Abstract
Background:
Sorafenib is a potent inhibitor against Raf kinase and several receptor tyrosine kinases that has been approved for the clinical treatment of advanced renal and liver cancer. Combining sorafenib with other agents has been shown to improve its antitumour efficacy by not only reducing the toxic side effects but also preventing primary and acquired resistance to sorafenib. We have previously observed that tetrandrine exhibits potent antitumour effects in human hepatocellular carcinoma. In this study, we investigated the synergistic antitumour activity of sorafenib in combination with tetrandrine.
Methods:
This was a two-part investigation that included the in vitro effects of sorafenib in combination with tetrandrine on cancer cells and the in vivo antitumour efficacy of this drug combination on tumour xenografts in nude mice.
Results:
Combined treatment showed a good synergistic antitumour effect yet spared nontumourigenic cells. The potential molecular mechanism may be mainly that it activated mitochondrial death pathway and induced caspase-dependent apoptosis in the cancer cells. Accumulation of intracellular reactive oxygen species (ROS) and subsequent activation of Akt may also be involved in apoptosis induction.
Conclusion:
The antitumour activity of sorafenib plus tetrandrine may be attributed to the induction of the intrinsic apoptosis pathway through ROS/Akt signaling. This finding provides a novel approach that may broaden the clinical application of sorafenib.
Keywords: sorafenib, tetrandrine, synergistic antitumour, apoptosis
Sorafenib, a biaryl urea, is an oral small molecule multikinase inhibitor that is effective against Raf kinase, vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), c-kit, c-Ret and FLT3 kinase (Wilhelm et al, 2006). It has been shown that sorafenib has a significant broad-spectrum, dose-dependent antitumour property against a wide variety of human tumours in preclinical models, including both in vitro cell culture models and in vivo xenograft models for prostate (Oh et al, 2012), colon (Walker et al, 2009), lung (Zhang et al, 2012), breast (Gradishar, 2012), ovarian (Matei et al, 2011) and pancreatic cancers (Huang and Sinicrope, 2010), as well as leukaemia (Zhang et al, 2008b) and melanoma (Eisen et al, 2006). In recent years, sorafenib has been approved for the treatment of advanced renal cell carcinoma and hepatocellular carcinoma (HCC) (Wilhelm et al, 2006; Iyer et al, 2010). The anti-cancer property of sorafenib lies in its potential to inhibit angiogenesis in tumour tissues and block cancer cell proliferation by inhibiting kinase activities, such as those of c-Raf, VEGFR2, VEGFR3 and PDGFR (Liu et al, 2006). It has also been reported that sorafenib induces apoptosis in a variety of human tumour cell lines by suppressing the activation of Bcl-2 family members, especially myeloid cell leukaemia sequence-1 (Mcl-1) (Zhang et al, 2008a; Huber et al, 2011). In addition, NF-κB (Kuo et al, 2012), Akt and rogen receptor (Oh et al, 2012) and signal transducer and activator of transcription-3 activities (Huang and Sinicrope, 2010) have all been reported to participate in sorafenib-induced apoptosis in cancer cells.
Apoptosis is a common mechanism for targeted chemotherapies that either directly induce cancer cell death or increase tumour cell sensitivity to known cytotoxic agents or radiation (Ricci and Zong, 2006). There are two established pathways that result in apoptosis: the extrinsic cell death pathway (cell death receptor pathway) and the intrinsic cell death pathway (the mitochondria-initiated pathway) (Elmore, 2007). Small molecule anti-cancer agents induce apoptosis in cancer cells mainly through the intrinsic pathway. Reports have shown that sorafenib induces apoptosis in several human cancer cell lines by downregulating the level of the antiapoptotic protein Mcl-1 (Rosato et al, 2007; Huber et al, 2011). However, alteration in susceptibility to apoptosis is a hallmark of cancer cells, which contributes to tumour development and enhances its resistance to conventional anti-cancer therapies, such as radiation and cytotoxic agents (Ziegler et al, 2011). Targeting multiple signaling pathways with synergistic chemotherapy drugs is a potential novel therapeutic strategy for many types of cancer. Combination therapies involving sorafenib have been shown to improve the antitumour efficacy of sorafenib in a limited number of preclinical studies (Hikita et al, 2010; Pawlik et al, 2011; Wang et al, 2012). These synergistic treatments could not only alleviate primary resistance to sorafenib but also prevent acquired resistance. Therefore, evaluating additional combinations of sorafenib and various chemotherapeutic agents will potentially improve sorafenib efficacy and lead to novel therapeutic applications.
Tetrandrine, a bisbenzylisoquinoline alkaloid isolated from the Chinese medicinal herb Stephaniae tetrandrae has been broadly used in China to treat patients with arthritis, hypertension, inflammation and even silicosis (Shen et al, 2010; Wu et al, 2010). We have previously demonstrated that tetrandrine at high concentrations induces apoptosis through the ROS/Akt pathway (Liu et al, 2011) and, at low concentrations, induces autophagy through autophagy-related gene 7 and ROS/extracellular-signal-regulated kinase (ERK) in human HCC cells (Gong et al, 2012a), suggesting that tetrandrine may be a promising agent for the treatment of cancer.
Based on our previous studies, here we investigate the synergistic antitumour activity of sorafenib in combination with tetrandrine. The results reveal that tetrandrine dramatically enhances sorafenib-induced apoptosis in human cancer cells in vitro and in vivo. We also show that ROS and Akt activity are involved in combination therapy-induced apoptosis. Therefore, our findings represent a novel effective therapeutic strategy for tumour treatment.
Materials and Methods
Cell lines and cell culture
The human hepatoma cell lines (BEL7402 and FHCC98), hepatoblastoma cell line (HepG2) and immortalised nonmalignant cell lines (L02 and HBL100) were cultured in Dulbecco's modified Eagle's medium (DMEM). The human colon cancer cells (HCT116, RKO, DLD1 and HCT116 Bax−/−) were cultured in McCoy's 5A medium. All cell culture media were supplemented with 10% fetal bovine serum, 1% penicillin and 1% streptomycin. Cells were maintained in a humidified 5% CO2 atmosphere at 37 °C.
