Abstract
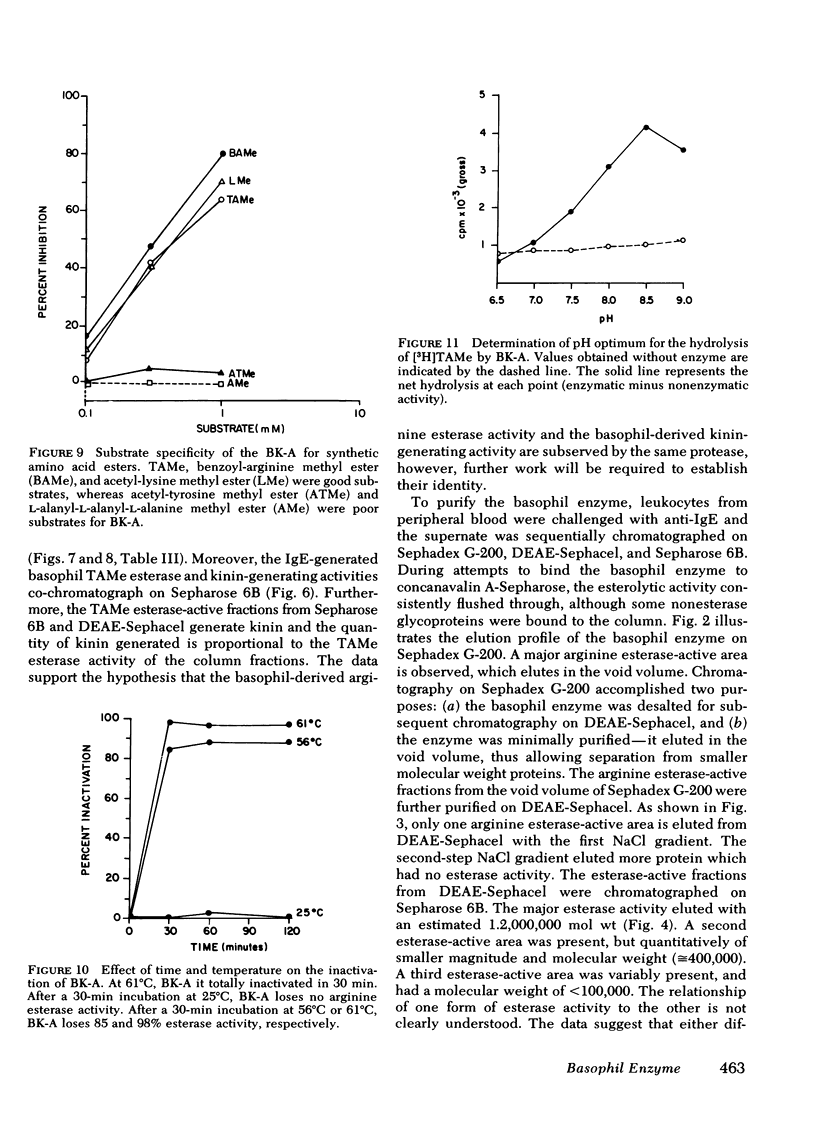
These studies describe the IgE-mediated relase of a basophil kallikrein-like enzyme that is an arginine esterase and is inhibited by plasma, diisopropylphosphofluoridate, and Trasylol. The substrate specificity for the synthetic amino acid ester substrates p-toluenesulfonyl-L-arginien methyl ester, benzoyl-arginine methyl ester, and acetyl-tyrosine methyl ester is similar for the basophil enzyme and plasma kallikrein. The interaction of arginine esterase-active fractions from ion-exchange (DEAE-Sephacel) and gel filtration (Sepharose 6B) chromatography, with human plasma kininogen, generates immunoreactive kinin. The basophil arginine esterase and kinin-generating activities co-chromatograph on Sepharose 6B and the quantity of kinin generated is, in general, proportional to the arginine esterase activity of the column fractions, suggesting that these two activities are subserved by the same protease. The ability of this protease to generate kinin equally well from heat- and acid-treated plasma, as from fresh human plasma, suggests that this protease has kallikrein-like activity. These data suggest that kallikrein-like activity can be generated from human basophils as a direct result of a primary IgE-mediated immune reaction, thus providing a potential link between reactions of immediate hypersensitivity and the plasma and(or) tissue kinin-generating systems.
