Abstract
Background:
Salivary adenoid cystic carcinoma (ACC) is an insidious slow-growing cancer with the propensity to recur and metastasise to distant sites. Basal-like breast carcinoma (BBC) is a molecular subtype that constitutes 15–20% of breast cancers, shares histological similarities and basal cell markers with ACC, lacks expression of ER (oestrogen receptor), PR (progesterone receptor), and HER2 (human epidermal growth factor receptor 2), and, similar to ACC, metastasises predominantly to the lung and brain. Both cancers lack targeted therapies owing to poor understanding of their molecular drivers.
Methods:
Gene expression profiling, immunohistochemical staining, western blot, RT-PCR, and in silico analysis of massive cancer data sets were used to identify novel markers and potential therapeutic targets for ACC and BBC. For the detection and comparison of gene signatures, we performed co-expression analysis using a recently developed web-based multi-experiment matrix tool for visualisation and rank aggregation.
Results:
In ACC and BBC we identified characteristic and overlapping SOX10 gene signatures that contained a large set of novel potential molecular markers. SOX10 was validated as a sensitive diagnostic marker for both cancers and its expression was linked to normal and malignant myoepithelial/basal cells. In ACC, BBC, and melanoma (MEL), SOX10 expression strongly co-segregated with the expression of ROPN1B, GPM6B, COL9A3, and MIA. In ACC and breast cancers, SOX10 expression negatively correlated with FOXA1, a cell identity marker and major regulator of the luminal breast subtype. Diagnostic significance of several conserved elements of the SOX10 signature (MIA, TRIM2, ROPN1, and ROPN1B) was validated on BBC cell lines.
Conclusion:
SOX10 expression in ACC and BBC appears to be a part of a highly coordinated transcriptional programme characteristic for cancers with basal/myoepithelial features. Comparison between ACC/BBC and other cancers, such as neuroblastomaand MEL, reveals potential molecular markers specific for these cancers that are likely linked to their cell identity. SOX10 as a novel diagnostic marker for ACC and BBC provides important molecular insight into their molecular aetiology and cell origin. Given that SOX10 was recently described as a principal driver of MEL, identification of conserved elements of the SOX10 signatures may help in better understanding of SOX10-related signalling and development of novel diagnostic and therapeutic tools.
Keywords: SOX10, salivary adenoid cystic carcinoma, basal-like breast carcinoma, melanoma, neural stem markers
Adenoid cystic carcinoma (ACC) of the salivary gland, the second most frequent salivary cancer, is notorious for nerve invasion and late recurrence (Papaspyrou et al, 2011). When compared with salivary mucoepidermoid and head and neck squamous cell carcinomas, ACC overexpressed a large cluster of neuronal genes grouped around TrkC/NTRK3, a tyrosine kinase neurotrophic receptor associated with neurogenesis and cancer (Ivanov et al, 2012). This observation suggested, for the first time, that ACC aberrantly expresses genes involved in neural stem cell differentiation. ACC was also found to express neurotrophin-3 (NT-3/NTF3), the TrkC ligand, suggesting that activation of TrkC through an autocrine signalling loop may contribute to tumour growth and dissemination.
Ectopic expression of TrkC revealed that NT-3/TrkC signalling can activate Ras, Akt, and Erk1/2 and promote metastatic behaviours, including increased motility, chemotaxis, invasion, and growth in soft agar (Ivanov et al, 2012). To further characterise the activation of the neurotropic gene expression programme in ACC, we focused on SOX10. SOX10 is of particular interest because of its roles as a marker of neural crest stem cells (NCSCs) and in the maintenance and migration of NCSCs (McKeown et al, 2005; Drerup et al, 2009; Miyahara et al, 2011). Remarkably, TrkC and Sox10 may be functionally linked, as inactivating mutations in NTRK3, NTF3, and SOX10 were identified as independent drivers of Hirschsprung disease (Pingault et al, 1998; Ruiz-Ferrer et al, 2008; Fernandez et al, 2009; Sanchez-Mejias et al, 2009), a genetic condition linked to the inability of NCSCs to migrate, differentiate, and develop into the enteric nervous system (Iwashita et al, 2003).
In addition to its role in neural crest development, SOX10 has also been identified as a driver of melanoma (MEL) progression, a cancer that develops from melanocytes that are neural crest derivatives (Shakhova et al, 2012). In this study, we report the overexpression of SOX10 in ACC and establish it as a sensitive ACC marker. Using our ACC expression array data and available public data sets, we characterise SOX10 gene signature in basal-like breast carcinoma (BBC) and compare it with ACC. BBC is perhaps the least understood breast cancer subtype that largely overlaps with triple-negative breast cancers (TNBCs), lacks obvious molecular markers, and has no effective targeted therapeutic approach (Dey et al, 2012; Gelmon et al, 2012). Together, these data suggest that a large portion of ACC and BBC may share neurologic signalling pathways associated with SOX10 activation in MEL and that these molecular similarities are of potential therapeutic importance.
Materials and methods
Head and neck cancer specimens
Original expression array data were obtained on clinical specimens from 25 patients treated at Vanderbilt Ingram University Medical Center: ACC (n=7), mucoepidermoid carcinoma (MEC, n=6), adenocarcinoma (n=2), and head and neck squamous carcinoma (n=10) (for clinical details, see (Ivanov et al, 2012)). The validation set of ACC specimens (n=13) was obtained from the Salivary Gland Tumor Biorepository (MD Anderson Cancer Center, Houston, TX, USA).
Cell lines
A375, HCC38, HCC1569, MCF7, and T47D were obtained directly from ATCC (Manassas, VA, USA). MX-1 cells were purchased from the NCI tumour repository (Frederick, MD, USA).
Expression array analyses
Collection and processing of expression array data has been described previously (Ivanov et al, 2012). Analysis of data sets from public domains available from the ArrayExpress Archive (http://www.ebi.ac.uk/arrayexpress/) was performed using MEM (http://biit.cs.ut.ee/mem/index.cgi).
Western blot analysis and antibodies
Anti-human Sox10 antibodies (NBP1-68983; Novus Biologicals, Littleton, CO, USA) and cell lysates produced from snap-frozen VUMC and UVA specimens were used, as well as 13 additional specimens from MD Anderson Cancer Center specimens as described (Ivanov et al, 2012).
