent a more powerful predictive recurrence marker than the type of gastric atrophy.
Although H. pylori infection clearly delineated EGC development when H. pylori-naive-negative and H. pylori-positive patients were compared (Hanaoka et al, 2010), H. pylori eradication only reduced the risk of EGC recurrence to one-third (Fukase et al, 2008). In the present study, CD44v9 positivity could predict EGC recurrence in a smaller population than was required for H. pylori infection status to predict recurrence. With regard to the infection status of H. pylori at the initial ESD in all of the present cases (Supplementary Figure 4), 32 of 52 patients in the CD44v9-negative group and 7 of 13 patients in the CD44v9-positive group were H. pylori positive (Supplementary Table 2). After ESD, the success of H. pylori eradication therapy was confirmed in 21 of 32 (65.6%) patients in the CD44v9-negative group (5.6±6.1 months after ESD) and in all 7 patients (100%) in the CD44v9-positive group (6.1±3.0 months after ESD). Therefore, H. pylori positivity as a confounding factor could only have affected the CD44v9-negative group, suggesting that the present results were reliable even after accounting for the H. pylori-associated effects. Thus, CD44v9 positivity can predict the development of multiple EGC even after accounting for the presence or absence of H. pylori infection status.
Although the incidence of gastric mucin-producing tumours was significantly higher in cases of tumours with recurrence than in cases of tumours without recurrence of advanced gastric cancer (Tajima et al, 2004), a correlation between the subclassification of mucin phenotypes and the intragastric recurrence rate was not found in the present study. Furthermore, miR-21 and PDCD4 expression did not predict the risk of developing multiple EGCs.
A limitation of this study is that the cut-off values for CD44v9 and the other biomarkers were determined on the basis of their optimal performance in this specific population of 65 patients. Risk prediction tools are known to suffer from optimism bias, and their performance in new populations does not always match the success of that in the derivation population. Therefore, prospective validation of these biomarkers using separate patient populations is required.
In conclusion, the data presented in our study suggest that the recurrence rate of EGC following ESD is higher in cases of CD44v9-positive tumours than in cases of CD44v9-negative tumours. CD44 variant 9 expression in primary EGC represents a useful biomarker for predicting patients' potential risk of developing multiple EGCs after curative resection with ESD.
Acknowledgments
This study was supported by a Grant-in-Aid for Young Scientists (B) (40570932 to KH) and a Grant-in-Aid for Scientific Research (B) (22300169, to HSu) from the Japan Society for the Promotion of Science (JSPS), a grant from the Smoking Research Foundation (to HSu), and the Keio Gijuku Academic Development Fund (to HSu).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65 (14:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- Chen W, Zhang X, Chu C, Cheung WL, Ng L, Lam S, Chow A, Lau T, Chen M, Li Y, Nie Y, Wong BC, Pang R.2013Identification of CD44+ cancer stem cells in human gastric cancer Hepatogastroenterology 60(127): DOI:10.5754/hge12881. [DOI] [PubMed] [Google Scholar]
- Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65 (23:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104 (24:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, Joshua B, Kaplan MJ, Wapnir I, Dirbas FM, Somlo G, Garberoglio C, Paz B, Shen J, Lau SK, Quake SR, Brown JM, Weissman IL, Clarke MF. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458 (7239:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassan M, Pizzi M, Giacomelli L, Mescoli C, Ludwig K, Pucciarelli S, Rugge M. PDCD4 nuclear loss inversely correlates with miR-21 levels in colon carcinogenesis. Virchows Arch. 2011;458 (4:413–419. doi: 10.1007/s00428-011-1046-5. [DOI] [PubMed] [Google Scholar]
- Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372 (9636:392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10 (1:1–11. doi: 10.1007/s10120-006-0408-1. [DOI] [PubMed] [Google Scholar]
- Hanaoka N, Uedo N, Shiotani A, Inoue T, Takeuchi Y, Higashino K, Ishihara R, Iishi H, Haruma K, Tatsuta M. Autofluorescence imaging for predicting development of metachronous gastric cancer after Helicobacter pylori eradication. J Gastroenterol Hepatol. 2010;25 (12:1844–1849. doi: 10.1111/j.1440-1746.2010.06442.x. [DOI] [PubMed] [Google Scholar]
- Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114 (6:1169–1179. doi: 10.1016/s0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- Imaeda H, Iwao Y, Ogata H, Ichikawa H, Mori M, Hosoe N, Masaoka T, Nakashita M, Suzuki H, Inoue N, Aiura K, Nagata H, Kumai K, Hibi T. A new technique for endoscopic submucosal dissection for early gastric cancer using an external grasping forceps. Endoscopy. 2006;38 (10:1007–1010. doi: 10.1055/s-2006-925264. [DOI] [PubMed] [Google Scholar]
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc- and thereby promotes tumor growth. Cancer Cell. 2011;19 (3:387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- Ito S, Azuma T, Murakita H, Hirai M, Miyaji H, Ito Y, Ohtaki Y, Yamazaki Y, Kuriyama M, Keida Y, Kohli Y. Profile of Helicobacter pylori cytotoxin derived from two areas of Japan with different prevalence of atrophic gastritis. Gut. 1996;39 (6:800–806. doi: 10.1136/gut.39.6.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimoto T, Shiraishi R, Iwakiri R, Fujimoto K, Takahashi H, Hamajima H, Mizuta T, Ideguchi H, Toda S, Kitajima Y, Ozaki I, Matsuhashi S. Expression patterns of the tumor suppressor PDCD4 and correlation with beta-catenin expression in gastric cancers. Oncol Rep. 2011;26 (6:1385–1392. doi: 10.3892/or.2011.1450. [DOI] [PubMed] [Google Scholar]
- Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1 (3:87–97. [Google Scholar]
- Motoyama K, Inoue H, Mimori K, Tanaka F, Kojima K, Uetake H, Sugihara K, Mori M. Clinicopathological and prognostic significance of PDCD4 and microRNA-21 in human gastric cancer. Int J Oncol. 2010;36 (5:1089–1095. doi: 10.3892/ijo_00000590. [DOI] [PubMed] [Google Scholar]
- Nagano O, Murakami D, Hartmann D, De Strooper B, Saftig P, Iwatsubo T, Nakajima M, Shinohara M, Saya H. Cell-matrix interaction via CD44 is independently regulated by different metalloproteinases activated in response to extracellular Ca2+ influx and PKC activation. J Cell Biol. 2004;165 (6:893–902. doi: 10.1083/jcb.200310024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T, Oda I, Gotoda T, Hamanaka H, Eguchi T, Yokoi C, Saito D. Metachronous gastric cancers after endoscopic resection: how effective is annual endoscopic surveillance. Gastric cancer. 2006;9 (2:93–98. doi: 10.1007/s10120-006-0372-9. [DOI] [PubMed] [Google Scholar]
- Nasu J, Doi T, Endo H, Nishina T, Hirasaki S, Hyodo I. Characteristics of metachronous multiple early gastric cancers after endoscopic mucosal resection. Endoscopy. 2005;37 (10:990–993. doi: 10.1055/s-2005-870198. [DOI] [PubMed] [Google Scholar]
- Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325 (16:1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325 (16:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414 (6859:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Rocco A, Liguori E, Pirozzi G, Tirino V, Compare D, Franco R, Tatangelo F, Palaia R, D'Armiento FP, Pollastrone G, Affuso A, Bottazzi EC, Masone S, Persico G, Nardone G. CD133 and CD44 cell surface markers do not identify cancer stem cells in primary human gastric tumors. J Cell Physiol. 2012;227 (6:2686–2693. doi: 10.1002/jcp.23013. [DOI] [PubMed] [Google Scholar]