Chemical reagents and antibodies
Tetrandrine was purchased from Shanghai Ronghe Medical (Shanghai, China). Sorfenib was purchased from Bayer Pharmaceutical Corporation (West Haven, CT, USA). Z-VAD-fmk was purchased from R&D Systems (Minneapolis, MN, USA). DCFH-DA was obtained from Invitrogen (Carlsbad, CA, USA). N-acetyl-ℒ-cysteine (NAC) was purchased from Sigma (St. Louis, MO, USA). Rhodamine 123 (Rh123), cyclosporin A (CsA), caspase-8 antibody, GAPDH antibody and HRP-conjugated secondary antibodies (goat anti-rabbit and goat anti-mouse) were purchased from Beyotime (Nantong, China). Caspase-3, caspase-9, PARP, Mcl-1, Bcl-2, c-FLIP, Puma, Bax, phospho-Akt(Ser473), Akt, phospho-ERK(Thr202/Tyr204), ERK, phospho-p38(Thr180/Tyr182) and p38 antibodies were from Cell Signaling Technologies(Beverly, MA, USA). Bim, Bid and Bcl-xL antibodies were from Proteintech Group (Chicago, IL, USA).
Cell viability and colony-formation assay
Cells were seeded in 96-well plates, treated as indicated in the figure legends after 12 h and then were allowed to grow for 72 h. DMSO was used as vehicle. Cell viability was observed by the trypan blue dye-exclusion assay and cells were counted using a haemocytometer. To determine the long-term effects of drug treatment on cell colony formation, cells were seeded in six-well plates at 2000 cells per well and treated as indicated in the figure legends. After rinsing with fresh medium, cells were allowed to grow for 14 days to form colonies, which were stained with crystal violet (0.5% w/v), photographed with a scanner and then counted.
Apoptosis assay by flow cytometry
Apoptosis was determined by the flow cytometric measurement of sub-G1 cell populations. Cells were harvested and washed with PBS, followed by fixation with 70% alcohol overnight at 4 °C. Fixed cells were collected, washed with PBS and then stained with 4 μl of 10 mg ml−1 propidium iodide (PI) and 10 μl of 1 mg ml−1 RNase. Stained cells were assessed on a flow cytometer (Beckman, Indianapolis, CA, USA). The results were analysed by the FlowJo software (Tree Star, San Carlos, CA, USA).
Measurement of intracellular ROS level and mitochondrial membrane potential
FACS analysis was carried out to study intracellular ROS and mitochondrial membrane potential. Briefly, following different treatments as indicated in figure legends, cells were collected, washed with PBS and resuspended in serum-free medium containing the corresponding dye. Intracellular ROS levels were measured by the addition of 10 μℳ 5-(and-6)-carboxy-2', 7'-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA; Invitrogen) at 37 °C for 20 min. Mitochondrial membrane potential was determined by measuring the retention of the Rh123 dye. The cells were washed again and subjected to flow cytometry analysis. The data were processed with FlowJo.
Western blot analysis
Harvested cells were washed with PBS, lysed with 1% SDS to break down the membranes and then immediately heated to 95 °C for 20 min. The samples were centrifuged at 12 000 g for 15 min to collect the supernatant, and the protein concentrations were assessed with a bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA). Samples were run on SDS-PAGE gels and immunoblotted with the antibodies mentioned above.
Plasmids and transient transfection
The constitutively active Akt plasmid (pUSE-CA-Akt) and the empty vector (pUSE) were purchased from Upstate (Lake Placid, NY, USA). Cells were seeded in 24-well plates overnight and transfected for 36 h using FuGENE HD transfection reagent following the manufacturer's instructions (Roche, Indianapolis, IN, USA).
Tumour xenograft and TUNEL assay
Animal experiments were conducted according to the guidelines of the Laboratory Animal Center of Wuhan University College of Life Sciences. Six-week-old male athymic nude mice (BALB/c, nu/nu) were purchased from the Model Animal Research Center (Changsha, China). All qualified mice were injected in the right flank with 2 × 107 HCT116 cells suspended in 0.2 ml of PBS. Tumour growth and body weight of the mice were monitored every other day. Five days later, mice bearing tumours reaching about 50 mm3 were randomly divided into four experimental categories (N=7) to receive the following treatments by gavage every other day for 3 weeks: (i) 0.1% sodium carboxyl methylcellulose; (ii) 25 mg kg−1 body weight of sorafenib; (iii) 30 mg kg−1 body weight of tetrandrine; and (iv) 25 mg kg−1 body weight of sorafenib and 30 mg kg−1 body weight of tetrandrine. Tumour volumes were calculated with the following formula: π/6 × large diameter × (small diameter)2. After the treatment, the mice were killed, and the tumours were excised for analysis.
The TUNEL assay was used to detect DNA strand breaks labeled with fluorescein. The tumour tissue samples were sectioned and treated according to the manufacturer's directions (Roche) and then inspected under a fluorescence microscope (Olympus, Tokyo, Japan) to identify blue DAPI staining at 460 nm and green fluorescence (apoptotic cells) at 520 nm.
Malondialdehyde (MDA) assay
Tumour tissues were extracted from all mice killed after the 3-week treatment. For the MDA assay, tissue proteins of tumour xenograft were prepared according to the description in the Lipid Peroxidation MDA assay kit (Beyotimes, Nantong, China). The MDA concentration of each sample was detected by multimode microplate readers (Spectram-Max M5; Molecular Devices, Sunnyvale, CA, USA) at 532 nm, using 490 nm as a control.
Statistical analysis
Results are expressed as the mean±s.d. of three independent experiments unless otherwise indicated. Levels of significance were evaluated by two-tailed paired Student's t-test, and P<0.05 was considered statistically significant.
Results
Combination of sorafenib and tetrandrine showed synergistic antitumour activity
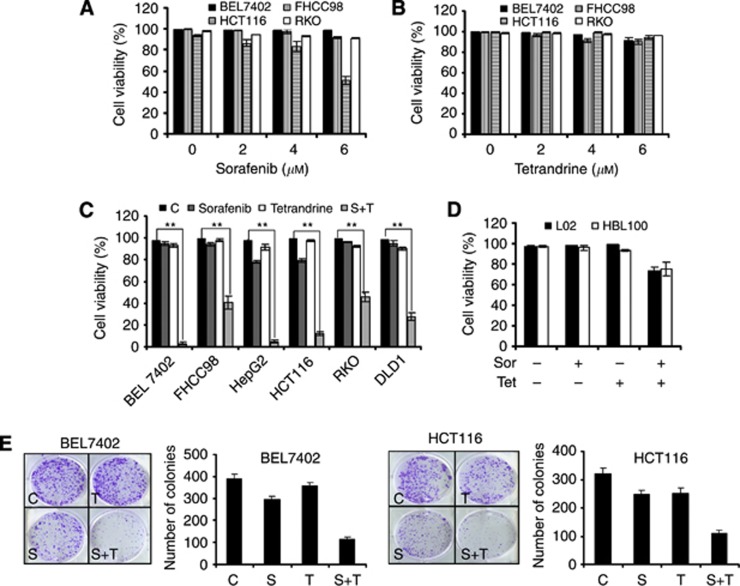
Although sorafenib could induce apoptosis in various human tumour cell lines, many cancer cells showed resistance to the treatment. Figure 1A shows that human HCC cell lines BEL7402 and FHCC98 and human colorectal carcinoma cell lines RKO and HCT116 did not exhibit a significant increase in cell death after a 72-h treatment with 2–6 μℳ of sorafenib (except for HCT116 in 6 μℳ). Similarly, tetrandrine also did not kill cancer cells at low concentrations (Figure 1B), which is consistent with our previous reports (Gong et al, 2012a). Interestingly, a synergistic antitumour activity was observed at 4 μℳ sorafenib in combination with 6 μℳ tetrandrine in the cancer cells after 72 h of treatment (Figure 1C and Supplementary Figures S1 and S2). In contrast, immortalised non-malignant human mammary epithelial cells (HBL100) and normal human hepatic cells (L02) were less sensitive to this combined treatment (Figure 1D). Further analysis of long-term cell survival by the colony-formation assay showed that the combination of sorafenib and tetrandrine dramatically decreased the number of colonies formed by BEL7402 and HCT116 cells (Figure 1E). In addition, the cell-cycle detection results suggested that no significant changes had been found on the levels of cell cycle for combination of sorafenib and tetrandrine, which indicated that the cell-cycle alteration is not a mechanism to have an effect on the number of colonies (Supplementary Figure S3). These data demonstrate that the combination treatment is therapeutically effective against cancerous cells and minimally toxic to normal cells.
