Abstract
Background:
Breast cancer is the most common malignancy in women. Although chemotherapeutic agents, such as paclitaxel, are effective treatments for the majority of breast cancer patients, recurrence is frequent and often leads to death. Thus, there is an urgent need to identify novel therapeutic targets that sensitise tumour cells to existing chemotherapy agents.
Methods:
The levels of leukotriene B4 receptor-2 (BLT2) in multidrug-resistant MCF-7/DOX cells were determined using quantitative PCR and FACS analysis. The potential role of BLT2 in the paclitaxel resistance of MCF-7/DOX cells was assessed using a pharmacological inhibitor and small interfering RNA knockdown, and the BLT2-associated resistance mechanism was assessed.
Results:
The expression levels of BLT2 were markedly upregulated in MCF-7/DOX cells. The inhibition of BLT2 by pre-treatment with LY255283 or siBLT2 knockdown significantly sensitised MCF-7/DOX cells to paclitaxel and induced significant levels of apoptotic death, suggesting that BLT2 mediates paclitaxel resistance. We also demonstrated that BLT2-induced paclitaxel resistance was associated with the upregulation of P-glycoprotein. Finally, co-treatment with a BLT2 inhibitor and paclitaxel markedly reduced tumour growth in an MCF-7/DOX in vivo model.
Conclusion:
Together, our results demonstrate that BLT2 is a novel therapeutic target that sensitises drug-resistant breast cancer cells to paclitaxel.
Keywords: breast cancer, paclitaxel resistance, multidrug resistance, BLT2, P-glycoprotein
Multidrug resistance (MDR) is a considerable problem in progressive breast cancer patients undergoing treatment with chemotherapy. Among the various chemotherapeutic agents, paclitaxel, a member of the taxane group (mitotic inhibitors and anti-microtubule agents), has been shown to have therapeutic benefits against breast cancer (Rowinsky, 1997). The response rate to paclitaxel, however, markedly decreases to 20–40% when used as a second- or third-line chemotherapy regimen (Crown et al, 2004); nearly half of the treated individuals exhibit resistance and do not respond to treatment. Therefore, the identification of molecular mechanisms of paclitaxel resistance in breast cancer is of considerable interest. It has been reported that the expression of the multidrug transporter P-glycoprotein (P-gp), encoded by multidrug resistance gene 1 (MDR1), is one cause of clinical drug resistance to taxanes (Gottesman et al, 1996; Duan et al, 2004). In addition, MDR1 is overexpressed in tumour cells with multidrug-resistant phenotypes. However, the regulation of MDR1 expression and stability is not fully understood.
Leukotriene B4 (LTB4) is a potent proinflammatory lipid mediator that has a role in the pathogenesis of several inflammatory diseases (Peters-Golden and Henderson, 2007). Recent studies have suggested that LTB4 and its receptors critically regulate tumour progression by promoting cell proliferation, survival, migration, and metastasis (Hennig et al, 2008; Rocconi et al, 2008; Sveinbjornsson et al, 2008; Choi et al, 2010; Kim et al, 2010). For example, LTB4-related inflammatory signalling has been shown to stimulate cell proliferation by activating extracellular signal-regulated kinase (ERK) in several types of cancer cells, such as colon and pancreatic cancer cell lines (Tong et al, 2005; Ihara et al, 2007). In addition, elevated levels of LTB4 and its receptors have been observed in many types of tumours, including pancreatic cancer, colon cancer, ovarian cancer, and neuroblastomas (Yoo et al, 2004; Rocconi et al, 2008; Sveinbjornsson et al, 2008). Moreover, LY293111, an antagonist of the LTB4 receptor leukotriene B4 receptor-1 (BLT1), inhibited the growth and induced the apoptosis of human pancreatic cancer and lymphoma cells (Tong et al, 2002; Zhang et al, 2005). Expression of the LTB4 receptor BLT2 in ovarian and breast cancer tissue was also found to be increased in advanced-stage tumours and to be associated with a poor clinical outcome (Rocconi et al, 2008; Choi et al, 2010; Seo et al, 2012). Despite these observations, implicating BLT2 as a potential marker for aggressive cancer, the role of BLT2 in drug resistance has remained uncharacterised.
We have now found that BLT2 expression is markedly upregulated in the multidrug-resistant MCF-7/DOX cells and that BLT2 has a principal role in mediating the paclitaxel resistance of these cells. We also show that ERK acts downstream of BLT2 signalling and regulates P-gp levels. Together, our results indicate that BLT2 confers chemoresistance and is a novel target for the treatment of drug-resistant breast cancer.
Materials and methods
Materials
Fetal bovine serum (FBS), RPMI 1640, DMEM, and non-essential amino acids were obtained from Life Technologies (Gaithersburg, MD, USA). AA861, baicalein, U75302, and LY255283 were obtained from Cayman Chemical (Ann Arbor, MI, USA). Bovine serum albumin (BSA) and dimethyl sulphoxide (DMSO) were obtained from Sigma-Aldrich (St Louis, MO, USA). PD98059 was from Calbiochem (La Jolla, CA, USA), and paclitaxel was obtained from AG Scientific, Inc. (San Diego, CA, USA). Antibodies to poly ADP-ribose polymerase (PARP) and ERK were obtained from Cell Signaling Technology (Danvers, MA, USA), and antibodies to MDR1, 5-lipoxygenase (5-LO), and 12-lipoxygenase (12-LO) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All other chemicals were obtained from standard sources and were of molecular biology grade or higher.
Cell culture
The human breast cancer cell line MCF-7 was obtained from the Korean Cell Line Bank (Seoul, Korea). The human ovarian cancer cell line OVCAR-8 is a kind gift from Dr Soo-Youl Kim (National Cancer Center, Goyang, Korea) and NCI/ADR-RES cells were obtained from Dr Yong-Nyun Kim (National Cancer Center). MCF-7, OVCAR-8, and NCI/ADR-RES cells were maintained in RPMI 1640 supplemented with 10% heat-inactivated FBS, 2 mℳ glutamine, penicillin (100 U ml−1), and streptomycin (100 U ml−1). Multidrug-resistant MCF-7/DOX cells were kindly provided by Dr Byeong Jae Lee (Seoul National University, Seoul, Korea) (Kim et al, 2003) and were maintained in DMEM supplemented with 10% heat-inactivated FBS, 2 mℳ glutamine, penicillin (100 U ml−1), and streptomycin (100 U ml−1). The MCF-7/DOX cells were isolated by the stepwise selection of MCF-7 cells exposed to increasing concentrations of doxorubicin (DOX) in Dr Lee's laboratory (Kim et al, 2003). All cells were maintained at 37 °C under an atmosphere of 5% CO2.
Cell growth and MTT cell viability assay
The cells were plated at a density of 4 × 105 cells per 35-mm dish. After 24 h, the cells were incubated in medium containing 0.5% FBS for 3 h. Then, the cells were treated with paclitaxel and incubated at 37 °C for 48 h. To measure cell growth, the treated cells were trypsinised and counted using the trypan blue exclusion method.