- Saito Y, Liang G, Egger G, Friedman JM, Chuang JC, Coetzee GA, Jones PA. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell. 2006;9 (6:435–443. doi: 10.1016/j.ccr.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Saito Y, Suzuki H, Tsugawa H, Nakagawa I, Matsuzaki J, Kanai Y, Hibi T. Chromatin remodeling at Alu repeats by epigenetic treatment activates silenced microRNA-512-5p with downregulation of Mcl-1 in human gastric cancer cells. Oncogene. 2009;28 (30:2738–2744. doi: 10.1038/onc.2009.140. [DOI] [PubMed] [Google Scholar]
- Satoh K, Kimura K, Taniguchi Y, Yoshida Y, Kihira K, Takimoto T, Kawata H, Saifuku K, Ido K, Takemoto T, Ota Y, Tada M, Karita M, Sakaki N, Hoshihara Y. Distribution of inflammation and atrophy in the stomach of Helicobacter pylori-positive and -negative patients with chronic gastritis. Am J Gastroenterol. 1996;91 (5:963–969. [PubMed] [Google Scholar]
- Seike M, Goto A, Okano T, Bowman ED, Schetter AJ, Horikawa I, Mathe EA, Jen J, Yang P, Sugimura H, Gemma A, Kudoh S, Croce CM, Harris CC. MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc Natl Acad Sci USA. 2009;106 (29:12085–12090. doi: 10.1073/pnas.0905234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiotani A, Uedo N, Iishi H, Yoshiyuki Y, Ishii M, Manabe N, Kamada T, Kusunoki H, Hata J, Haruma K. Predictive factors for metachronous gastric cancer in high-risk patients after successful Helicobacter pylori eradication. Digestion. 2008;78 (2–3:113–119. doi: 10.1159/000173719. [DOI] [PubMed] [Google Scholar]
- Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6 (5:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Masaoka T, Hosoda H, Nomura S, Ohara T, Kangawa K, Ishii H, Hibi T. Plasma ghrelin concentration correlates with the levels of serum pepsinogen I and pepsinogen I/II ratio: a possible novel and non-invasive marker for gastric atrophy. Hepatogastroenterology. 2004;51 (59:1249–1254. [PubMed] [Google Scholar]
- Suzuki H, Iwasaki E, Hibi T. Helicobacter pylori and gastric cancer. Gastric Cancer. 2009;12 (2:79–87. doi: 10.1007/s10120-009-0507-x. [DOI] [PubMed] [Google Scholar]
- Tajima Y, Yamazaki K, Nishino N, Morohara K, Yamazaki T, Kaetsu T, Suzuki S, Kawamura M, Kumagai K, Kusano M. Gastric and intestinal phenotypic marker expression in gastric carcinomas and recurrence pattern after surgery-immunohistochemical analysis of 213 lesions. Br J Cancer. 2004;91 (7:1342–1348. doi: 10.1038/sj.bjc.6602147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugawa H, Suzuki H, Saya H, Hatakeyama M, Hirayama T, Hirata K, Nagano O, Matsuzaki J, Hibi T. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe. 2012;12 (6:764–777. doi: 10.1016/j.chom.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Tsukashita S, Kushima R, Bamba M, Sugihara H, Hattori T. MUC gene expression and histogenesis of adenocarcinoma of the stomach. Int J Cancer. 2001;94 (2:166–170. doi: 10.1002/ijc.1460. [DOI] [PubMed] [Google Scholar]
- Ueda T, Volinia S, Okumura H, Shimizu M, Taccioli C, Rossi S, Alder H, Liu CG, Oue N, Yasui W, Yoshida K, Sasaki H, Nomura S, Seto Y, Kaminishi M, Calin GA, Croce CM. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 2010;11 (2:136–146. doi: 10.1016/S1470-2045(09)70343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WK, Lee CW, Cho CH, Fan D, Wu K, Yu J, Sung JJ. MicroRNA dysregulation in gastric cancer: a new player enters the game. Oncogene. 2010;29 (43:5761–5771. doi: 10.1038/onc.2010.352. [DOI] [PubMed] [Google Scholar]
- Yae T, Tsuchihashi K, Ishimoto T, Motohara T, Yoshikawa M, Yoshida GJ, Wada T, Masuko T, Mogushi K, Tanaka H, Osawa T, Kanki Y, Minami T, Aburatani H, Ohmura M, Kubo A, Suematsu M, Takahashi K, Saya H, Nagano O. Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nat Commun. 2012;3:883. doi: 10.1038/ncomms1892. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Li Z, Gao C, Chen P, Chen J, Liu W, Xiao S, Lu H. miR-21 plays a pivotal role in gastric cancer pathogenesis and progression. Lab Invest. 2008;88 (12:1358–1366. doi: 10.1038/labinvest.2008.94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.