Figure 1.
The combination of sorafenib and tetrandrine showed synergistic antitumour activity. Data are representative of values from at least three independent experiments. Cell viability was determined by the trypan blue dye-exclusion assay after treatment for 72 h. (A) Treatment of cancer cells with increasing concentrations of sorafenib (0, 2, 4, 6 μℳ) minimally affected cell viability. (B) No cytotoxic effect was observed with tetrandrine treatment (0, 2, 4, 6 μℳ) alone. (C) Sorafenib (4 μℳ) plus tetrandrine (6 μℳ) strongly decreased the viability of human tumour cell lines (**P<0.01). (D) Sorafenib (4 μℳ) plus tetrandrine (6 μℳ) minimally affected the viability of L02 and HBL100 cell lines. (E) Representative dishes from the colony-formation assay. The clonogenic assay was performed as described in Materials and Methods. The results shown here are representative of three independent experiments.
Combination of sorafenib and tetrandrine induced caspase-dependent apoptosis in cancer cells
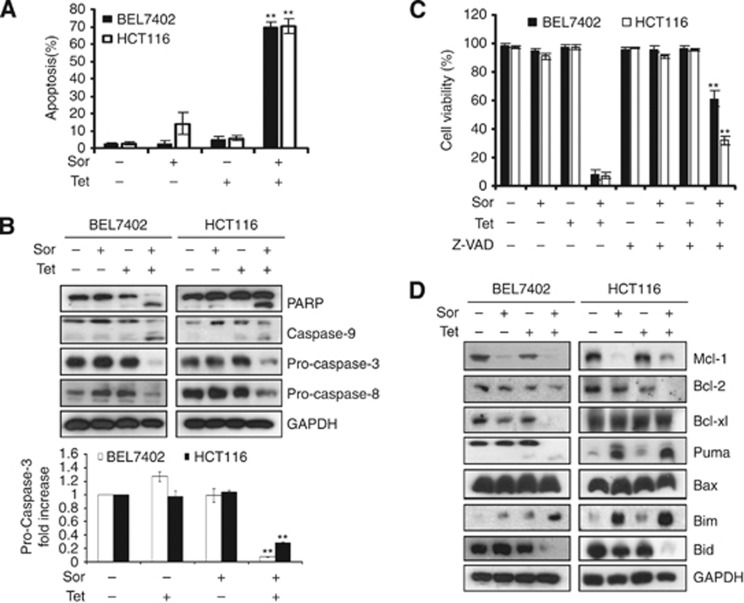
To determine whether the combination treatment with sorafenib and tetrandrine induces apoptosis in cancer cells, BEL7402 and HCT116 cells were treated with either 4 μℳ sorafenib or 6 μℳ tetrandrine alone or in combination and then stained with PI. Flow cytometry analysis revealed that cells underwent apoptosis after the 72-h combination treatment (Figure 2A). To further confirm these findings, we determined the effects of the combination treatment on the activation of various caspases, which is essential for both the extrinsic and intrinsic apoptotic pathways, and PARP cleavage, which serves as a marker of cells undergoing apoptosis. Western blot results showed that a combination of 4 μℳ sorafenib and 6 μℳ tetrandrine effectively induced PARP cleavage and activated caspase-8, caspase-9 and caspase-3 (Figure 2B). The results of caspase activity test further showed the combination treatment activated caspase-9 and caspase-3 (Supplementary Figure S4). Furthermore, pretreatment with the pan-caspase inhibitor z-VAD-fmk significantly blocked cell death in BEL7402 and HCT116 cells (Figure 2C), suggesting that the apoptotic response induced by sorafenib plus tetrandrine was at least partially caspase-dependent.
Figure 2.
Sorafenib plus tetrandrine induced caspase-dependent apoptosis in cancer cells. All experiments were conducted after treatment with sorafenib (4 μℳ) and tetrandrine (6 μℳ) for 72 h. (A) Sorafenib and tetrandrine combined to induce cell apoptosis. Apoptotic cells were detected by flow cytometry (**P<0.01). (B) Western blot analysis of PARP, caspase-9, pro-caspase-3 and pro-caspase-8 after cells were treated as described above. GAPDH was used as a loading control (**P<0.01). (C) Cell viability was assessed following a 72-h treatment with sorafenib (4 μℳ) and tetrandrine (6 μℳ) with or without a pre-treatment of 50 μℳ z-VAD-fmk (pan-caspase inhibitor) (**P<0.01). (D) Western blot analysis on whole-cell lysates with antibodies against mitochondrial Bcl-2 family members.
The Bcl-2 family of proteins, which includes the subfamilies of anti-apoptotic, pro-apoptotic and BH3-only proteins, has central roles in cell death regulation during chemotherapy (Hu et al, 2008; Leibowitz and Yu, 2010). In this study, we examined the protein levels of a few Bcl-2 family members and found that the combination of sorafenib and tetrandrine dramatically decreased Mcl-1 and Bcl-2 levels. The other proteins were regulated differently in BEL7402 and HCT116 cells (Figure 2D).