To determine cell viability, the cells were plated at 4 × 104 cells per well in 96-well plates. The cells were incubated in medium containing 0.5% for 3 h. The cells were left untreated or were pre-treated with the indicated inhibitors for 30 min, followed by treatment with paclitaxel for 48 h. The viable, adherent cells were stained with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT; 5 mg ml−1; USB Corporation, Cleveland, OH, USA) at 37 °C for 3 h. The media were removed, and the formazan crystals were dissolved by adding 100 μl DMSO per well. Finally, the density of formazan in each well was detected at 570 nm using a microplate reader. The cell viabilities were expressed as the ratios relative to untreated control cells.
Flow cytometric analysis of the sub-G1 population
The sub-G1 (apoptotic) population was assessed as previously described (Choi et al, 2010). The cells were treated as described for the MTT cell viability assay and then were fixed in 70% ethanol, resuspended in 0.5 ml phosphate-buffered saline (PBS), mixed with 0.5 ml DNA extraction buffer (19.2 mℳ Na2HPO4, 0.004% Triton X-100), and incubated for 5 min at room temperature. The cells were then isolated by centrifugation at 400 g for 5 min, stained for 30 min with PI (50 μg ml−1) in PBS containing RNase A (100 μg ml−1), and analysed using flow cytometry with a FACScan instrument and CellQuest software (Becton Dickinson, Franklin Lakes, NJ, USA). The cells with a DNA content less than that of cells in G1 phase (sub-G1) were considered apoptotic.
Semi-quantitative RT-PCR and real-time quantitative PCR analysis
Total RNA was extracted from cells with the use of the easy-BLUE RNA extraction kit (Intron, Sungnam, Korea) and then was dissolved in diethylpyrocarbonate-treated water and quantified by measuring the absorbance at 260 nm. The RNA (1.25 μg) was subjected to reverse transcription (RT) followed by PCR amplification of BLT1, BLT2, MDR1, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA with the use of an RT-PCR PreMix Kit (Intron). The primer sequences used are as follows: human BLT1 (forward, 5′-TATGTCTGCGGAGTCAGCATGTACGC-3′ reverse, 5′-CCTGTAGCCGACGCCCTATGTCCG-3′); human BLT2 (forward, 5′-AGCCTGGAGACTCTGACCGCTTTCG-3′ reverse, 5′-GACGTAGCACCGGGTTGACGCTA-3′); human MDR1 (forward, 5′-CCCATCATTGCAATAGCAGG-3′ reverse, 5′-GTTCAAACTTCTGCTCCTG-3′); and GAPDH (forward, 5′-CTGCACCACCAACTGCTTAGC-3′ reverse, 5′-CTTCACCACCTTCTTGATGTC-3′). The PCR products were purified by 1.5% agarose gel electrophoresis and visualised with ethidium bromide.
For real-time PCR analysis, total RNA (1.25 μg) extracted from cells with the use of the easy-BLUE RNA extraction kit (Intron) was subjected to RT with Moloney murine leukaemia virus reverse transcriptase (Invitrogen, Carlsbad, CA, USA). The BLT1, BLT2, and GAPDH cDNA libraries were amplified as described previously (Choi et al, 2010) with the use of a LightCyber 480 SYBR Green I Master instrument (Roche, Mannheim, Germany). The melting curves were analysed to ensure amplification specificity for the PCR products. The BLT1 and BLT2 mRNA levels were normalised to the corresponding level of GAPDH mRNA.
RNA interference
The BLT2 mRNA depletion using RNA interference (RNAi) was performed as described previously (Choi et al, 2010). The BLT2-specific and scrambled control small interfering RNAs (siRNAs) were designed previously (Hennig et al, 2008) and were obtained from Ambion (Austin, TX, USA).
Flow cytometric analysis for BLT2 expression
To quantify BLT2 expression on the cell surface, the cells were incubated in 60-mm dishes for 24 h. The cells were detached with trypsin, washed with PBS, and fixed in 2% paraformaldehyde. After exposure to 2% BSA for 30 min, the cells were incubated for 1 h at room temperature with rabbit polyclonal antibodies to BLT2 (MBL-2097, 1 : 100 dilution; Life Span, Des Plaines, IL, USA), washed three times with PBS, and incubated for 30 min at room temperature with fluorescein isothiocyanate-conjugated goat antibodies to rabbit immunoglobulin G (1 : 200 dilution; Molecular Probes, Eugene, OR, USA). The cells (10 000 per sample) were then subjected to flow cytometry with the use of a FACSCalibur instrument and CellQuest software (Becton Dickinson) to determine the mean fluorescence intensity.
Immunoblot analysis
The cells were collected and lysed in lysis buffer (20 mℳ Tris-HCl pH 7.5, 150 mℳ NaCl, 0.5% NP-40, 5 mℳ EDTA, 1% Triton X-100, and protease inhibitors). The proteins in the lysates were separated on SDS-PAGE gels and transferred to polyvinylidene difluoride membranes, after which the membranes were blocked with 5% non-fat dry milk in Tris-buffered saline and incubated with primary antibodies. The blots were developed with peroxidase-conjugated secondary antibodies, and the proteins were visualised using ECL reagents (Amersham, Arlington Heights, IL, USA) according to the manufacturer's recommendations.
Measurement of LTB4 and 12(S)-hydroxyeicosatetraenoic acid
MCF-7 and MCF-7/DOX cells were seeded in 35-mm dishes and incubated in medium containing 0.5% FBS for 48 h, after which the culture supernatants were collected, freeze-dried overnight, and reconstituted with the assay buffer supplied with the ELISA kits for LTB4 or 12(S)-hydroxyeicosatetraenoic acid (12(S)-HETE) obtained from Assay Designs (Ann Arbor, MI, USA). The LTB4 and 12(S)-HETE concentrations were measured according to the manufacturer's instructions.
DNA fragmentation assay
The cells were plated at a density of 4 × 106 cells per 100-mm dish. After 24 h, the cells were treated with U75302 (1 μℳ) and LY255283 (10 μℳ) for 30 min or were transfected with BLT2 siRNA for 24 h. Then, the cells were treated with paclitaxel for 48 h. Both attached and detached cells were collected and resuspended in lysis buffer (20 mℳ Tris-HCl pH 8.0, 0.1 mℳ ethylenediaminetetraacetic acid, 1% SDS and 0.5 mg ml−1 proteinase K) and incubated at 50 °C overnight. The DNA was extracted with phenol chloroform. The DNA samples were electrophoresed on a 1.8% agarose gel and visualised using ethidium bromide staining.