Full text
PDF

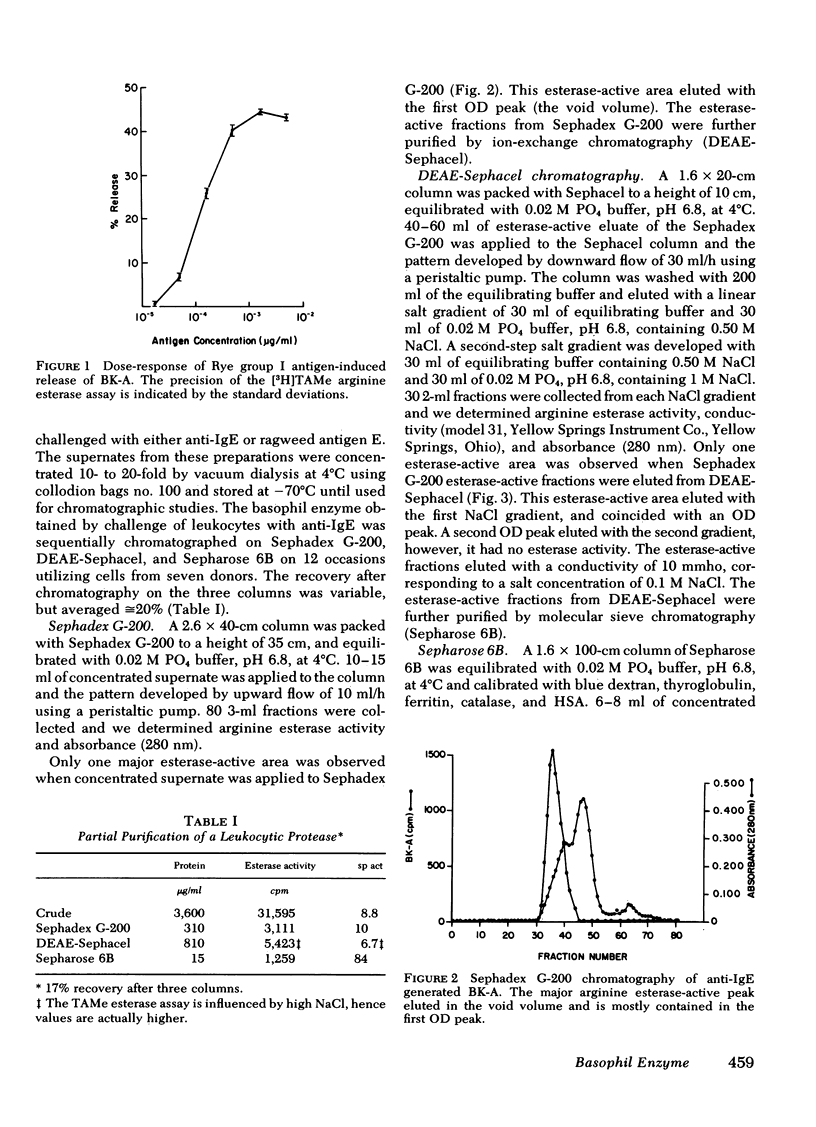
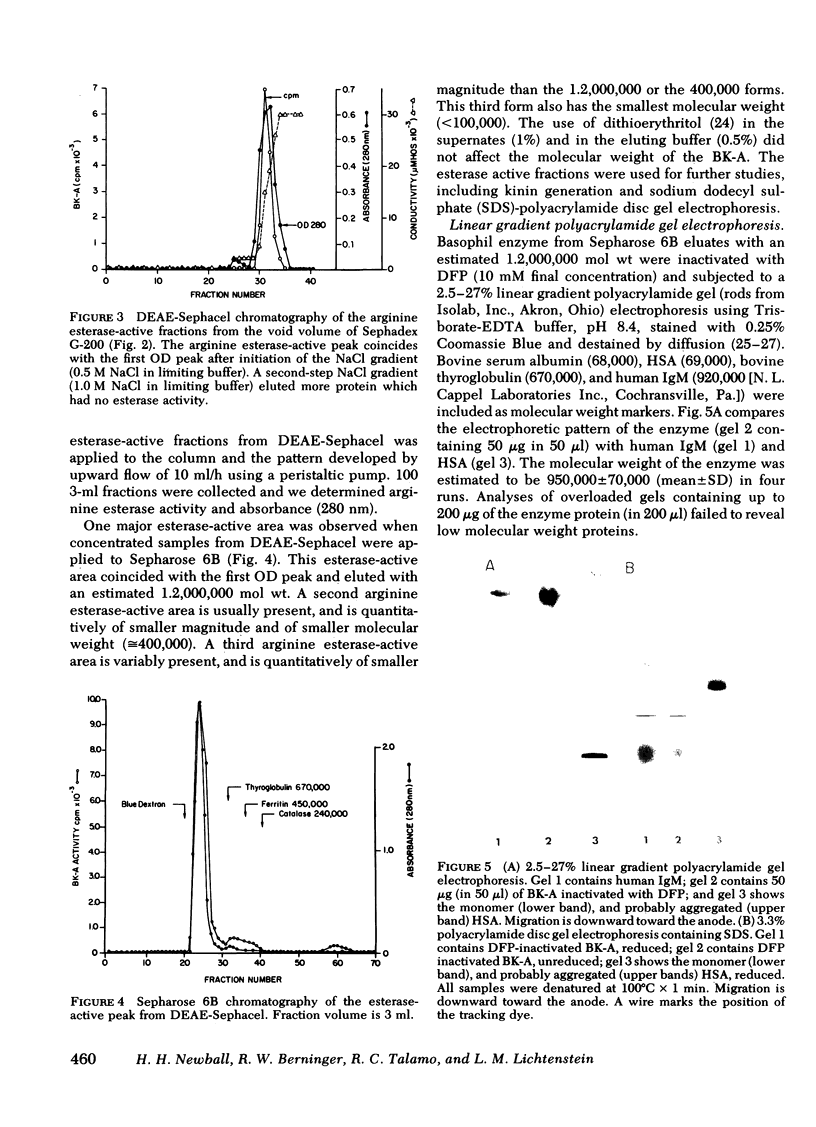

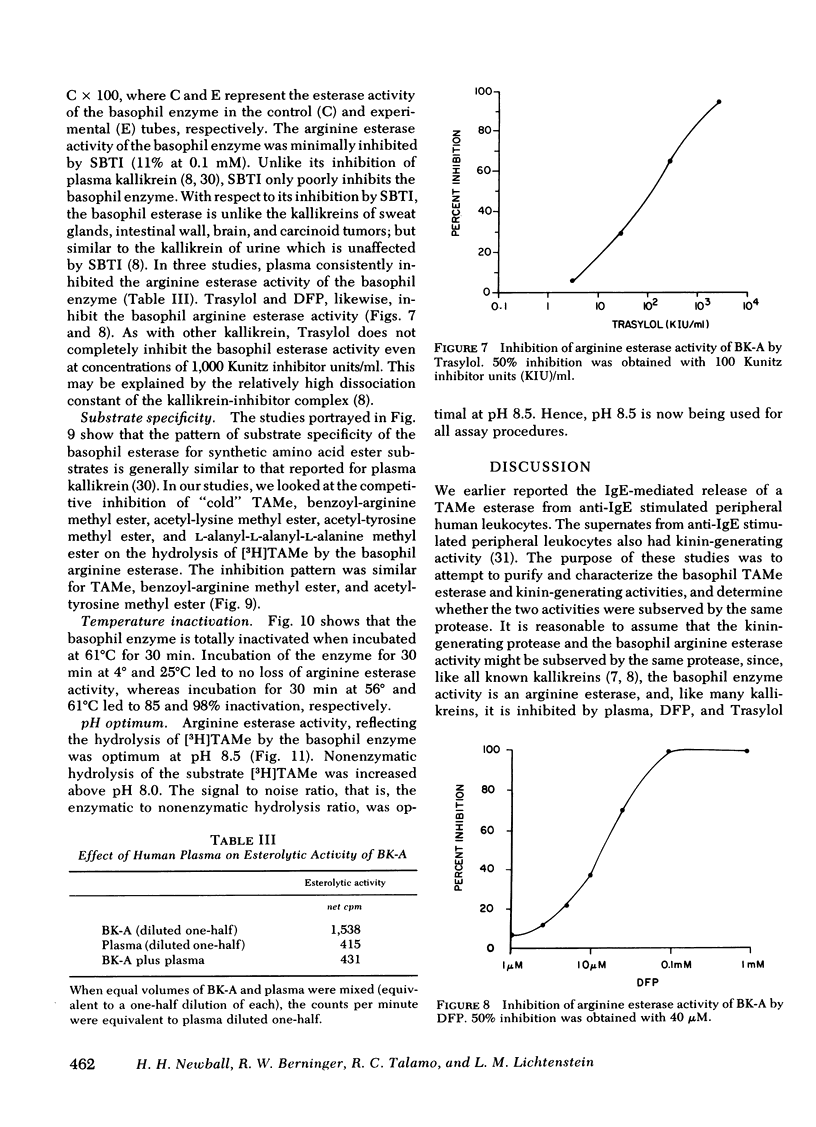


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. -O., Borg H., Mikaelsson M. Molecular weight estimations of proteins by electrophoresis in polyacrylamide gels of graded porosity. FEBS Lett. 1972 Feb 1;20(2):199–202. doi: 10.1016/0014-5793(72)80793-2. [DOI] [PubMed] [Google Scholar]
- Beaven V. H., Pierce J. V., Pisano J. J. A sensitive isotopic procedure for the assay of esterase activity: measurement of human urinary kallikrein. Clin Chim Acta. 1971 Mar;32(1):67–73. doi: 10.1016/0009-8981(71)90465-7. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. DITHIOTHREITOL, A NEW PROTECTIVE REAGENT FOR SH GROUPS. Biochemistry. 1964 Apr;3:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- Colman R. W., Bagdasarian A. Human kallikrein and prekallikrein. Methods Enzymol. 1976;45:303–322. doi: 10.1016/s0076-6879(76)45028-0. [DOI] [PubMed] [Google Scholar]
- Colman R. W. Formation of human plasma kinin. N Engl J Med. 1974 Sep 5;291(10):509–515. doi: 10.1056/NEJM197409052911008. [DOI] [PubMed] [Google Scholar]