Immunohistochemical studies
The salivary cancer TMA (45 1 mm cores, 14 cases in triplicates) was assembled in the laboratory of Dr Yarbrough by BB. Additional salivary cancer specimens (myoepithelial carcinoma, epimyoepithelial carcinoma, and basal cell adenoma) were obtained from the Department of Pathology, Yale School of Medicine. The breast cancer TMA that included triple-negative cases (YTMA-49-10, 0.6 mm core, n>300) was produced by the Yale Department of Pathology. Mouse embryo slides (stage E15) were obtained from Zyagen (San Diego, CA, USA). Staining with Sox10 antibodies (goat polyclonal, N-20; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was performed as described (Nonaka et al, 2008b).
Results
SOX10 is a novel and sensitive biological marker for ACC and other salivary cancers that originate from the acinar region
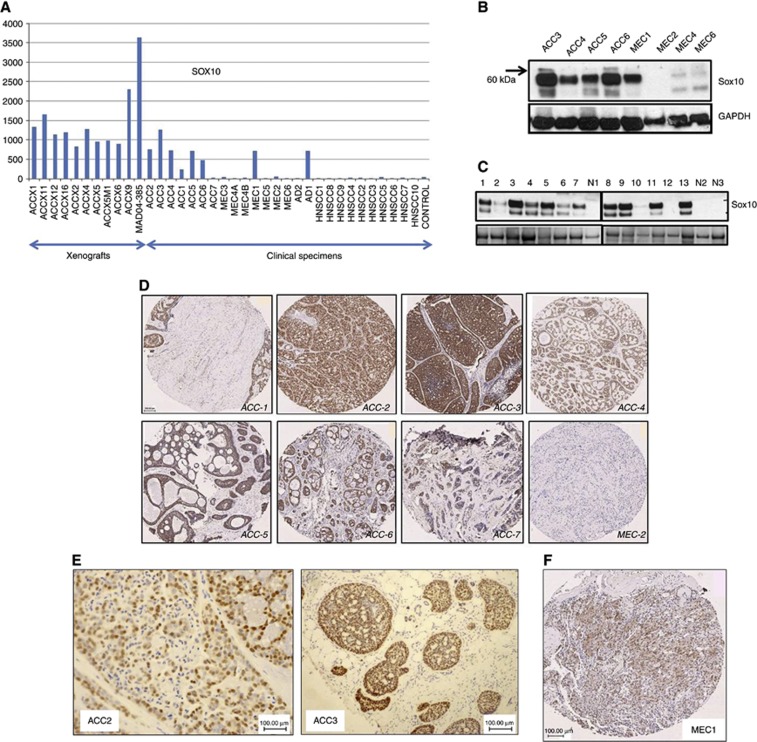
Analysis of expression array data from grossly dissected ACC and other head and neck tumours (Ivanov et al, 2012) revealed that SOX10 was expressed in 17 out of 18 ACC specimens (∼94%), including ACC xenografts produced from 11 patients (Figure 1A). SOX10 expression in primary ACC specimens was markedly higher than in normal salivary tissue (∼5-fold, ACC1; ∼25-fold, ACC3). SOX10 expression was maintained in the mouse ACC model reaching an ∼46-fold maximum in the MAD04-385 xenograft. At the protein level, SOX10 expression was confirmed in a subset of the same specimens by immunoblotting, with GAPDH serving as a loading control (Figure 1B). Studies were extended to an independent collection of clinical ACC specimens (gift of Adel El-Naggar, MD Anderson Cancer Center, n=13), wherein SOX10 was detected in all but one specimen (Figure 1C). Tumours examined in Figure 1A were immunostained and it was revealed that most ACC cancer cells were SOX10 positive (Figure 1D). Tumours with the lowest SOX10 expression as revealed by an expression array study were also those with the lowest percentage of tumour cells in the specimen (e.g., ACC1, ACC6, and ACC7). SOX10 staining in ACC tumour cells was intense in the nuclei and was also detectable in the cytoplasm in the majority of cells (∼80–90% of cells in all tumours examined, Figure 1E). Of six MEC specimens examined, only one was SOX10 positive (MEC1, Figure 1A), but, unlike ACC, staining of this tumour revealed only moderate nuclear/cytoplasmic expression (Figure 1F). Pathological re-evaluation of this case (performed by MP) classified this case as carcinoma NOS. In line with this conclusion, this peculiar SOX10-positive MEC1 case was characterised as an outlier in our previous expression array analysis (Ivanov et al, 2012).
Figure 1.
SOX10 expression in ACC. (A) Expression array data show high SOX10 levels in most primary and xenografted ACC specimens. (B) Validation of expression array data by western blot. (C) Expression of the SOX10 protein in 13 additional primary ACC specimens. (D) Immunohistochemical localisation of SOX10 expression in ACC1–7. (E) Nuclear expression of SOX10 in ACC cells. (F) Nuclear–cytoplasmic SOX10 expression detected in one out of six MEC specimens studied.
To explore the diagnostic value of Sox10 beyond ACC, we analysed two cases of myoepithelial carcinoma, three cases of epithelial–myoepithelial carcinoma, and one basal cell adenoma. In all these cases, Sox10 staining was observed in >80% of cancer cells. Differentiation between the myoepithelial and epithelial components in epithelial–myoepithelial carcinoma with p63, calponin, and CK7 confirmed that SOX10 is expressed in the myoepithelial component (data not shown).