ROS were involved in cellular apoptosis induced by the combination of sorafenib and tetrandrine
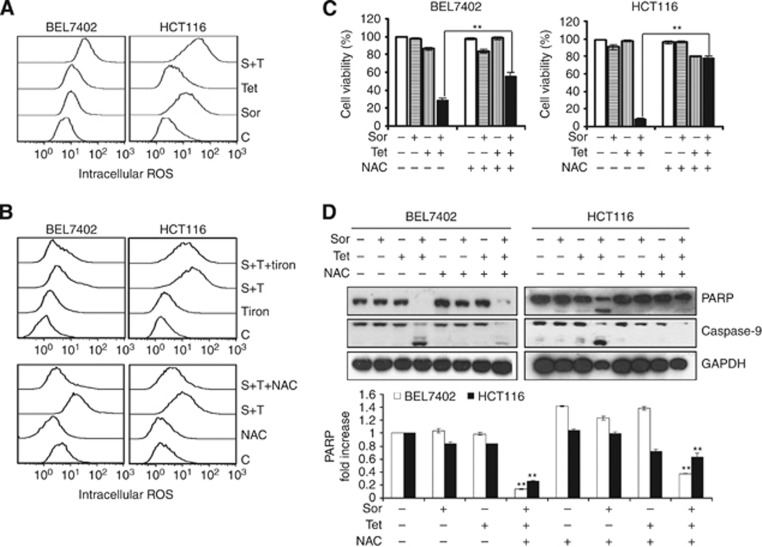
ROS are important products of the mitochondria. They take part in the regulation of physiological cell signaling but might cause cell death if produced excessively (Vandamme et al, 2012; Gong et al, 2012b). Intracellular ROS generation is crucial for chemotherapeutic agent-induced apoptosis in various cancer cells (O'Connor et al, 2012). Sorafenib alone dose-dependently induces the generation of ROS in tumour cells in vitro and in vivo (Coriat et al, 2012). Therefore, we next determined whether the combination drug treatment could produce abnormal levels of ROS in BEL7402 and HCT116 cells. Using H2DCFDA-based detection and flow cytometry, ROS accumulation was observed after treating cells with either single agents or the combination of sorafenib and tetrandrine. However, the combination treatment resulted in significantly higher levels of intracellular ROS compared with the single-agent treatments (Figure 3A). To determine whether excessive ROS generation is involved in apoptosis, BEL7402 and HCT116 cell viabilities were assessed after the combination treatment with sorafenib and tetrandrine in the presence or absence of ROS scavengers NAC and Tiron. The results indicated that not only was ROS generation markedly abrogated by NAC after the combination treatment but NAC also rescued cells from combination treatment-induced cell death (partial rescue for BEL7402 cells and nearly complete rescue for HCT116 cells, Figure 3B and C). Additional experiments showed that combination treatment-induced cleavage of PARP and caspase-9 was also inhibited by pretreatment with NAC, consistent with our cell survival data (Figure 3C and D). Thus, these results suggest that intracellular ROS have an essential role in cellular apoptosis induced by sorafenib plus tetrandrine.
Figure 3.
Intracellular ROS generation was involved in cellular apoptosis induced by sorafenib plus tetrandrine. (A) Effects of sorafenib plus tetrandrine on intracellular ROS levels after 72 h of treatment. (B) BEL7402 and HCT116 cells were pretreated with 15 mℳ NAC or 10 mℳ Tiron for 1 h and then with the combination of sorafenib and tetrandrine for 72 h. (C) Cell viability was determined in the presence of 15 mℳ NAC after 1 h of treatment (**P<0.01). Values represent mean±s.d. (N=3). (D) Western blot analysis of PARP and caspase-9 levels.
Mitochondrial depolarisation was required in combination treatment-induced apoptosis
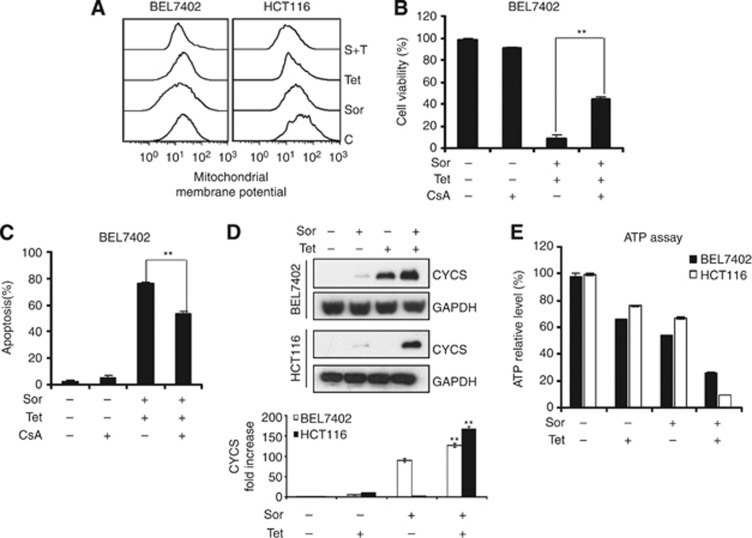
Mitochondria have a critical role in regulating cell physiology and cell survival, thus making them integral to many human diseases (Osiewacz et al, 2010). Excessive production of ROS leads to mitochondrial membrane depolarisation, causing a reduction in the membrane potential (Δψm) and an increase in the permeability of the outer membrane (Zorov et al, 2006). Consequently, proapoptotic proteins such as cytochrome c are released and ATP synthesis is decreased (Garcia-Ruiz et al, 2000; Tait and Green, 2010), which are considered as triggers for the intrinsic cell death pathway. A variety of chemotherapeutic agents, as well as radiation, can directly target mitochondria and induce apoptosis (Huang et al, 2009; Santana et al, 2009). Therefore, we examined whether mitochondrial events were associated with apoptosis induction by sorafenib plus tetrandrine in cancer cells. FACS analysis showed that the combination treatment decreased membrane potential in both the cell lines, but the effect was more dramatic in HCT116 cells than in BEL7402 cells (Figure 4A). However, when the cells were pre-incubated with a mitochondrial membrane potential stabiliser, CsA, cells' death was partially abrogated (Figure 4B and C). Therefore, we believe that mitochondrial depolarisation is necessary in combination treatment-induced apoptosis. In addition, we also observed that cytochrome c was released into the cytoplasm from the mitochondria, and ATP production was reduced when cells were treated with sorafenib plus tetrandrine (Figure 4D and E). Therefore, cellular apoptosis induced by the combination treatment is mitochondrially mediated.
Figure 4.