- Colman R. W., Mattler L., Sherry S. Studies on the prekallikrein (kallikreinogen)--kallikrein enzyme system of human plasma. II. Evidence relating the kaolin-activated arginine esterase to plasma kallikrein. J Clin Invest. 1969 Jan;48(1):23–32. doi: 10.1172/JCI105971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINIZ C. R., CARVALHO I. F. A micromethod for determination of bradykininogen under several conditions. Ann N Y Acad Sci. 1963 Feb 4;104:77–89. doi: 10.1111/j.1749-6632.1963.tb17654.x. [DOI] [PubMed] [Google Scholar]
- Engleman E. G., Greenbaum L. M. Kinin-forming activity of human lymphocytes. Biochem Pharmacol. 1971 Apr;20(4):922–924. doi: 10.1016/0006-2952(71)90058-x. [DOI] [PubMed] [Google Scholar]
- Greenbaum L. M., Prakash A., Semente G., Johnston M. The leukokinin system; its role in fluid accumulation in malignancy and inflammation. Agents Actions. 1973 Dec;3(5):332–334. doi: 10.1007/BF01986490. [DOI] [PubMed] [Google Scholar]
- Habal F. M., Movat H. Z., Burrowes C. E. Isolation of two functionally different kininogens from human plasma--separation from proteinase inhibitors and interaction with plasma kallikrein. Biochem Pharmacol. 1974 Aug 15;23(16):2291–2303. doi: 10.1016/0006-2952(74)90558-9. [DOI] [PubMed] [Google Scholar]
- Imanari T., Wilcox G. M., Pisano J. J. Sensitive radiochemical esterolytic assays for urokinase. Clin Chim Acta. 1976 Sep 6;71(2):267–276. doi: 10.1016/0009-8981(76)90540-4. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Soto C. S., Ishizaka K. Mechanisms of passive sensitization. 3. Number of IgE molecules and their receptor sites on human basophil granulocytes. J Immunol. 1973 Aug;111(2):500–511. [PubMed] [Google Scholar]