Sox10 is expressed in embryonic and differentiated salivary tissues
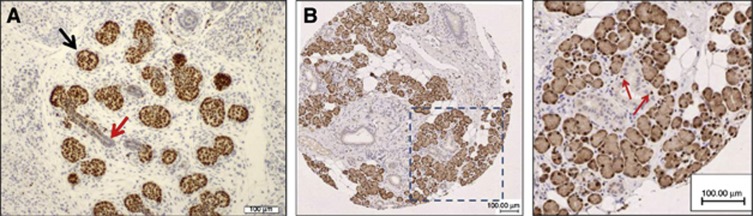
SOX10 is recognised as a marker and principal regulator of NCSCs (Britsch et al, 2001; Potterf et al, 2001; Nonaka et al, 2008b). To determine whether SOX10 is expressed in developing and mature salivary glands, mouse E15 embryonic tissue and human adult salivary and other tissues were immunostained. Presumptive acinar cells, but not ductal epithelium, expressed SOX10, suggesting that SOX10 may be involved primarily in the development and differentiation of acinar structures (Figure 2A). In line with this observation, SOX10 expression was similarly detected in the nuclei of human adult salivary gland acinar cells, as well as in the nuclei of myoepithelial cells (Figure 2B). As expected, Sox10 antibodies also stained the nuclei of melanocytes of normal skin and cutaneous MEL (Supplementary Figure 1). Altogether, these observations suggest that SOX10 has important roles in the embryogenesis and function of salivary tissue.
Figure 2.
Immunohistochemical analysis of SOX10 expression in mouse embryonic (A) and human adult (B) salivary glands. The red arrow in A points to the developing duct, whereas the black arrow shows the acinus.
Sox10 expression in basal-type breast carcinoma
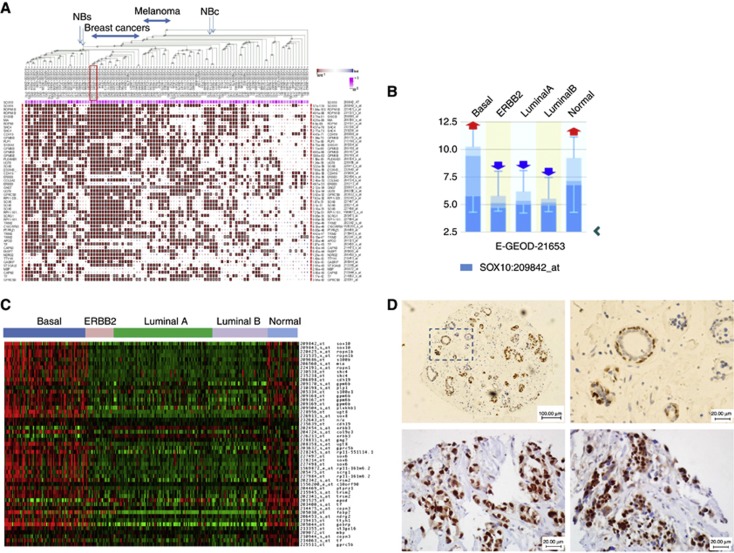
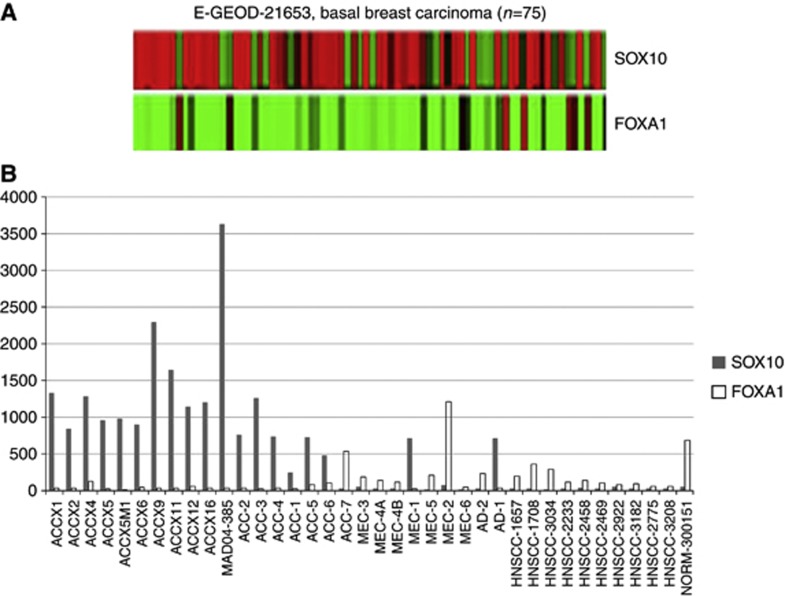
To better understand the significance of SOX10 expression in cancer in general and in ACC in particular, we explored SOX10 expression in 1764 publicly available U133 Plus 2.0 cancer data sets (http://biit.cs.ut.ee/mem/) using a novel noise-resistant rank aggregation and visualisation algorithm developed by Adler et al (2009) and Kolde et al (2012), which allows simultaneous comparison of gene expression across massive data sets. Robust SOX10 signatures with the involvement of hundreds of genes were detected in breast cancers (22 studies, 10−80<P<10−30 for top 50 genes), MEL, neuroblastoma, (Figure 3A and Supplementary Table 1), and glioma, but not in other cancers (data not shown). In breast cancer studies with stratified molecular subtypes, SOX10 and its signature strongly co-segregated with the basal subtype. Thus, analysis of the data set E-GEOD-21653 that compared expression profiles of basal, ERBB2, and luminal subtypes (total n=266) revealed that 52 of 73 basal-type specimens (71%) expressed SOX10 (Figure 3B). When compared with luminal subtypes, the basal subtype showed at least a 16-fold upregulation of SOX10 (Figure 3C, P<10−10). A similar rate of SOX10-positive BBC cases (73%) was confirmed in the other study (E-GEOD-20711, n=90, data not shown). SOX10 expression in breast cancers was validated by immunostaining on a TMA containing normal and malignant breast tissues, including TNBCs that largely overlap with BBC (Figure 3D). In normal breast tissue, SOX10 was expressed in the nuclei of basal/myoepithelial and some luminal cells. In TNBC cancer, nuclear SOX10 expression was seen on average in >60% of malignant cells. Together, these data suggest that SOX10 is expressed in normal breast tissue as well as in BBC/TNBC breast cancers serving as a marker of cell identity. When our article was in preparation, SOX10 expression in BBC was independently reported by Cimino-Mathews et al (2013).
Figure 3.
Characterisation of SOX10 signature in BBC. (A) Rank aggregation analysis identifies genes whose activity co-segregates with that of SOX10 in BBC, MEL and neuroblastoma (NBc=neuroblastoma cell lines; NBs=clinical specimens). Two breast cancer studies that stratify specimens by molecular subtypes are marked in a red frame. (B) SOX10 overexpression in BBC. (C) The heat map for the E-GEOD-21653 study shows SOX10 signature expression in a great majority of basal-like specimens but not in other breast cancer subtypes. (D) Validation of SOX10 expression in normal (upper panel) and malignant (bottom panel, YTMA-49-10 TNBC cases 1840 (left) and 1843) breast tissues.