Mitochondrial depolarisation was necessary in combination treatment-induced apoptosis. Cells were treated with sorafenib (4 μℳ) in combination with tetrandrine (6 μℳ) for 72 h. (A) Mitochondrial membrane potential was determined by Rh123 staining and flow cytometry. (B) BEL7402 cells were treated with sorafenib and tetrandrine alone or in combination with 2 μℳ CsA for 72 h, and cell viability was assessed by the trypan blue dye-exclusion assay (**P<0.01). (C) BEL7402 cells were treated in the presence or absence of 2 μℳ CsA for 72 h and then subjected to flow cytometry analysis of cellular apoptosis (**P<0.01). (D) Western blot analysis of cytochrome c release from mitochondria (**P<0.01). (E) Detection of relative ATP level by ATP Assay Kit according to the manufacturer's protocol on a luminometer. The ATP level of untreated cells was set at 100%.
Combination treatment induces cancer cell apoptosis through inhibition of Akt activation
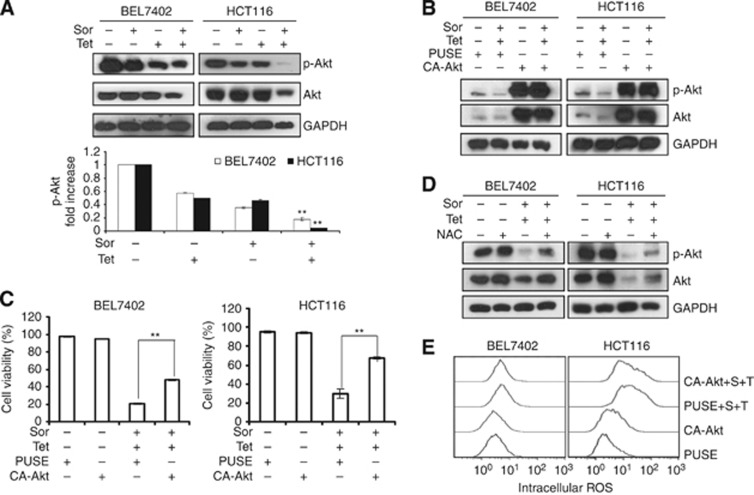
Our previous reports have demonstrated that tetrandrine can significantly inhibit the activity of Akt (Liu et al, 2011). Akt is a critical kinase that regulates a variety of biological processes, including survival, proliferation, apoptosis and differentiation through downstream signal transduction cascades (Li et al, 2006; Zhang et al, 2011; Sheppard et al, 2012). To examine the role of Akt activity in combination treatment-induced cell apoptosis, we first evaluated the level of phosphorylated Akt, which represents the active form. As shown in Figure 5A, the combination treatment dramatically suppressed Akt activation. However, ectopic expression of Akt (Figure 5B) partly abrogated combination treatment-induced apoptosis in BEL7402 and HCT116 cells (Figure 5C). These results suggested that Akt was most likely involved in cellular apoptosis induced by sorafenib plus tetrandrine.
Figure 5.
Combination treatment induced cancer cell apoptosis through inhibition of Akt activation. BEL7402 and HCT116 cells were treated with sorafenib (4 μℳ) and tetrandrine (6 μℳ) for 72 h in all the experiments. (A) Protein lysates from cells treated with sorafenib and tetrandrine were subjected to western blot analysis for Akt kinase (**P<0.01). (B) Akt protein level was assessed by western blot after cells were transfected with either the vehicle plasmid (pUSE) or the constitutively active Akt-expressing plasmid (CA-Akt) in the presence or absence of sorafenib (4 μℳ) and tetrandrine (6 μℳ) (**P<0.01). (C) Cell viability was determined after cells were transfected with the vehicle plasmid (pUSE) or the Akt-expressing plasmid (CA-Akt). (D) Western blot analysis of Akt in BEL7402 and HCT116 cells treated with sorafenib plus tetrandrine after a 1-h pretreatment with 15 mℳ NAC. (E) Cells transfected with pUSE or CA-Akt were incubated with sorafenib (4 μℳ) and tetrandrine (6 μℳ) for 72 h and then intracellular ROS was detected by flow cytometry.
To determine the relationship between ROS generation and Akt inhibition in the combination treatment-induced cell apoptosis, we performed western blot analysis of Akt activity in the presence of an ROS scavenger. The results indicated that NAC partially restored both total and phosphorylated Akt levels when cells were treated with sorafenib plus tetrandrine (Figure 5D). By contrast, when we upregulated Akt activity through ectopic expression, ROS levels were not obviously diminished in the presence of sorafenib plus tetrandrine (Figure 5B and E). These results suggested that ROS acted upstream of the Akt signaling pathways in our treatment model.
Combination of sorafenib and tetrandrine showed synergistic antitumour activity in an in vivo xenograft model
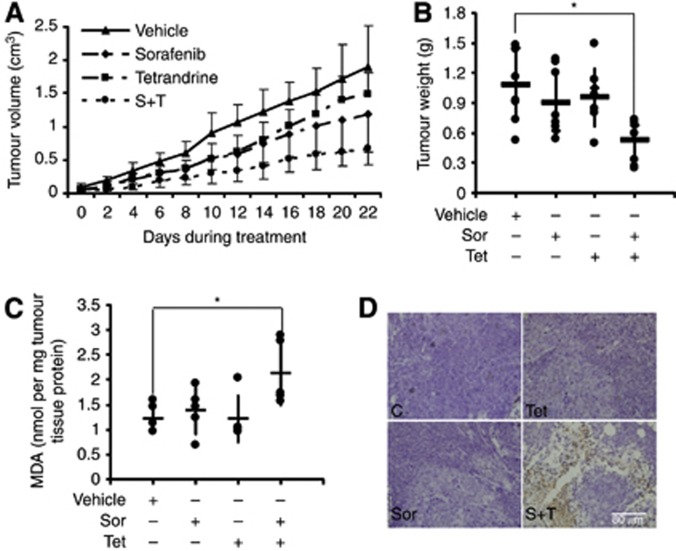
To investigate the synergistic antitumour effects of sorafenib plus tetrandrine in vivo, nude mice bearing established HCT116 tumour xenografts were gavaged with vehicle, sorafenib (25 mg kg−1 body weight), tetrandrine (30 mg kg−1 body weight) or both compounds (sorafenib 25 mg kg−1 body weight and tetrandrine 30 mg kg−1 body weight ) every other day for 22 days. The results showed that the combination treatment strongly inhibited tumour growth compared with vehicle or single-agent treatments (Figure 6A). Consistent with tumour volumes, the average tumour weights associated with vehicle, sorafenib, tetrandrine and the combination therapy were 1.08, 0.90, 0.96 and 0.52 g (*P<0.05), respectively (Figure 6B). Moreover, we found no losses in body weights (Supplementary Figure S5). Therefore, these results showed that the combination therapy was synergistically effective against tumours and caused minimal damage to normal cells. Moreover, the level of the lipid peroxidation product MDA, used as a presumptive measure of ROS-mediated injury, was increased in the combination therapy group compared with the other groups (Figure 6C). TUNEL assay further showed a significant increase in the number of apoptotic cells in the tumour tissues from mice treated with both compounds in comparison with tissues from mice that received single-agent treatments (Figure 6D). Taken together, these data suggested that the in vivo antitumour activity of sorafenib plus tetrandrine was reflected by a highly synergistic ability to induce cancer cell apoptosis.