Xenograft study
This study was approved by the Ethics Committee of Korea University, and all experimental animals used in this study were treated according to the guidelines approved by the Institutional Animal Care and Use Committee of Korea University. We used 6-week-old female nude mice (Charles River, Wilmington, MA, USA) to generate an experimental orthotopic breast cancer model. Mice were injected unilaterally with 1.0 × 107 cells in 100 μl PBS containing 50% Matrigel (BD Bioscience, Bedford, MA, USA) into the fourth right mammary fat pad by subcutaneous injection at the base of the nipple. Tumour growth was monitored at least twice weekly using vernier callipers. When the tumours grew to ∼5 mm in diameter, the mice (N=6 per group) were randomly divided into the DMSO vehicle control, LY255283-treated, paclitaxel-treated, or the LY255283+paclitaxel-treated groups. Then, the appropriate mice were injected with LY255283 (2.5 mg kg−1) or DMSO by i.p. injection for 4 weeks (twice a week (Saturday and Wednesday)), whereas paclitaxel was administered at 15 mg kg−1 i.p. for 4 weeks (once a week (Wednesday)). All mice were killed 1 week after the end of treatment. The tumours were excised and weighed.
Data analyses and statistics
The results are presented as the means±s.d. Analyses were performed with Student's t-test using SigmaPlot 8.0 software. P-values less than 0.05 were considered significant.
Results
Upregulation of BLT2 and its ligands in multidrug-resistant MCF-7/DOX cells
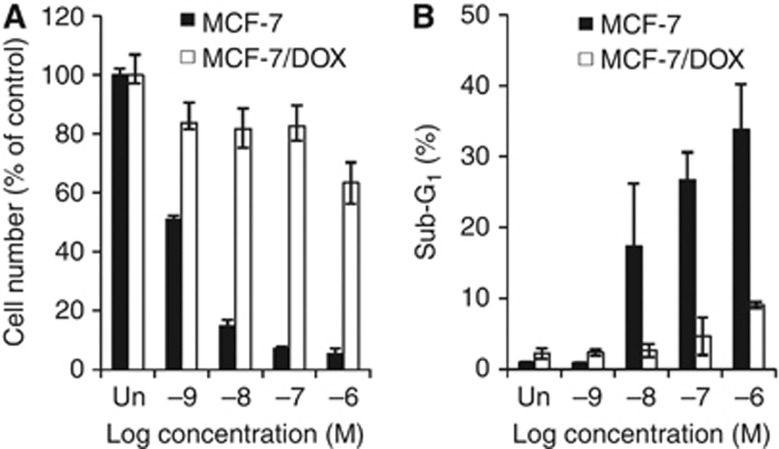
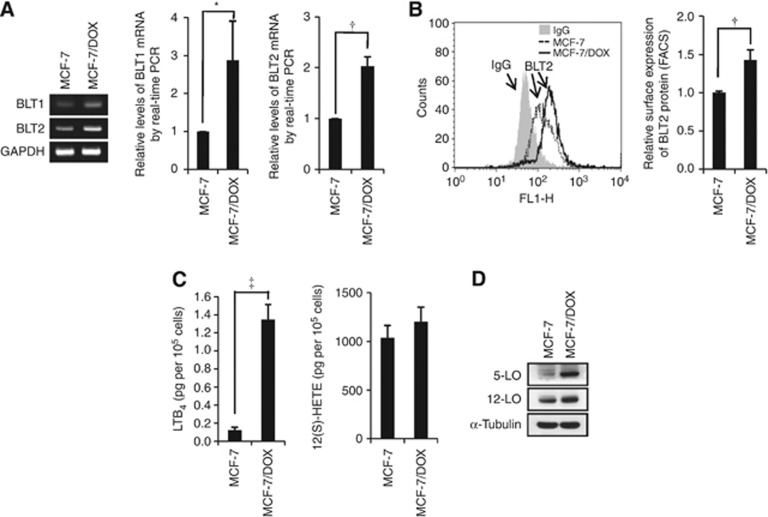
As reported, MCF-7/DOX cells display resistance to chemotherapeutic agents, such as paclitaxel. As shown in Figure 1, although the parental MCF-7 cells showed a dose-dependent decrease in viable cell numbers (Figure 1A) and a dose-dependent increase in apoptotic population (Figure 1B) after paclitaxel treatment, the MCF-7/DOX cells showed significant resistance to paclitaxel treatment. To elucidate the role of BLT2 in paclitaxel resistance, we first analysed BLT2 expression levels in MCF-7 and MCF-7/DOX cells. Both semi-quantitative RT-PCR and quantitative real-time PCR analysis revealed that mRNA levels of BLT2 as well as BLT1 were markedly increased (approximately two-fold) in MCF-7/DOX cells compared with MCF-7 cells (Figure 2A). Consistent with these results, the abundance of BLT2 on the cell surface, as determined by flow cytometry, was significantly higher in MCF-7/DOX cells than in MCF-7 cells (Figure 2B). Together, these results suggest that BLT2 expression is markedly upregulated in MCF-7/DOX cells. We next determined the levels of the BLT2 ligands LTB4 and 12(S)-HETE. The amount of LTB4 in the culture supernatants was substantially greater in MCF-7/DOX cells than in MCF-7 cells, and the amount of 12(S)-HETE was also increased in MCF-7/DOX cells, albeit less than those of LTB4 (Figure 2C). Consistent with these results, immunoblot analyses revealed that the amounts of 5-LO and 12-LO, key enzymes in the synthesis of LTB4 and 12(S)-HETE, respectively, were increased in MCF-7/DOX cells (Figure 2D). Together, these results indicate that the expression of BLT2 and its ligands are markedly upregulated in paclitaxel-resistant MCF-7/DOX cells.
Figure 1.
The effect of paclitaxel on MCF-7/DOX cell survival. (A) MCF-7 and MCF-7/DOX cells were treated with increasing concentrations of paclitaxel ranging from 1 nℳ to 1 μℳ for 48 h, and the viable cell numbers were determined using trypan blue staining. (B) MCF-7 and MCF-7/DOX cells treated as in (A) were assayed for apoptotic population by flow cytometric analysis of the sub-G1 population. All quantitative data are shown as the means±s.d. from three independent experiments.
Figure 2.
Upregulation of BLT2 and its ligands in multidrug-resistant MCF-7/DOX cells. (A) Semi-quantitative RT-PCR (left panel) and quantitative real-time PCR (centre and right panels) were performed to analyse BLT1 and BLT2 mRNA levels in MCF-7 and MCF-7/DOX cells. The data are representative of three independent experiments. (B) The expression levels of BLT2 on the cell surface were determined by flow cytometric analysis. (C) MCF-7 (2.5 × 105) and MCF-7/DOX (2.5 × 105) cells were cultured in 35-mm dishes for 48 h, after which the amounts of LTB4 and 12(S)-HETE in the culture supernatants were measured using ELISA assays. (D) The expression levels of 5-LO and 12-LO were determined using immunoblot assays. The data are representative of three independent experiments. All quantitative data are expressed relative to the values for the MCF-7 cells and are shown as the means±s.d. from three independent experiments. *P<0.05, †P<0.01, ‡P<0.005.