Genes commonly co-expressed with SOX10 in ACC, BBC, and MEL
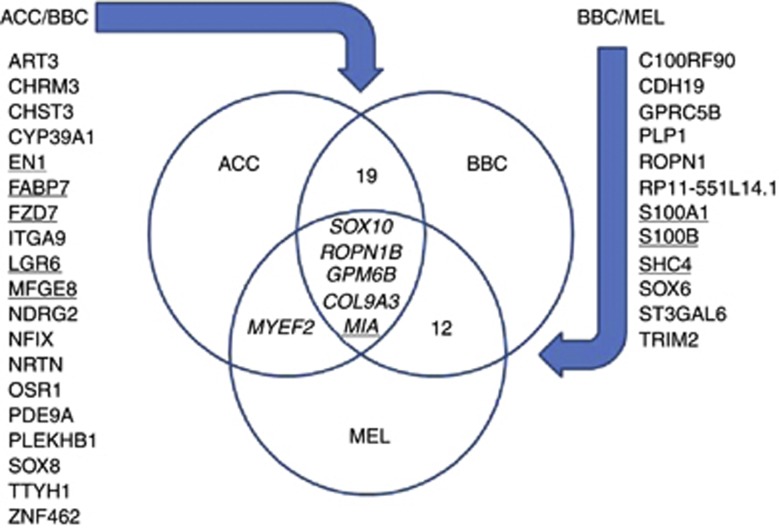
To identify critical genes that may co-function with SOX10, we performed comparative analysis of SOX10 signatures in ACC, BBC, and MEL. For each of these cancers, 160 top genes that showed the highest co-segregation with SOX10 were selected (Supplementary Table 1). A comparison of these lists revealed that ACC and BBC had 24 common genes (15%), BBC and MEL had 17 (∼11%), and ACC and MEL had 5 genes in common (∼3%). Remarkably, some of the genes from the ACC/BBC and BBC/MEL overlaps (Figure 4) have been previously described as markers of poor prognosis in MEL (MIA (Diaz-Lagares et al, 2011), S100A1 (Nonaka et al, 2008a; Sviatoha et al, 2010), S100B (Sviatoha et al, 2010; Diaz-Lagares et al, 2011) and SHC4/RaLP (Fagiani et al, 2007)), BBC (FABP7 (Alshareeda et al, 2012), FZD7 (King et al, 2012) and MFGE8 (Carrascosa et al, 2012)), and ACC (EN1 (Bell et al, 2012)), suggesting their utility in a plurality of cancers. However, clinical significance of four ‘core' genes that co-segregated with SOX10 in all three cancers, ROPN1B, GPM6B, COL9A3, and MIA, as well as many other genes found in the overlaps (e.g., CDH19, PLP1, and TRIM2) remains to be explored. To our knowledge, none of these genes have been previously studied in the context of SOX10 expression.
Figure 4.
Common elements of SOX10 signatures in ACC, BBC, and MEL. ROPN1B, COL9A3, GPM6B, and MIA are strongly co-expressed with SOX10 in all three cancers. This and other overlaps contain prospective clinical targets with several of them already known as clinically significant (underlined).
SOX10 signature is recapitulated in BBC cell lines
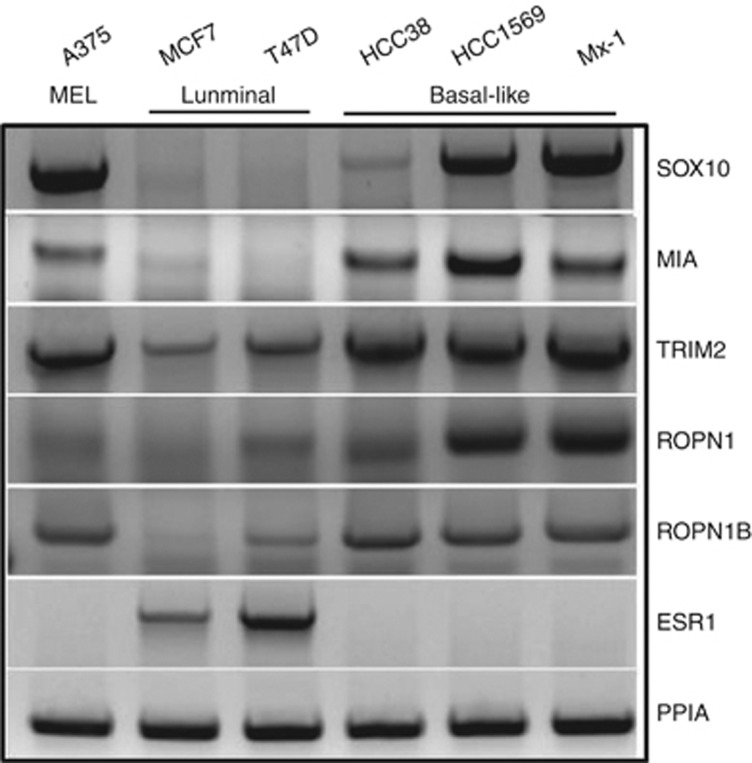
To validate our in silico findings, we assessed the expression of SOX10 signature elements in A375 MEL and breast cancer luminal (MCF, T47D) and basal-like (HCC38, HCC1569, and MX-1) cell lines. In this experiment, MEL and BBC cells expressed SOX10 and its several co-expression partners that we assessed (MIA, TRIM2, ROPN1, and ROPN1B), whereas oestrogen receptor (ESR1)-positive luminal MCF7 and T47D cell lines expressed only limited amounts of TRIM2 and none of the other SOX10 signature elements (Figure 5).
Figure 5.
Expression of SOX10 signature components in MEL and BBC cell lines. End-point RT-PCR shows that MEL and BBC cancer cell lines recapitulate the expression of SOX10 and elements of its signature, whereas ESR1-positive luminal breast cancer cell lines are SOX10 negative.