Figure 6.
Sorafenib and tetrandrine showed synergistic antitumour activity in the in vivo xenograft model. HCT116 cells were inoculated into mice to establish a tumour model as indicated in Materials and Methods. Mice bearing tumours (7 mice per group) were treated with vehicle, sorafenib (25 mg kg−1), tetrandrine (30 mg kg−1) or sorafenib plus tetrandrine every other day for 3 weeks. (A) Mean tumour volumes at given time points. (B) The weights of extracted tumours are presented on a scatter plot; the bars represent the s.d. *P<0.05. (C) Tumour tissue proteins exacted from HCT116 xenografts were subjected to the MDA assay. *P<0.05. (D) Apoptosis analysis of tumour tissues by TUNEL staining.
Discussion
The Ras-Raf-MEK-ERK pathway has a critical role in tumourigenesis and targeted therapies, because it represents a common downstream pathway for several key tyrosine kinase receptors that regulate tumour cell proliferation, apoptosis and differentiation (Friday and Adjei, 2008; Shin-Kang et al, 2011; Santarpia et al, 2012). Sorafenib is a type of tyrosine kinase inhibitor used in the clinical treatment of certain solid cancers (Walker et al, 2009; Gradishar, 2012; Oh et al, 2012; Zhang et al, 2012), mainly exerting its antitumour effect through the induction of apoptosis. In the present study, we demonstrated that sorafenib in combination with tetrandrine induced caspase-dependent apoptosis in liver and colon cancer cells, yet non-malignant cells are less sensitive to the treatment. Mechanistically, this combination treatment-induced cancer cell apoptosis occurred mainly through the mitochondrial death pathway, as manifested by the activation of caspase 3 and caspase 9, early releasing of cytochrome c, loss of the mitochondrial membrane potential and the accumulation of intracellular ROS that were primarily generated in the mitochondria. Consistent with the in vitro results, sorafenib plus tetrandrine also showed considerable synergistic antitumour activity and low toxicity in our in vivo xenograft model.
The cells are undergoing multiple forms of dynamic process after drug treatment. And there are diverse mechanisms targeting the cell death pathway. According to our study, ROS and mitochondrial depolarisation were involved in cellular apoptosis induced by the combination of sorafenib and tetrandrine. But NAC and CsA only partially improved the cell viability induced by the combination treatment which indicated that probably there are other ROS-independent mechanisms and non-mitochondrial dysfunction for the cell death induced by the combination therapy (Figure 3C and D and Figure 4B and C), which need to be further explored. Our previous studies demonstrated that tetrandrine treatment resulted in apoptosis at a high concentration (20–30 μℳ) (Liu et al, 2011) and autophagy at a low concentration (5–10 μℳ) in human HCC cells (Gong et al, 2012a). Autophagy is a double-edged sword for tumours. It contributes to cancer cell survival under low nutrient conditions and resistance to anti-cancer treatments, while both autophagy-inhibiting and -inducing genes might be promising molecular targets for anti-cancer agents (Shintani and Klionsky, 2004; Kondo et al, 2005; Maiuri et al, 2009). In the present experimental model, tetrandrine alone also induced considerable cellular autophagy, but the combination treatment mainly induced apoptosis. Whether the apoptotic response was the result of tetrandrine-induced autophagy is not yet known, but cell death could not be blocked by the autophagy inhibitor 3-methyladenine or chloroquine (Supplementary Figure S6), which suggests that tetrandrine combination with sorafenib-induced cell death is probably unrelated to autophagic cell death.
Generation of intracellular ROS seemed to be the main mechanism of apoptosis induction by the combination treatment because the ROS scavenger NAC could efficiently prevent cell death. In addition, Akt might also be involved in apoptosis, because sorafenib plus tetrandrine dramatically suppressed the phosphorylation of Akt, and ectopic overexpression of Akt partly rescued the cells from death. Moreover, activation of Akt most likely acted downstream of ROS production. Indeed, besides inhibiting Akt activation, the combination treatment also repressed the activation of certain MAP kinases, including ERK and p38 (data not shown). It has been established that sorafenib blocks the Raf-MEK pathway and inhibits ERK activation. To mimic sorafenib-mediated inhibition of ERK, PD98059, a potent and selective inhibitor of MEK/ERK kinase, was used in combination with tetrandrine to treat BEL7402 and HCT116 cells (Supplementary Figure S7A). The two compounds acted synergistically to kill the cancer cells, implying that suppression of ERK activation may have a role in the antitumour activity of sorafenib plus tetrandrine. The detailed mechanism behind ERK inhibition still needs further investigation. In comparison, a combination of the p38 kinase inhibitor SB 203580 and tetrandrine could not induce apoptosis, suggesting that apoptotic induction by sorafenib plus tetrandrine does not involve p38 inactivation (Supplementary Figure S7B).
Bcl-2 family proteins have a pivotal role in the regulation of the intrinsic apoptosis pathway (Pritchard et al, 2011; Vogler, 2012). Several groups have shown that sorafenib kills human cancer cells through a mechanism involving downregulation of the antiapoptotic Bcl-2 family member Mcl-1 (Katz et al, 2009; Huber et al, 2011). In the present study, we also investigated the effects of sorafenib and tetrandrine alone or in combination on Bcl-2 family proteins. We found that sorafenib downregulated Mcl-1 and upregulated Bim, which are consistent with other reports. Sorafenib or tetrandrine alone had little effect on the expression of the Bcl-2 protein, but the combination treatment significantly reduced Bcl-2 expression (Figure 2D). Whether Bcl-2 inhibition is related to apoptosis induction needs to be further investigated. Previous reports indicate that the absence of Bax blocks apoptosis and increases drug resistance in many cancers (McCurrach et al, 1997; Chipuk et al, 2004; Letai, 2008). In this study, we found that HCT116 cells with Bax knocked out (Bax−/−) were not sensitive to the combination treatment with sorafenib and tetrandrine as the HCT116 wild-type cells. Therefore, Bax is most likely partly involved in our therapeutic model (Supplementary Figure S8).