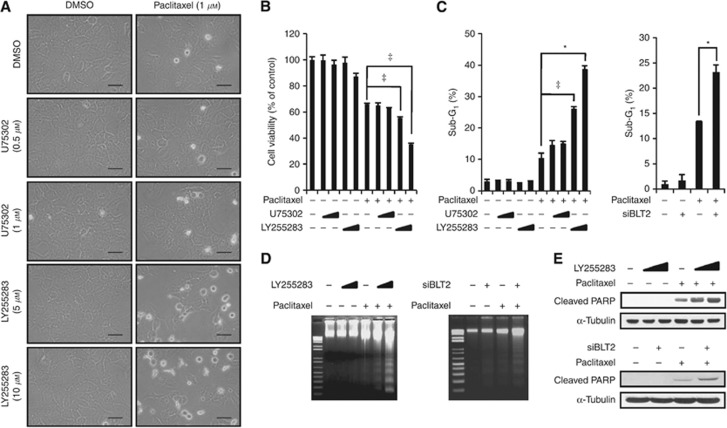
Suppression of BLT2 with a pharmacological inhibitor or depletion after RNAi knockdown significantly attenuates paclitaxel resistance in MCF-7/DOX cells
To investigate a possible role for upregulated BLT2 in paclitaxel resistance, MCF-7/DOX cells were pre-treated with the BLT2 inhibitor LY255283 for 30 min and then were treated with paclitaxel for 48 h. Pre-treatment with LY255283 in MCF-7/DOX cells caused significant morphological changes typical of apoptotic cells, whereas pre-treatment with the BLT1 inhibitor U75302 failed to trigger a change in morphology (Figure 3A). We next analysed cell viability using an MTT assay. As shown in Figure 3B, BLT2 inhibition by LY255283 enhanced cell death in response to paclitaxel treatment in MCF-7/DOX cells, whereas U75302 had no such effect. To further analyse the consequence of BLT2 inhibition in paclitaxel-induced apoptosis, we performed sub-G1 analysis (Figure 3C), DNA fragmentation assays (Figure 3D), and PARP cleavage assays (Figure 3E) after exposure to LY255283 or BLT2 depletion by RNAi. Transfection with BLT2 siRNA resulted in selective knockdown of BLT2 mRNA but did not affect the amount of BLT1 mRNA (Supplementary Figure 1). The suppression of BLT2 significantly attenuated paclitaxel resistance in MCF-7/DOX cells, suggesting that BLT2 may be associated with paclitaxel chemoresistance.
Figure 3.
The BLT2 suppression with a pharmacological inhibitor or RNAi knockdown significantly attenuated paclitaxel resistance of MCF-7/DOX cells. (A) MCF-7/DOX cells were incubated in the presence of 0.5% serum for 3 h and then were pre-treated with U75302 (0.5 or 1 μℳ), LY255283 (5 or 10 μℳ), or DMSO for 30 min before paclitaxel (1 μℳ) treatment for 48 h. The cells were visualised using an Olympus BX51 microscope at × 40 magnification. Scale bars, 50 μm. (B) MCF-7/DOX cells treated as in A were assayed for cell viability using an MTT assay. (C) MCF-7/DOX cells were incubated with U75302 (0.5 or 1 μℳ), LY255283 (5 or 10 μℳ), or DMSO for 30 min or were transfected with siBLT2 or control siRNAs for 24 h. Then, the cells were treated with or without paclitaxel (1 μℳ) for 48 h for determination of cell apoptosis by flow cytometric analysis of the sub-G1 population. All quantitative data are shown as the means±s.d. of three independent experiments. *P<0.05, ‡P<0.005. (D and E) MCF-7/DOX cells were incubated with LY255283 (5 or 10 μℳ) or DMSO for 30 min, or were transfected with siBLT2 or control siRNAs for 24 h, after which the cells were treated with or without paclitaxel (1 μℳ) for 48 h for determination of cell apoptosis using a DNA fragmentation assay (D) and PARP cleavage (E). The data are representative of three independent experiments.
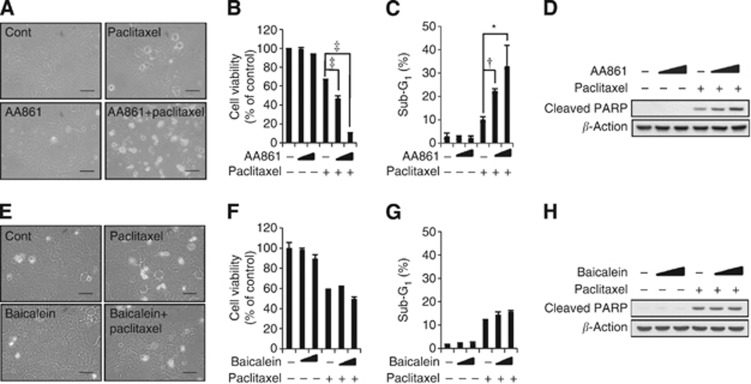
Inhibition of 5-LO significantly attenuates paclitaxel resistance in MCF-7/DOX cells
The BLT2 is a G-protein-coupled receptor that is expressed on the cell surface and interacts with specific ligands, such as LTB4 and 12(S)-HETE (Yokomizo et al, 1997, 2000). Thus, we examined the involvement of BLT2 ligands in paclitaxel resistance using AA861, a 5-LO inhibitor, or baicalein, a 12-LO inhibitor. Of the inhibitors tested, only AA861 significantly enhanced cell death in the presence of paclitaxel, whereas baicalein had no effect (Figure 4A and E). To further analyse the consequence of 5-LO inhibition in paclitaxel-induced apoptosis, we performed MTT assays, sub-G1 analysis, and cleaved PARP immunoblot assays. We found that AA861 but not baicalein dramatically reduced cell viability in the presence of paclitaxel (Figure 4B and F). In addition, AA861 increased the sub-G1 fraction (Figure 4C) and cleaved PARP levels (Figure 4D) in response to paclitaxel, whereas baicalein had no effect (Figure 4G and H). Together, these results suggested that the LTB4–BLT2 signalling pathway is associated with MCF-7/DOX paclitaxel resistance.
Figure 4.
The inhibition of 5-LO significantly attenuated paclitaxel resistance in MCF-7/DOX cells. (A and E) MCF-7/DOX cells were incubated in the presence of 0.5% serum for 3 h and then were pre-treated with AA861 (10 μℳ) (A) or baicalein (20 μℳ) (E) for 30 min before paclitaxel (1 μℳ) treatment for 48 h. Cell morphology was visualised using an Olympus BX51 microscope at × 40 magnification. Scale bars, 50 μm. (B and F) MCF-7/DOX cells were pre-treated with AA861 (5 or 10 μℳ) (B), baicalein (10 or 20 μℳ) (F), or DMSO for 30 min, and then were treated with or without paclitaxel (1 μℳ) for 48 h. Cell viability was quantified using an MTT assay. (C and G) MCF-7/DOX cells were pre-treated with AA861 (5 or 10 μℳ) (C), baicalein (10 or 20 μℳ) (G), or DMSO for 30 min, and were then treated with or without paclitaxel (1 μℳ) for 48 h for determination of cell apoptosis by flow cytometric analysis of the sub-G1 population. All quantitative data are the mean±s.d. of three independent experiments. *P<0.05, †P<0.01, ‡P<0.005. (D and H) MCF-7/DOX cells were pre-treated with AA861 (5 or 10 μℳ) (D), baicalein (10 or 20 μℳ) (H), or DMSO for 30 min, and were then treated with or without paclitaxel (1 μℳ) for 48 h for determination of cell apoptosis by PARP cleavage. The data are representative of three independent experiments.