Genes whose expression negatively correlates with SOX10 expression in breast and salivary cancers
To further explore SOX10 specificity to the basal-like breast cancer subtype, we performed correlation analyses of the TCGA Invasive Breast Carcinoma data set (Agilent mRNA expression microarrays, n=547) (Cancer Genome Atlas Network, 2012) and identified FOXA1, ESR1, GATA3, XBP1, and CA12 as top-rank genes whose expression negatively correlated with SOX10 (Table 1). Noteworthy, FOXA1 showed the strongest negative correlation with SOX10, and this observation was also confirmed on the E-GEOD-21653 BBC data set (Figure 6A) as well as on our ACC expression array (Ivanov et al, 2012) data set (Figure 6B). The opposing expression of FOXA1 and SOX10 was consistent with reports that FOXA1 supports luminal breast cancer morphology (Nakshatri and Badve, 2009) and suppresses the basal-like phenotype (Bernardo et al, 2013). In addition, FOXA1 cooperates with ESR1 as a pioneer factor that maintains luminal identity in breast cancer (Zhang et al, 2010). Pioneer factors are chromatin remodellers with the capacity to modulate cellular identity by defining the genomic regions accessible for other transcription factors (Jozwik and Carroll, 2012). Three other genes from Table 1, GATA3, XBP1, and CA12, are each linked to the ESR1 and FOXA1 activities (Lacroix and Leclercq, 2004; Barnett et al, 2008; Nakshatri and Badve, 2009; Bernardo et al, 2010). Together, these data suggest that FOXA1 and SOX10 expression is mutually exclusive in breast and salivary cancers and is linked with maintenance of distinct molecular subtypes.
Table 1. Genes whose expression negatively correlates with SOX10 in the TCGA-invasive breast cancer study.
| Genes | R-value | P-value |
|---|---|---|
|
FOXA1 |
−0.63624 |
3.70E-62 |
|
MLPH |
−0.61778 |
1.01E-57 |
|
ESR1a |
−0.60447 |
1.06E-54 |
|
SIDT1 |
−0.59409 |
1.93E-52 |
|
AGR2 |
−0.59118 |
8.01E-52 |
|
PRR15 |
−0.58674 |
6.84E-51 |
|
GATA3 |
−0.58602 |
9.65E-51 |
|
XBP1 |
−0.58458 |
1.92E-50 |
|
LRFN2 |
−0.57267 |
4.95E-48 |
|
CYB561D2 |
−0.57248 |
5.39E-48 |
|
P4HTM |
−0.57196 |
6.85E-48 |
|
TBC1D9 |
−0.56207 |
5.76E-46 |
|
CA12 |
−0.55819 |
3.14E-45 |
|
FAAH2 |
−0.55712 |
5.00E-45 |
| AR | −0.55371 | 2.17E-44 |
Underlined genes cooperate in ESR1 signalling.
Figure 6.
Mutually exclusive expression of SOX10 and FOXA1 in breast and salivary cancers. Expression array data on head and neck cancers (A) and heat map for the E-GEOD-21653 study (B) show inverse SOX10 and FOXA1 expression in ACC and breast cancer, respectively.
Discussion
The transcriptional factor SOX10 appears to support stem-like properties in normal tissues and cancer cells. In normal tissue, it maintains stem cells in their undifferentiated state and controls differentiation (Wegner, 2005; Kelsh, 2006; Wong et al, 2006), whereas in MEL it serves as a marker of the stem-like CD271-positive cells (Civenni et al, 2011). In ACC, as we demonstrated previously (Ivanov et al, 2012), SOX10 expression correlates with the neural stem markers TrkC, MAP2, SALL2, and SLITRK6. In this study we establish SOX10 as a novel sensitive ACC marker, which is expressed normally during salivary gland differentiation and markedly upregulated in a great majority of ACC cells. Thus, in differentiating salivary cells and ACC, SOX10 may function in a way similar to that in differentiating melanocytes and MEL. We also characterise SOX10 as a marker of BBC, a molecular subtype of breast cancer that lacks expression of oestrogen, progesterone, and HER2 receptors (human epidermal growth factor receptor 2) (Valentin et al, 2012) and, similar to ACC, expresses basal cytokeratins (Nielsen et al, 2004) and other genes linked to myoepithelial cells (Treilleux and Morellon-Mialhe, 2009). The diagnostic value of SOX10 in BBC was confirmed by others in a recently submitted study (Cimino-Mathews et al, 2013). Unlike previously described TrkC, which is highly specific for the myoepithlial cells/cancers of salivary gland and myoepithelial cells of breast tissue, Sox10 expression in salivary tissue is not restricted to the myoepithelial cells and tumours that show myoepithelial differentiation but is also seen in acinar cells, acinic tumours, and, occasionally, in the basal cells of the intercalated duct (data not shown). Thus, Sox10 shows a broader specificity than TrkC and may be helpful for the diagnosis of salivary cancers that originate from the acinar and intercalated duct areas of the salivary gland.
Characterisation of SOX10 as a basal-like breast cancer marker in both ACC and BBC supports the hypothesis that cancer cells hijack the inherent plasticity of normal stem cells (Raouf, 2010) and stimulates more studies into the therapeutic and biological importance of SOX10 expression. Moreover, as we demonstrate here, the expression of large sets of genes strongly co-segregates with SOX10 in these cancers, greatly increasing the reliability of molecular diagnostics. These novel potential markers and targets, once validated, may significantly increase the accuracy of FNA diagnosis in ACC and BBC. Importantly, some of these genes have been already validated as diagnostic and prognostic markers.