- Jonasson O., Becker E. L. Release of kallikrein from guinea pig lung during anaphylaxis. J Exp Med. 1966 Mar 1;123(3):509–522. doi: 10.1084/jem.123.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING T. P., NORMAN P. S., CONNELL J. T. ISOLATION AND CHARACTERIZATION OF ALLERGENS FROM RAGWEED POLLEN. II. Biochemistry. 1964 Mar;3:458–468. doi: 10.1021/bi00891a026. [DOI] [PubMed] [Google Scholar]
- LICHTENSTEIN L. M., OSLER A. G. STUDIES ON THE MECHANISMS OF HYPERSENSITIVITY PHENOMENA. IX. HISTAMINE RELEASE FROM HUMAN LEUKOCYTES BY RAGWEED POLLEN ANTIGEN. J Exp Med. 1964 Oct 1;120:507–530. doi: 10.1084/jem.120.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh D. G., Milner F. H., Johnson P. The allergenic activity and stability of purified allergens from the pollen of common rye grass (lolium perenne). Int Arch Allergy Appl Immunol. 1966;29(6):521–535. doi: 10.1159/000229739. [DOI] [PubMed] [Google Scholar]
- Movat H. Z., Steinberg S. G., Habal F. M., Ranadive N. S. Demonstration of a kinin-generating enzyme in the lysosomes of human polymorphonuclear leukocytes. Lab Invest. 1973 Dec;29(6):669–684. [PubMed] [Google Scholar]