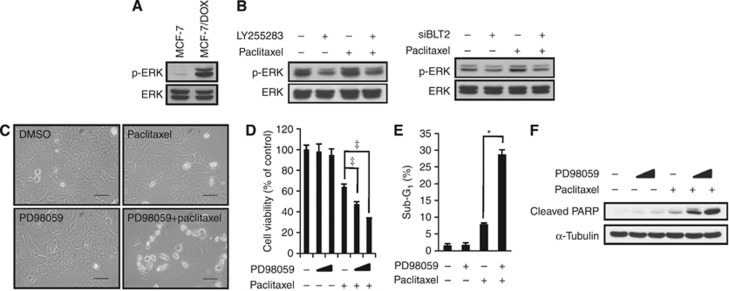
Extracellular signal-regulated kinase lies downstream of BLT2 and mediates paclitaxel resistance in MCF-7/DOX cells
Activation of the MEK/ERK pathway is a common cause of apoptosis resistance (McCubrey et al, 2007). In addition, LTB4-related inflammatory signalling has been shown to activate ERK in several types of cancer cells (Tong et al, 2005; Ihara et al, 2007). We, therefore, next examined whether ERK might function downstream of BLT2 to mediate paclitaxel resistance in MCF-7/DOX cells. Immunoblot assays revealed that the amounts of ERK phosphorylation (activated forms) were markedly increased in MCF-7/DOX cells compared with the parental MCF-7 cells (Figure 5A). Furthermore, LY255283 treatment or depletion of BLT2 by RNAi markedly inhibited ERK phosphorylation (Figure 5B). These observations suggest that ERK activation occurs downstream of BLT2 signalling in MCF-7/DOX cells. To evaluate whether this BLT2-dependent ERK activation contributed to the paclitaxel resistance of MCF-7/ADR cells, we examined the effects of treatment with PD98059, an ERK inhibitor. Inhibition of ERK by PD98059 enhanced paclitaxel-induced death in MCF-7/DOX cells (Figure 5C and D). Similarly, we observed enhanced cell death by analysing the sub-G1 fraction of cells (Figure 5E) and PARP cleavage (Figure 5F) levels. Together, these data indicate that BLT2–ERK signalling is associated with paclitaxel resistance in MCF-7/DOX cells.
Figure 5.
ERKs are downstream of BLT2 and mediate paclitaxel resistance in MCF-7/DOX cells. (A) Immunoblot analyses were performed to detect ERK phosphorylation in MCF-7 and MCF-7/DOX cells. The data are representative of three independent experiments. (B) MCF-7/DOX cells were pre-treated with LY255283 (10 μℳ) for 30 min before paclitaxel (1 μℳ) treatment for 6 h (left panel), or the cells were transfected with BLT2 or control siRNAs for 24 h before paclitaxel (1 μℳ) treatment for 6 h (right panel). The cells were then subjected to immunoblot analysis as in (A). The data are representative of three independent experiments. (C) MCF-7/DOX cells were pre-treated with PD98059 (10 μℳ) for 30 min before paclitaxel (1 μℳ) treatment for 48 h. Cell morphology was visualised using an Olympus BX51 microscope at × 40 magnification. Scale bars, 50 μm. (D) MCF-7/DOX cells were pre-treated with PD98059 (10 or 20 μℳ) for 30 min before paclitaxel (1 μℳ) treatment for 48 h. Viable cells were detected using an MTT assay. (E) MCF-7/DOX cells were pre-treated with PD98059 (10 μℳ) for 30 min and were then treated with or without paclitaxel (1 μℳ) for 48 h. Apoptotic cells were quantified by flow cytometric analysis of the sub-G1 population. All quantitative data are the means±s.d. of three independent experiments. *P<0.05, ‡P<0.005. (F) MCF-7/DOX cells treated as in (D) were assayed for apoptosis levels, as determined by PARP cleavage. The data are representative of three independent experiments.
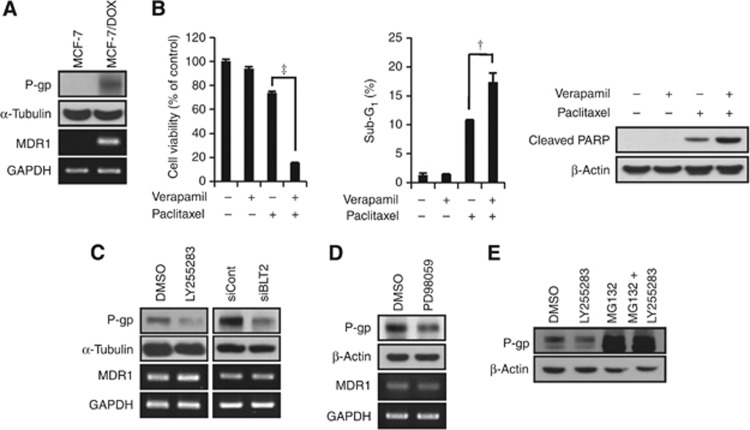
The ‘BLT2–ERK' cascade regulates MDR1 protein stability in MCF-7/DOX cells
The MDR1 product, P-gp, pumps a variety of anticancer agents, including taxanes, out of cells (Riordan et al, 1985; Gottesman et al, 1995) and confers resistance to these anticancer agents. We therefore examined the possible role of MDR1 in the regulation of the paclitaxel resistance of MCF-7/DOX cells. The MDR1 mRNA and protein levels were markedly increased in MCF-7/DOX cells compared with the parental MCF-7 cells (Figure 6A). Treatment with verapamil, an MDR1 inhibitor, significantly reduced paclitaxel resistance in MCF-7/DOX cells (Figure 6B), suggesting that P-gp confers paclitaxel resistance in MCF-7/DOX cells. Furthermore, P-gp levels in MCF-7/DOX cells were markedly reduced after exposure to LY255283 or BLT2 depletion by RNAi, whereas MDR1 mRNA was hardly affected (Figure 6C). These observations suggest that BLT2 regulates P-gp levels in MCF-7/DOX cells. The MEK–ERK pathway has been suggested to regulate the P-gp levels through ubiquitination in MCF-7/MDR cells (Katayama et al, 2007). In addition, the stability of P-gp is regulated by the ubiquitin–proteasome system (Zhang et al, 2004). Therefore, we determined whether the reduced levels of P-gp after BLT2 suppression were mediated through ERK. Previously, in Figure 5 we showed that BLT2 inhibition significantly downregulates ERK. As shown in Figure 6D, the ERK inhibitor PD98059 clearly suppressed P-gp levels without affecting its transcription. To assess whether the BLT2 inhibitor LY255283 represses P-gp levels through proteasomal degradation, MCF-7/DOX cells were incubated with the proteasome inhibitor MG132 for 12 h. MG132 dramatically increased P-gp levels and apparently abolished the reduction of P-gp levels induced by LY255283 treatment (Figure 6E). Collectively, these results suggest that the BLT2–ERK cascade regulates P-gp levels, most likely through the ubiquitin–proteasome system.