Although SOX10 activity in MEL is essential for cell survival and growth (Shakhova et al, 2012), targeting of transcription factors is challenging. As SOX10 expression in each of three cancers appears to be part of a highly coordinated expression of hundreds of genes, a better understanding of molecular mechanisms, signalling pathways, and critical drivers that orchestrate such expression may provide a more efficient and broader means for tumour suppression. As we show, analysis of the overlaps between SOX10 gene signatures is instrumental for identification of common elements of the SOX10 network. Remarkably, two out of four genes that consistently co-expressed with SOX10, GPM6B, and COL9A3 (Figure 4) have been previously reported to bind EGFR (Deribe et al, 2009), a commonly recognised BBC marker and regulator (Carey et al, 2010). Thus, it would be interesting to explore the possible involvement of this receptor in SOX10 signalling. Two other closest SOX10 co-expression partners are ROPN1B and MIA. Although little is known about ropporin ROPN1B, its function is most likely mediated through its R2D2 motif, which is implicated in cAMP-dependent PKA signalling (Newell et al, 2008). As PKA activity is critically involved in melanocyte proliferation and stimulates the proliferation of MEL cells (Mantovani et al, 2008), it is essential to investigate the ROPN1B role in ACC and BBC. Unlike ROPN1, the MEL inhibitory activity protein MIA is a well-established diagnostic and prognostic serum marker and therapeutic target in MEL (Schmidt and Bosserhoff, 2009; Perrotta et al, 2010; Kluger et al, 2011; Schmidt et al, 2012). However, to our knowledge, its link with SOX10 has not been previously established. Studies on serum derived from ACC and BBC patients are warranted in order to assess the clinical value of MIA in these cancers.
Overall, our findings bring attention to previously unrecognised transcriptional networks and signalling pathways related to SOX10 activation in various cancers and help to identify common and cancer type-specific biomarkers and prospective therapeutic targets whose expression strongly co-segregates with SOX10.
Acknowledgments
This study was supported by funds from the Adenoid Cystic Carcinoma Research Foundation to SI and WGY, and by grant number 1RC1DE020332-01 from the National Institute of Dental and Craniofacial Research to WGY. This work was also supported in part by funds from the Department of Surgery, Yale School of Medicine, and the Vanderbilt Ingram Cancer Center, the Vanderbilt Bill Wilkerson Center for Otolaryngology and Communication Sciences, the Robert J Kleberg Jr and Helen C Kleberg Foundation, and by an endowment to the Barry Baker Laboratory for Head and Neck Oncology. We acknowledge Dr Adel El-Naggar (MD Anderson Cancer Center) and the NIH National Institute of Dental and Craniofacial Research and the NIH Office of Rare Diseases Research grant number U01DE019765 (PI, Adel El-Naggar) for providing useful discussion and tumour specimens. Authors are grateful to Dr. Luis A Chiriboga and Stephanie A Krauter for help with IHC staining. The Experimental Pathology and Immunohistochemistry core laboratory is supported in part by NYULCI Center Support Grant NIH/NCI 5 P30CA16087-31.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490 (7418:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler P, Kolde R, Kull M, Tkachenko A, Peterson H, Reimand J, Vilo J. Mining for coexpression across hundreds of datasets using novel rank aggregation and visualization methods. Genome Biol. 2009;10 (12:R139. doi: 10.1186/gb-2009-10-12-r139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshareeda AT, Rakha EA, Nolan CC, Ellis IO, Green AR. Fatty acid binding protein 7 expression and its sub-cellular localization in breast cancer. Breast Cancer Res Treat. 2012;134 (2:519–529. doi: 10.1007/s10549-012-2083-8. [DOI] [PubMed] [Google Scholar]
- Barnett DH, Sheng S, Charn TH, Waheed A, Sly WS, Lin CY, Liu ET, Katzenellenbogen BS. Estrogen receptor regulation of carbonic anhydrase XII through a distal enhancer in breast cancer. Cancer Res. 2008;68 (9:3505–3515. doi: 10.1158/0008-5472.CAN-07-6151. [DOI] [PubMed] [Google Scholar]
- Bell D, Bell A, Roberts D, Weber RS, El-Naggar AK. Developmental transcription factor EN1--a novel biomarker in human salivary gland adenoid cystic carcinoma. Cancer. 2012;118 (5:1288–1292. doi: 10.1002/cncr.26412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo GM, Bebek G, Ginther CL, Sizemore ST, Lozada KL, Miedler JD, Anderson LA, Godwin AK, Abdul-Karim FW, Slamon DJ, Keri RA. FOXA1 represses the molecular phenotype of basal breast cancer cells. Oncogene. 2013;32 (5:554–563. doi: 10.1038/onc.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo GM, Lozada KL, Miedler JD, Harburg G, Hewitt SC, Mosley JD, Godwin AK, Korach KS, Visvader JE, Kaestner KH, Abdul-Karim FW, Montano MM, Keri RA. FOXA1 is an essential determinant of ERalpha expression and mammary ductal morphogenesis. Development. 2010;137 (12:2045–2054. doi: 10.1242/dev.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, Birchmeier C, Wegner M. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001;15 (1:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience. Nat Rev Clin Oncol. 2010;7 (12:683–692. doi: 10.1038/nrclinonc.2010.154. [DOI] [PubMed] [Google Scholar]
- Carrascosa C, Obula RG, Missiaglia E, Lehr HA, Delorenzi M, Frattini M, Ruegg C, Mariotti A. MFG-E8/lactadherin regulates cyclins D1/D3 expression and enhances the tumorigenic potential of mammary epithelial cells. Oncogene. 2012;31 (12:1521–1532. doi: 10.1038/onc.2011.356. [DOI] [PubMed] [Google Scholar]