- Newball H. H., Talamo R. C., Lichtenstein L. M. Anaphylactic relase of a basophil kallikrein-like activity. II. A mediator of immediate hypersensitivity reactions. J Clin Invest. 1979 Aug;64(2):466–475. doi: 10.1172/JCI109484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newball H. H., Talamo R. C., Lichtenstein L. M. Release of leukocyte kallikrein mediated by IgE. Nature. 1975 Apr 17;254(5501):635–636. doi: 10.1038/254635a0. [DOI] [PubMed] [Google Scholar]
- Talamo R. C., Haber E., Austen K. F. A radioimmunoassay for bradykinin in plasma and synovial fluid. J Lab Clin Med. 1969 Nov;74(5):816–827. [PubMed] [Google Scholar]
- WEBSTER M. E., PIERCE J. V. Action of the kallikreins on synthetic ester substrates. Proc Soc Exp Biol Med. 1961 May;107:186–191. doi: 10.3181/00379727-107-26575. [DOI] [PubMed] [Google Scholar]
- Weber K., Kuter D. J. Reversible denaturation of enzymes by sodium dodecyl sulfate. J Biol Chem. 1971 Jul 25;246(14):4504–4509. [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Wintroub B. U., Goetzl E. J., Austen K. F. A neutrophil-dependent pathway for the generation of a neutral peptide mediator: partial characterization of components and control by alpha-1-antitrypsin. J Exp Med. 1974 Sep 1;140(3):812–824. doi: 10.1084/jem.140.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]