Figure 6.
The ‘BLT2–ERK' cascade regulates MDR1 protein levels in MCF-7/DOX cells. (A) Immunoblot assays and semi-quantitative RT-PCR analyses of P-gp and MDR1 mRNA were performed using MCF-7 and MCF-7/DOX cells. (B) MCF-7/DOX cells were pre-treated with verapamil (10 μℳ) for 30 min and were then treated with or without paclitaxel (1 μℳ) for 48 h. Cell viability and the percentages of cells in the sub-G1 population were determined using an MTT assay (left panel) and flow cytometric analysis (centre panel). In addition, cleaved PARP was determined using an immunoblot assay (right panel). All quantitative data are the means±s.d. of three independent experiments. †P<0.01, ‡P<0.005. (C) MCF-7/DOX cells were incubated with LY255283 (10 μℳ) or DMSO for 12 h, or transfected with BLT2 or control siRNAs for 48 h, and then subjected to immunoblot analysis and semi-quantitative RT-PCR of P-gp and MDR1 mRNA. (D) MCF-7/DOX cells were treated with PD98059 (10 μℳ) for 8 h, after which the amounts of P-gp and MDR1 mRNA were determined using an immunoblot assay and semi-quantitative RT-PCR. (E) MCF-7/DOX cells were incubated in the presence of 0.5% serum for 3 h and then incubated with LY255283 (10 μℳ) plus MG132 (10 μℳ) for 12 h. Cell lysates were subjected to immunoblot analysis with antibodies against P-gp and β-actin (loading control).
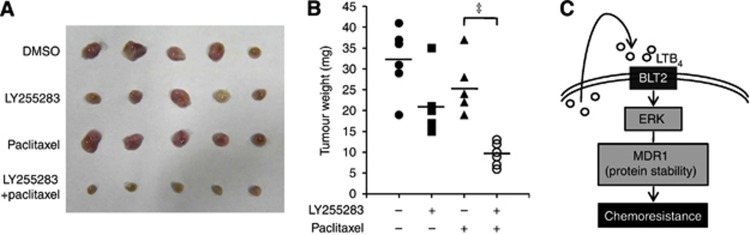
The BLT2 inhibition significantly reduces tumour progression in an orthotopic breast tumour model
To extend our in vitro findings and to determine whether BLT2 could be an effective therapeutic target for paclitaxel-resistant breast cancer, we examined the effects of LY255283 in a breast tumour animal model by orthotopically implanting MCF-7/DOX cells into mice. Paclitaxel (15 mg kg−1) treatment (once per week for 4 weeks) showed only marginal inhibition of tumour growth; however, co-injection of LY255283 (2.5 mg kg−1, twice per week) significantly potentiated paclitaxel-mediated tumour growth inhibition (Figure 7A and B). The mice showed no toxic side effects during the observation period. These results implicate BLT2 in the paclitaxel resistance of breast cancer cells in vivo.
Figure 7.
Inhibition of BLT2 significantly reduced tumour progression in an orthotopic breast tumour model. (A) Representative photographs of tumour extracted from the mice treated with or without paclitaxel (15 mg kg−1, i.p., once a week for 4 weeks) and LY255283 (2.5 mg kg−1, i.p., twice a week for 4 weeks). (B) Breast tumour weight was determined in mice administrated LY255283 or DMSO along with paclitaxel (N=6 per group). Data are presented as scatter dot plot and line indicate mean. Data are means±s.d. of values from six mice of each group. ‡P<0.005. (C) A proposed scheme for the involvement of the BLT2 cascade in paclitaxel resistance of breast cancer cells. LTB4–BLT2 pathway activation induces ERK activation. Then, ERK activation regulates MDR1 protein stability, which leads to paclitaxel resistance.
Discussion
In the present study, we found that BLT2 was markedly upregulated in the multidrug-resistant MCF-7/DOX cells. Selective BLT2 suppression with LY255283 treatment or RNAi-mediated knockdown resulted in the attenuation of MCF-7/DOX paclitaxel resistance, thus implicating BLT2 as a potential determinant of drug resistance. In addition, we characterised the BLT2 signal pathway as one of the potential mechanisms for the regulation of paclitaxel resistance and found that ERK functions downstream of BLT2 to regulate P-gp levels. Finally, our orthotopic in vivo results showed that in the presence of paclitaxel, the resistance phenotype was diminished by a BLT2 inhibitor, thus demonstrating the therapeutic effect of BLT2 suppression. Together, our findings suggest that a BLT2–ERK signalling cascade regulates the levels of P-gp and contributes to paclitaxel resistance in MCF-7/DOX cells.
The MCF-7/DOX cells were isolated by the stepwise selection of MCF-7 cells exposed to increasing concentrations of doxorubicin (Kim et al, 2003). Similarly, another doxorubicin-selective cell line MCF-7/ADR-RES (now renamed NCI/ADR-RES) was established (Scudiero et al, 1998). Recently, it was reported that NCI/ADR-RES cells are derived from the ovarian cancer cell line OVCAR-8 and express higher levels of P-gp and MDR1 (Scudiero et al, 1998; Liscovitch and Ravid, 2007). To determine whether BLT2 is associated with paclitaxel resistance in this cell type, we repeated the experiments using NCI/ADR-RES cells and obtained results that were identical to those obtained with MCF-7/DOX cells (Supplementary Figure 2). On the basis of these results, we are quite confident that BLT2 is associated with paclitaxel resistance in both MCF-7/DOX and NCI/ADR-RES cells.