- Cimino-Mathews A, Subhawong AP, Elwood H, Warzecha HN, Sharma R, Park BH, Taube JM, Illei PB, Argani P. Neural crest transcription factor Sox10 is preferentially expressed in triple-negative and metaplastic breast carcinomas. Hum Pathol. 2013;44 (6:959–965. doi: 10.1016/j.humpath.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civenni G, Walter A, Kobert N, Mihic-Probst D, Zipser M, Belloni B, Seifert B, Moch H, Dummer R, van den Broek M, Sommer L. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 2011;71 (8:3098–3109. doi: 10.1158/0008-5472.CAN-10-3997. [DOI] [PubMed] [Google Scholar]
- Deribe YL, Wild P, Chandrashaker A, Curak J, Schmidt MH, Kalaidzidis Y, Milutinovic N, Kratchmarova I, Buerkle L, Fetchko MJ, Schmidt P, Kittanakom S, Brown KR, Jurisica I, Blagoev B, Zerial M, Stagljar I, Dikic I. Regulation of epidermal growth factor receptor trafficking by lysine deacetylase HDAC6. Sci Signal. 2009;2 (102:ra84. doi: 10.1126/scisignal.2000576. [DOI] [PubMed] [Google Scholar]
- Dey N, Smith BR, Leyland-Jones B. Targeting basal-like breast cancers. Curr Drug Targets. 2012;13 (12:1510–1524. doi: 10.2174/138945012803530116. [DOI] [PubMed] [Google Scholar]
- Diaz-Lagares A, Alegre E, Arroyo A, Gonzalez-Cao M, Zudaire ME, Viteri S, Martin-Algarra S, Gonzalez A. Evaluation of multiple serum markers in advanced melanoma. Tumour Biol. 2011;32 (6:1155–1161. doi: 10.1007/s13277-011-0218-x. [DOI] [PubMed] [Google Scholar]
- Drerup CM, Wiora HM, Topczewski J, Morris JA. Disc1 regulates foxd3 and sox10 expression, affecting neural crest migration and differentiation. Development. 2009;136 (15:2623–2632. doi: 10.1242/dev.030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiani E, Giardina G, Luzi L, Cesaroni M, Quarto M, Capra M, Germano G, Bono M, Capillo M, Pelicci P, Lanfrancone L. RaLP, a new member of the Src homology and collagen family, regulates cell migration and tumor growth of metastatic melanomas. Cancer Res. 2007;67 (7:3064–3073. doi: 10.1158/0008-5472.CAN-06-2301. [DOI] [PubMed] [Google Scholar]
- Fernandez RM, Sanchez-Mejias A, Mena MD, Ruiz-Ferrer M, Lopez-Alonso M, Antinolo G, Borrego S. A novel point variant in NTRK3, R645C, suggests a role of this gene in the pathogenesis of Hirschsprung disease. Ann Hum Genet. 2009;73 (1:19–25. doi: 10.1111/j.1469-1809.2008.00479.x. [DOI] [PubMed] [Google Scholar]
- Gelmon K, Dent R, Mackey JR, Laing K, McLeod D, Verma S. Targeting triple-negative breast cancer: optimising therapeutic outcomes. Ann Oncol. 2012;23 (9:2223–2234. doi: 10.1093/annonc/mds067. [DOI] [PubMed] [Google Scholar]
- Ivanov SV, Panaccione A, Brown B, Guo Y, Moskaluk CA, Wick MJ, Brown JL, Ivanova AV, Issaeva N, El-Naggar AK, Yarbrough WG.2012TrkC signaling is activated in adenoid cystic carcinoma and requires NT-3 to stimulate invasive behavior Oncogenee-pub ahead of print 1 October 2012; doi: 10.1038/onc.2012.377 . [DOI] [PubMed]
- Iwashita T, Kruger GM, Pardal R, Kiel MJ, Morrison SJ. Hirschsprung disease is linked to defects in neural crest stem cell function. Science. 2003;301 (5635:972–976. doi: 10.1126/science.1085649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozwik KM, Carroll JS. Pioneer factors in hormone-dependent cancers. Nat Rev Cancer. 2012;12 (6:381–385. doi: 10.1038/nrc3263. [DOI] [PubMed] [Google Scholar]
- Kelsh RN. Sorting out Sox10 functions in neural crest development. Bioessays. 2006;28 (8:788–798. doi: 10.1002/bies.20445. [DOI] [PubMed] [Google Scholar]
- King TD, Suto MJ, Li Y. The Wnt/beta-catenin signaling pathway: a potential therapeutic target in the treatment of triple negative breast cancer. J Cell Biochem. 2012;113 (1:13–18. doi: 10.1002/jcb.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger HM, Hoyt K, Bacchiocchi A, Mayer T, Kirsch J, Kluger Y, Sznol M, Ariyan S, Molinaro A, Halaban R. Plasma markers for identifying patients with metastatic melanoma. Clin Cancer Res. 2011;17 (8:2417–2425. doi: 10.1158/1078-0432.CCR-10-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolde R, Laur S, Adler P, Vilo J. Robust rank aggregation for gene list integration and meta-analysis. Bioinformatics. 2012;28 (4:573–580. doi: 10.1093/bioinformatics/btr709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix M, Leclercq G. About GATA3, HNF3A, and XBP1, three genes co-expressed with the oestrogen receptor-alpha gene (ESR1) in breast cancer. Mol Cell Endocrinol. 2004;219 (1-2:1–7. doi: 10.1016/j.mce.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Mantovani G, Bondioni S, Lania AG, Rodolfo M, Peverelli E, Polentarutti N, Veliz Rodriguez T, Ferrero S, Bosari S, Beck-Peccoz P, Spada A. High expression of PKA regulatory subunit 1A protein is related to proliferation of human melanoma cells. Oncogene. 2008;27 (13:1834–1843. doi: 10.1038/sj.onc.1210831. [DOI] [PubMed] [Google Scholar]
- McKeown SJ, Lee VM, Bronner-Fraser M, Newgreen DF, Farlie PG. Sox10 overexpression induces neural crest-like cells from all dorsoventral levels of the neural tube but inhibits differentiation. Dev Dyn. 2005;233 (2:430–444. doi: 10.1002/dvdy.20341. [DOI] [PubMed] [Google Scholar]
- Miyahara K, Kato Y, Koga H, Dizon R, Lane GJ, Suzuki R, Akazawa C, Yamataka A. Visualization of enteric neural crest cell migration in SOX10 transgenic mouse gut using time-lapse fluorescence imaging. J Pediatr Surg. 2011;46 (12:2305–2308. doi: 10.1016/j.jpedsurg.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Nakshatri H, Badve S. FOXA1 in breast cancer. Expert Rev Mol Med. 2009;11:e8. doi: 10.1017/S1462399409001008. [DOI] [PubMed] [Google Scholar]