Emerging evidence suggests that the inflammatory tumour microenvironment has an important role in modulating drug resistance (DeNardo et al, 2011; Shree et al, 2011); however, underlying mechanism has been still largely unknown. In the present study, our results point to LTB4–BLT2 as a novel mediator of chemoresistance. The LTB4 is suggested to act mostly within the local inflammatory microenvironment and, in fact, arachidonic acid (AA) is one of the most abundant fatty acids in breast. The LTB4, derived from AA metabolism via 5-LO, has been associated with promotion of carcinogenesis (Ye et al, 2005; Yang et al, 2008), tumour progression (Freedman et al, 2007; Larre et al, 2008), and apoptosis resistance (Serhan et al, 2008). The BLT2 is a G-protein-coupled receptor that is expressed on the cell surface and interacts with specific ligands, such as LTB4 and 12(S)-HETE. Although various inflammatory functions of BLT1 have been extensively characterised, few biological functions of BLT2 have been identified, although recent studies have suggested that it has a role in several inflammatory diseases and cancer progression (Hennig et al, 2008; Rocconi et al, 2008; Sveinbjornsson et al, 2008; Choi et al, 2010; Kim et al, 2010). Our results suggest that among the BLT2 ligands, LTB4 is the principal ligand responsible for BLT2 stimulation in paclitaxel resistance, because the LTB4 synthesis inhibitor (AA861) suppressed the paclitaxel resistance of MCF-7/DOX cells, whereas the 12(S)-HETE synthesis inhibitor (baicalein) had no effect. We propose that a 5-LO–LTB4–BLT2 signalling pathway is responsible for paclitaxel resistance in MCF-7/DOX cells.
Our studies suggest that ERK lies downstream of BLT2 in mediating breast cancer drug resistance. We examined two other members of the MAPK pathway, JNK and p38, and observed that LY255283 treatment and RNAi-mediated BLT2 knockdown significantly suppressed the levels of p-ERK1/2 in MCF-7/DOX cells without affecting the levels of JNK and p38 (data not shown). Our results suggest that ERKs regulate the P-gp levels. To date, the mechanisms underlying the regulation of P-gp levels have not been well clarified, although P-gp expression has been suggested to be primarily regulated at the transcriptional level (Shtil, 2001). However, a recent study indicated that inhibition of the MEK–ERK pathway suppressed P-gp levels by promoting P-gp degradation (Zhang et al, 2004). Indeed, our results show that PD98059 inhibits P-gp levels; however, the transcript levels of MDR1 were not affected by LY255283 treatment or RNAi-mediated BLT2 knockdown (Figure 6C), suggesting that BLT2–ERK regulate MDR1 protein levels. At steady state, P-gp is located at the plasma membrane and undergoes endocytosis and recycling (Kim et al, 1997). In addition, previous studies have shown that the ubiquitination–proteasome, but not lysosome, pathway is involved in P-gp turnover (Katayama et al, 2007). Consistent with this report, we observed that the proteasome inhibitor MG132 dramatically increased P-gp levels and apparently abolished the reduction of P-gp levels induced by LY255283 treatment (Figure 6E). Therefore, these studies suggest that suppressing P-gp expression by enhancing its degradation might be another effective strategy to modulate P-gp-mediated drug resistance. Previously, it was shown that MDR cell lines overexpress the epidermal growth factor receptor (EGFR) as well as P-gp (Yang et al, 1997). The EGFR has been described as an important regulatory effector that is able to stimulate ERK/MAPK signalling. Moreover, we recently observed that BLT2 induces EGFR transactivation (Ryu et al, 2010). Thus, we speculate that BLT2 may induce EGFR transactivation, thereby mediating ERK activation. A detailed mechanism for the potential link between BLT2 and EGFR remains to be further characterised. As summarised in Figure 7C, we have shown that a BLT2–ERK cascade upregulates P-gp levels in MCF-7/DOX cells and thereby contributes to paclitaxel resistance.
Our findings have thus revealed a previously unrecognised role of BLT2 in paclitaxel resistance. Our results contribute to a better understanding of the molecular mechanisms of chemoresistance, as well as provide potential targets for the development of new therapeutics.
Acknowledgments
This work was supported by grants from the Senior Researcher Project (number 2012R1A2A2A01044526) and the Bio and Medical Technology Development Program (2012M3A9C5048709) of the National Research Foundation (NRF) funded by the Ministry of Education, Science, and Technology (MEST), Republic of Korea, and also by the grant from the National R&D Program for Cancer Control (1220020), Ministry for Health and Welfare, Republic of Korea.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Choi JA, Lee JW, Kim H, Kim EY, Seo JM, Ko J, Kim JH. Pro-survival of estrogen receptor-negative breast cancer cells is regulated by a BLT2-reactive oxygen species-linked signaling pathway. Carcinogenesis. 2010;31:543–551. doi: 10.1093/carcin/bgp203. [DOI] [PubMed] [Google Scholar]
- Crown J, O'Leary M, Ooi WS. Docetaxel and paclitaxel in the treatment of breast cancer: a review of clinical experience. Oncologist. 2004;9 (Suppl 2:24–32. doi: 10.1634/theoncologist.9-suppl_2-24. [DOI] [PubMed] [Google Scholar]
- DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirstrom K, West BL, Coussens LM. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Z, Brakora KA, Seiden MV. Inhibition of ABCB1 (MDR1) and ABCB4 (MDR3) expression by small interfering RNA and reversal of paclitaxel resistance in human ovarian cancer cells. Mol Cancer Ther. 2004;3:833–838. [PubMed] [Google Scholar]
- Freedman RS, Wang E, Voiculescu S, Patenia R, Bassett RL, Jr, Deavers M, Marincola FM, Yang P, Newman RA. Comparative analysis of peritoneum and tumor eicosanoids and pathways in advanced ovarian cancer. Clin Cancer Res. 2007;13:5736–5744. doi: 10.1158/1078-0432.CCR-07-0583. [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Hrycyna CA, Schoenlein PV, Germann UA, Pastan I. Genetic analysis of the multidrug transporter. Annu Rev Genet. 1995;29:607–649. doi: 10.1146/annurev.ge.29.120195.003135. [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Pastan I, Ambudkar SV. P-glycoprotein and multidrug resistance. Curr Opin Genet Dev. 1996;6:610–617. doi: 10.1016/s0959-437x(96)80091-8. [DOI] [PubMed] [Google Scholar]
- Hennig R, Osman T, Esposito I, Giese N, Rao SM, Ding XZ, Tong WG, Buchler MW, Yokomizo T, Friess H, Adrian TE. BLT2 is expressed in PanINs, IPMNs, pancreatic cancer and stimulates tumour cell proliferation. Br J Cancer. 2008;99:1064–1073. doi: 10.1038/sj.bjc.6604655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara A, Wada K, Yoneda M, Fujisawa N, Takahashi H, Nakajima A. Blockade of leukotriene B4 signaling pathway induces apoptosis and suppresses cell proliferation in colon cancer. J Pharmacol Sci. 2007;103:24–32. doi: 10.1254/jphs.fp0060651. [DOI] [PubMed] [Google Scholar]
- Katayama K, Yoshioka S, Tsukahara S, Mitsuhashi J, Sugimoto Y. Inhibition of the mitogen-activated protein kinase pathway results in the down-regulation of P-glycoprotein. Mol Cancer Ther. 2007;6:2092–2102. doi: 10.1158/1535-7163.MCT-07-0148. [DOI] [PubMed] [Google Scholar]
- Kim EY, Seo JM, Kim C, Lee JE, Lee KM, Kim JH. BLT2 promotes the invasion and metastasis of aggressive bladder cancer cells through a reactive oxygen species-linked pathway. Free Radic Biol Med. 2010;49:1072–1081. doi: 10.1016/j.freeradbiomed.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Kim H, Barroso M, Samanta R, Greenberger L, Sztul E. Experimentally induced changes in the endocytic traffic of P-glycoprotein alter drug resistance of cancer cells. Am J Physiol. 1997;273:C687–C702. doi: 10.1152/ajpcell.1997.273.2.C687. [DOI] [PubMed] [Google Scholar]
- Kim S, Kim SS, Bang YJ, Kim SJ, Lee BJ. In vitro activities of native and designed peptide antibiotics against drug sensitive and resistant tumor cell lines. Peptides. 2003;24:945–953. doi: 10.1016/s0196-9781(03)00194-3. [DOI] [PubMed] [Google Scholar]
- Larre S, Tran N, Fan C, Hamadeh H, Champigneulles J, Azzouzi R, Cussenot O, Mangin P, Olivier JL. PGE2 and LTB4 tissue levels in benign and cancerous prostates. Prostaglandins Other Lipid Mediat. 2008;87:14–19. doi: 10.1016/j.prostaglandins.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Liscovitch M, Ravid D. A case study in misidentification of cancer cell lines: MCF-7/AdrR cells (re-designated NCI/ADR-RES) are derived from OVCAR-8 human ovarian carcinoma cells. Cancer Lett. 2007;245:350–352. doi: 10.1016/j.canlet.2006.01.013. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters-Golden M, Henderson WRJr. Leukotrienes. N Engl J Med. 2007;357:1841–1854. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- Riordan JR, Deuchars K, Kartner N, Alon N, Trent J, Ling V. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. Nature. 1985;316:817–819. doi: 10.1038/316817a0. [DOI] [PubMed] [Google Scholar]
- Rocconi RP, Kirby TO, Seitz RS, Beck R, Straughn JM, Jr, Alvarez RD, Huh WK. Lipoxygenase pathway receptor expression in ovarian cancer. Reprod Sci. 2008;15:321–326. doi: 10.1177/1933719108316390. [DOI] [PubMed] [Google Scholar]
- Rowinsky EK. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu Rev Med. 1997;48:353–374. doi: 10.1146/annurev.med.48.1.353. [DOI] [PubMed] [Google Scholar]
- Ryu HC, Kim C, Kim JY, Chung JH, Kim JH. UVB radiation induces apoptosis in keratinocytes by activating a pathway linked to “BLT2-reactive oxygen species”. J Invest Dermatol. 2010;130:1095–1106. doi: 10.1038/jid.2009.436. [DOI] [PubMed] [Google Scholar]
- Scudiero DA, Monks A, Sausville EA. Cell line designation change: multidrug-resistant cell line in the NCI anticancer screen. J Natl Cancer Inst. 1998;90:862. doi: 10.1093/jnci/90.11.862. [DOI] [PubMed] [Google Scholar]
- Seo JM, Park S, Kim JH. Leukotriene B4 receptor-2 promotes invasiveness and metastasis of ovarian cancer cells through signal transducer and activator of transcription 3 (STAT3)-dependent up-regulation of matrix metalloproteinase 2. J Biol Chem. 2012;287:13840–13849. doi: 10.1074/jbc.M111.317131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shree T, Olson OC, Elie BT, Kester JC, Garfall AL, Simpson K, Bell-McGuinn KM, Zabor EC, Brogi E, Joyce JA. Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev. 2011;25:2465–2479. doi: 10.1101/gad.180331.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtil AA. Signal transduction pathways and transcriptional mechanisms as targets for prevention of emergence of multidrug resistance in human cancer cells. Curr Drug Targets. 2001;2:57–77. doi: 10.2174/1389450013348957. [DOI] [PubMed] [Google Scholar]
- Sveinbjornsson B, Rasmuson A, Baryawno N, Wan M, Pettersen I, Ponthan F, Orrego A, Haeggstrom JZ, Johnsen JI, Kogner P. Expression of enzymes and receptors of the leukotriene pathway in human neuroblastoma promotes tumor survival and provides a target for therapy. FASEB J. 2008;22:3525–3536. doi: 10.1096/fj.07-103457. [DOI] [PubMed] [Google Scholar]
- Tong WG, Ding XZ, Hennig R, Witt RC, Standop J, Pour PM, Adrian TE. Leukotriene B4 receptor antagonist LY293111 inhibits proliferation and induces apoptosis in human pancreatic cancer cells. Clin Cancer Res. 2002;8:3232–3242. [PubMed] [Google Scholar]
- Tong WG, Ding XZ, Talamonti MS, Bell RH, Adrian TE. LTB4 stimulates growth of human pancreatic cancer cells via MAPK and PI-3 kinase pathways. Biochem Biophys Res Commun. 2005;335:949–956. doi: 10.1016/j.bbrc.2005.07.166. [DOI] [PubMed] [Google Scholar]
- Yang JM, Sullivan GF, Hait WN. Regulation of the function of P-glycoprotein by epidermal growth factor through phospholipase C. Biochem Pharmacol. 1997;53:1597–1604. doi: 10.1016/s0006-2952(97)82451-3. [DOI] [PubMed] [Google Scholar]
- Yang P, Sun Z, Chan D, Cartwright CA, Vijjeswarapu M, Ding J, Chen X, Newman RA. Zyflamend reduces LTB4 formation and prevents oral carcinogenesis in a 7,12-dimethylbenz[alpha]anthracene (DMBA)-induced hamster cheek pouch model. Carcinogenesis. 2008;29:2182–2189. doi: 10.1093/carcin/bgn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye YN, Wu WK, Shin VY, Bruce IC, Wong BC, Cho CH. Dual inhibition of 5-LOX and COX-2 suppresses colon cancer formation promoted by cigarette smoke. Carcinogenesis. 2005;26:827–834. doi: 10.1093/carcin/bgi012. [DOI] [PubMed] [Google Scholar]
- Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- Yokomizo T, Kato K, Terawaki K, Izumi T, Shimizu T. A second leukotriene B(4) receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J Exp Med. 2000;192:421–432. doi: 10.1084/jem.192.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo MH, Song H, Woo CH, Kim H, Kim JH. Role of the BLT2, a leukotriene B4 receptor, in Ras transformation. Oncogene. 2004;23:9259–9268. doi: 10.1038/sj.onc.1208151. [DOI] [PubMed] [Google Scholar]
- Zhang W, McQueen T, Schober W, Rassidakis G, Andreeff M, Konopleva M. Leukotriene B4 receptor inhibitor LY293111 induces cell cycle arrest and apoptosis in human anaplastic large-cell lymphoma cells via JNK phosphorylation. Leukemia. 2005;19:1977–1984. doi: 10.1038/sj.leu.2403929. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wu JY, Hait WN, Yang JM. Regulation of the stability of P-glycoprotein by ubiquitination. Mol Pharmacol. 2004;66:395–403. doi: 10.1124/mol.104.001966. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.