- Newell AE, Fiedler SE, Ruan JM, Pan J, Wang PJ, Deininger J, Corless CL, Carr DW. Protein kinase A RII-like (R2D2) proteins exhibit differential localization and AKAP interaction. Cell Motil Cytoskeleton. 2008;65 (7:539–552. doi: 10.1002/cm.20279. [DOI] [PubMed] [Google Scholar]
- Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, Akslen LA, Ragaz J, Gown AM, Gilks CB, van de Rijn M, Perou CM. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10 (16:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- Nonaka D, Chiriboga L, Rubin BP. Differential expression of S100 protein subtypes in malignant melanoma, and benign and malignant peripheral nerve sheath tumors. J Cutan Pathol. 2008a;35 (11:1014–1019. doi: 10.1111/j.1600-0560.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- Nonaka D, Chiriboga L, Rubin BP. Sox10: a pan-schwannian and melanocytic marker. Am J Surg Pathol. 2008b;32 (9:1291–1298. doi: 10.1097/PAS.0b013e3181658c14. [DOI] [PubMed] [Google Scholar]
- Papaspyrou G, Hoch S, Rinaldo A, Rodrigo JP, Takes RP, van Herpen C, Werner JA, Ferlito A. Chemotherapy and targeted therapy in adenoid cystic carcinoma of the head and neck: a review. Head Neck. 2011;33 (6:905–911. doi: 10.1002/hed.21458. [DOI] [PubMed] [Google Scholar]
- Perrotta R, Bevelacqua Y, Malaguarnera G, Paladina I, Giordano M, Malaguarnera M. Serum markers of cutaneous melanoma. Front Biosci (Elite Ed) 2010;2:1115–1122. doi: 10.2741/e170. [DOI] [PubMed] [Google Scholar]
- Pingault V, Bondurand N, Kuhlbrodt K, Goerich DE, Prehu MO, Puliti A, Herbarth B, Hermans-Borgmeyer I, Legius E, Matthijs G, Amiel J, Lyonnet S, Ceccherini I, Romeo G, Smith JC, Read AP, Wegner M, Goossens M. SOX10 mutations in patients with Waardenburg-Hirschsprung disease. Nat Genet. 1998;18 (2:171–173. doi: 10.1038/ng0298-171. [DOI] [PubMed] [Google Scholar]
- Potterf SB, Mollaaghababa R, Hou L, Southard-Smith EM, Hornyak TJ, Arnheiter H, Pavan WJ. Analysis of SOX10 function in neural crest-derived melanocyte development: SOX10-dependent transcriptional control of dopachrome tautomerase. Dev Biol. 2001;237 (2:245–257. doi: 10.1006/dbio.2001.0372. [DOI] [PubMed] [Google Scholar]
- Raouf A. Basal-like breast cancers: the phenotypic disparity between the cancer-initiating cells and tumor histology. Breast Cancer Res. 2010;12 (6:316. doi: 10.1186/bcr2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ferrer M, Fernandez RM, Antinolo G, Lopez-Alonso M, Borrego S. NTF-3, a gene involved in the enteric nervous system development, as a candidate gene for Hirschsprung disease. J Pediatr Surg. 2008;43 (7:1308–1311. doi: 10.1016/j.jpedsurg.2008.02.076. [DOI] [PubMed] [Google Scholar]
- Sanchez-Mejias A, Fernandez RM, Lopez-Alonso M, Antinolo G, Borrego S. Contribution of RET, NTRK3 and EDN3 to the expression of Hirschsprung disease in a multiplex family. J Med Genet. 2009;46 (12:862–864. doi: 10.1136/jmg.2009.067819. [DOI] [PubMed] [Google Scholar]
- Schmidt J, Bosserhoff AK. Processing of MIA protein during melanoma cell migration. Int J Cancer. 2009;125 (7:1587–1594. doi: 10.1002/ijc.24508. [DOI] [PubMed] [Google Scholar]
- Schmidt J, Riechers A, Stoll R, Amann T, Fink F, Spruss T, Gronwald W, Konig B, Hellerbrand C, Bosserhoff AK. Targeting melanoma metastasis and immunosuppression with a new mode of melanoma inhibitory activity (MIA) protein inhibition. PloS One. 2012;7 (5:e37941. doi: 10.1371/journal.pone.0037941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhova O, Zingg D, Schaefer SM, Hari L, Civenni G, Blunschi J, Claudinot S, Okoniewski M, Beermann F, Mihic-Probst D, Moch H, Wegner M, Dummer R, Barrandon Y, Cinelli P, Sommer L. Sox10 promotes the formation and maintenance of giant congenital naevi and melanoma. Nat Cell Biol. 2012;14 (8:882–890. doi: 10.1038/ncb2535. [DOI] [PubMed] [Google Scholar]
- Sviatoha V, Tani E, Kleina R, Sperga M, Skoog L. Immunohistochemical analysis of the S100A1, S100B, CD44 and Bcl-2 antigens and the rate of cell proliferation assessed by Ki-67 antibody in benign and malignant melanocytic tumours. Melanoma Res. 2010;20 (2:118–125. doi: 10.1097/CMR.0b013e3283350554. [DOI] [PubMed] [Google Scholar]
- Treilleux I, Morellon-Mialhe B. [Basal-like breast cancer: a review] Ann Pathol. 2009;29 (3:180–186. doi: 10.1016/j.annpat.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Valentin MD, da Silva SD, Privat M, Alaoui-Jamali M, Bignon YJ. Molecular insights on basal-like breast cancer. Breast Cancer Res Treatment. 2012;134 (1:21–30. doi: 10.1007/s10549-011-1934-z. [DOI] [PubMed] [Google Scholar]
- Wegner M. Secrets to a healthy Sox life: lessons for melanocytes. Pigment Cell Res. 2005;18 (2:74–85. doi: 10.1111/j.1600-0749.2005.00218.x. [DOI] [PubMed] [Google Scholar]
- Wong CE, Paratore C, Dours-Zimmermann MT, Rochat A, Pietri T, Suter U, Zimmermann DR, Dufour S, Thiery JP, Meijer D, Beermann F, Barrandon Y, Sommer L. Neural crest-derived cells with stem cell features can be traced back to multiple lineages in the adult skin. J Cell Biol. 2006;175 (6:1005–1015. doi: 10.1083/jcb.200606062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liang J, Li Y, Xuan C, Wang F, Wang D, Shi L, Zhang D, Shang Y. CCCTC-binding factor acts upstream of FOXA1 and demarcates the genomic response to estrogen. J Biol Chem. 2010;285 (37:28604–28613. doi: 10.1074/jbc.M110.149658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.