In summary, combining sorafenib with different targeted therapies has been shown to improve its antitumour efficacy in preclinical studies. Our present findings reveal that sorafenib acted highly synergistically with tetrandrine against tumour cells in both in vitro cell culture experiments and an in vivo xenograft model. The potential molecular mechanism is the induction of mitochondria-mediated apoptosis through the ROS/Akt pathway. Potentially, this type of combination treatment can not only reduce the toxic side effects of drugs on the patients but also prevent primary and acquired resistance to sorafenib. Therefore, our data provide a novel approach that broadens the application of sorafenib in clinical therapies.
Acknowledgments
This work was supported by the National Basic Research Program of China (2010CB529800), the National Nature Science Foundation of China (81072151, 81273540). Distinguished Youth Foundation of Hubei Province of China (2012FFA019), the Chinese 111 project (B06018) and the Major Scientific and Technological Special Project for the ‘Significant Creation of New Drugs' (2011ZX09102-001-32, 2010ZX09401).
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303 (5660:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- Coriat CCR, Chéreau C, Mir O, Alexandre J, Ropert S, Weill B, Chaussade S, Goldwasser F, Batteux F. Sorafenib-induced hepatocellular carcinoma cell death depends on reactive oxygen species production in vitro and in vivo. Mol Cancer Ther. 2012;11 (10:2284–2293. doi: 10.1158/1535-7163.MCT-12-0093. [DOI] [PubMed] [Google Scholar]
- Eisen T, Ahmad T, Flaherty KT, Gore M, Kaye S, Marais R, Gibbens I, Hackett S, James M, Schuchter LM, Nathanson KL, Xia C, Simantov R, Schwartz B, Poulin-Costello M, O'Dwyer PJ, Ratain MJ. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer. 2006;95 (5:581–586. doi: 10.1038/sj.bjc.6603291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35 (4:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friday BB, Adjei AA. Advances in targeting the Ras/Raf/MEK/Erk mitogen-activated protein kinase cascade with MEK inhibitors for cancer therapy. Clin Cancer Res. 2008;14 (2:342–346. doi: 10.1158/1078-0432.CCR-07-4790. [DOI] [PubMed] [Google Scholar]
- Garcia-Ruiz C, Colell A, Paris R, Fernandez-Checa JC. Direct interaction of GD3 ganglioside with mitochondria generates reactive oxygen species followed by mitochondrial permeability transition, cytochrome c release, and caspase activation. Faseb J. 2000;14 (7:847–858. doi: 10.1096/fasebj.14.7.847. [DOI] [PubMed] [Google Scholar]
- Gong K, Chen C, Zhan Y, Chen Y, Huang Z, Li W. Autophagy-related gene 7 (ATG7) and reactive oxygen species/extracellular signal-regulated kinase regulate tetrandrine-induced autophagy in human hepatocellular carcinoma. J Biol Chem. 2012a;287 (42:35576–35588. doi: 10.1074/jbc.M112.370585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong K, Xie J, Yi H, Li W. CS055 (Chidamide/HBI-8000), a novel histone deacetylase inhibitor, induces G1 arrest, ROS-dependent apoptosis and differentiation in human leukaemia cells. Biocheml J. 2012b;443 (3:735–746. doi: 10.1042/BJ20111685. [DOI] [PubMed] [Google Scholar]
- Gradishar WJ. Sorafenib in locally advanced or metastatic breast cancer. Expert Opin Investig Drugs. 2012;21 (8:1177–1191. doi: 10.1517/13543784.2012.689824. [DOI] [PubMed] [Google Scholar]
- Hikita H, Takehara T, Shimizu S, Kodama T, Shigekawa M, Iwase K, Hosui A, Miyagi T, Tatsumi T, Ishida H, Li W, Kanto T, Hiramatsu N, Hayashi N. The Bcl-xL inhibitor, ABT-737, efficiently induces apoptosis and suppresses growth of hepatoma cells in combination with sorafenib. Hepatology (Baltimore, MD. 2010;52 (4:1310–1321. doi: 10.1002/hep.23836. [DOI] [PubMed] [Google Scholar]
- Hu ZY, Zhu XF, Zhong ZD, Sun J, Wang J, Yang D, Zeng YX. ApoG2, a novel inhibitor of antiapoptotic Bcl-2 family proteins, induces apoptosis and suppresses tumor growth in nasopharyngeal carcinoma xenografts. Int J Cancer. 2008;123 (10:2418–2429. doi: 10.1002/ijc.23752. [DOI] [PubMed] [Google Scholar]
- Huang F, Nie C, Yang Y, Yue W, Ren Y, Shang Y, Wang X, Jin H, Xu C, Chen Q. Selenite induces redox-dependent Bax activation and apoptosis in colorectal cancer cells. Free Radic Biol Med. 2009;46 (8:1186–1196. doi: 10.1016/j.freeradbiomed.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Huang S, Sinicrope FA. Sorafenib inhibits STAT3 activation to enhance TRAIL-mediated apoptosis in human pancreatic cancer cells. Mol Cancer Ther. 2010;9 (3:742–750. doi: 10.1158/1535-7163.MCT-09-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S, Oelsner M, Decker T, zum Buschenfelde CM, Wagner M, Lutzny G, Kuhnt T, Schmidt B, Oostendorp RA, Peschel C, Ringshausen I. Sorafenib induces cell death in chronic lymphocytic leukemia by translational downregulation of Mcl-1. Leukemia. 2011;25 (5:838–847. doi: 10.1038/leu.2011.2. [DOI] [PubMed] [Google Scholar]
- Iyer R, Fetterly G, Lugade A, Thanavala Y. Sorafenib: a clinical and pharmacologic review. Expert Opin Pharmacother. 2010;11 (11:1943–1955. doi: 10.1517/14656566.2010.496453. [DOI] [PubMed] [Google Scholar]
- Katz SI, Zhou L, Chao G, Smith CD, Ferrara T, Wang W, Dicker DT, El-Deiry WS. Sorafenib inhibits ERK1/2 and MCL-1(L) phosphorylation levels resulting in caspase-independent cell death in malignant pleural mesothelioma. Cancer Biol Ther. 2009;8 (24:2406–2416. doi: 10.4161/cbt.8.24.10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5 (9:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- Kuo YC, Lin WC, Chiang IT, Chang YF, Chen CW, Su SH, Chen CL, Hwang JJ. Sorafenib sensitizes human colorectal carcinoma to radiation via suppression of NF-kappaB expression in vitro and in vivo. Biomed Pharmacother. 2012;66 (1:12–20. doi: 10.1016/j.biopha.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Leibowitz B, Yu J. Mitochondrial signaling in cell death via the Bcl-2 family. Cancer Biol Ther. 2010;9 (6:417–422. doi: 10.4161/cbt.9.6.11392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai AG. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8 (2:121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- Li MH, Cha YN, Surh YJ. Peroxynitrite induces HO-1 expression via PI3K/Akt-dependent activation of NF-E2-related factor 2 in PC12 cells. Free Radic Biol Med. 2006;41 (7:1079–1091. doi: 10.1016/j.freeradbiomed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Liu C, Gong K, Mao X, Li W. Tetrandrine induces apoptosis by activating reactive oxygen species and repressing Akt activity in human hepatocellular carcinoma. Int J Cancer. 2011;129 (6:1519–1531. doi: 10.1002/ijc.25817. [DOI] [PubMed] [Google Scholar]
- Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66 (24:11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Tasdemir E, Criollo A, Morselli E, Vicencio JM, Carnuccio R, Kroemer G. Control of autophagy by oncogenes and tumor suppressor genes. Cell Death Differ. 2009;16 (1:87–93. doi: 10.1038/cdd.2008.131. [DOI] [PubMed] [Google Scholar]
- Matei D, Sill MW, Lankes HA, DeGeest K, Bristow RE, Mutch D, Yamada SD, Cohn D, Calvert V, Farley J, Petricoin EF, Birrer MJ. Activity of sorafenib in recurrent ovarian cancer and primary peritoneal carcinomatosis: a gynecologic oncology group trial. J Clin Oncol. 2011;29 (1:69–75. doi: 10.1200/JCO.2009.26.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurrach ME, Connor TM, Knudson CM, Korsmeyer SJ, Lowe SW. bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis. Proc Natl Acad Sci USA. 1997;94 (6:2345–2349. doi: 10.1073/pnas.94.6.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor AE, Mc Gee MM, Likar Y, Ponomarev V, Callanan JJ, O'Shea DF, Byrne AT, Gallagher WM. Mechanism of cell death mediated by a BF2-chelated tetraaryl-azadipyrromethene photodynamic therapeutic: dissection of the apoptotic pathway in vitro and in vivo. Int J Cancer. 2012;130 (3:705–715. doi: 10.1002/ijc.26073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SJ, Erb HH, Hobisch A, Santer FR, Culig Z. Sorafenib decreases proliferation and induces apoptosis of prostate cancer cells by inhibition of the androgen receptor and Akt signaling pathways. Endocr Relat Cancer. 2012;19 (3:305–319. doi: 10.1530/ERC-11-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osiewacz HD, Brust D, Hamann A, Kunstmann B, Luce K, Muller-Ohldach M, Scheckhuber CQ, Servos J, Strobel I. Mitochondrial pathways governing stress resistance, life, and death in the fungal aging model Podospora anserina. Ann N Y Acad Sci. 2010;1197:54–66. doi: 10.1111/j.1749-6632.2010.05190.x. [DOI] [PubMed] [Google Scholar]
- Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind JF. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol. 2011;29 (30:3960–3967. doi: 10.1200/JCO.2011.37.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard JR, Gilbert LA, Meacham CE, Ricks JL, Jiang H, Lauffenburger DA, Hemann MT. Bcl-2 family genetic profiling reveals microenvironment-specific determinants of chemotherapeutic response. Cancer Res. 2011;71 (17:5850–5858. doi: 10.1158/0008-5472.CAN-11-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci MS, Zong WX. Chemotherapeutic approaches for targeting cell death pathways. Oncologist. 2006;11 (4:342–357. doi: 10.1634/theoncologist.11-4-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato RR, Almenara JA, Coe S, Grant S. The multikinase inhibitor sorafenib potentiates TRAIL lethality in human leukemia cells in association with Mcl-1 and cFLIPL down-regulation. Cancer Res. 2007;67 (19:9490–9500. doi: 10.1158/0008-5472.CAN-07-0598. [DOI] [PubMed] [Google Scholar]
- Santana DP, Faria PA, Paredes-Gamero EJ, Caires AC, Nantes IL, Rodrigues T. Palladacycles catalyse the oxidation of critical thiols of the mitochondrial membrane proteins and lead to mitochondrial permeabilization and cytochrome c release associated with apoptosis. Biochem J. 2009;417 (1:247–256. doi: 10.1042/BJ20080972. [DOI] [PubMed] [Google Scholar]
- Santarpia L, Lippman SM, El-Naggar AK. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16 (1:103–119. doi: 10.1517/14728222.2011.645805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen DF, Tang QZ, Yan L, Zhang Y, Zhu LH, Wang L, Liu C, Bian ZY, Li H. Tetrandrine blocks cardiac hypertrophy by disrupting reactive oxygen species-dependent ERK1/2 signalling. Br J Pharmacol. 2010;159 (4:970–981. doi: 10.1111/j.1476-5381.2009.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard K, Kinross KM, Solomon B, Pearson RB, Phillips WA. Targeting PI3 kinase/AKT/mTOR signaling in cancer. Crit Rev Oncog. 2012;17 (1:69–95. doi: 10.1615/critrevoncog.v17.i1.60. [DOI] [PubMed] [Google Scholar]
- Shin-Kang S, Ramsauer VP, Lightner J, Chakraborty K, Stone W, Campbell S, Reddy SA, Krishnan K. Tocotrienols inhibit AKT and ERK activation and suppress pancreatic cancer cell proliferation by suppressing the ErbB2 pathway. Free Radic Biol Med. 2011;51 (6:1164–1174. doi: 10.1016/j.freeradbiomed.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306 (5698:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11 (9:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- Vandamme M, Robert E, Lerondel S, Sarron V, Ries D, Dozias S, Sobilo J, Gosset D, Kieda C, Legrain B, Pouvesle JM, Pape AL. ROS implication in a new antitumor strategy based on non-thermal plasma. Int J Cancer. 2012;130 (9:2185–2194. doi: 10.1002/ijc.26252. [DOI] [PubMed] [Google Scholar]
- Vogler M. BCL2A1: the underdog in the BCL2 family. Cell Death Differ. 2012;19 (1:67–74. doi: 10.1038/cdd.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T, Mitchell C, Park MA, Yacoub A, Graf M, Rahmani M, Houghton PJ, Voelkel-Johnson C, Grant S, Dent P. Sorafenib and vorinostat kill colon cancer cells by CD95-dependent and -independent mechanisms. Mol Pharmacol. 2009;76 (2:342–355. doi: 10.1124/mol.109.056523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Jia D, Duan F, Sun Z, Liu X, Zhou L, Sun L, Ren S, Ruan Y, Gu J. Combined anti-tumor effects of IFN-alpha and sorafenib on hepatocellular carcinoma in vitro and in vivo. Biochem Biophys Res Commun. 2012;422 (4:687–692. doi: 10.1016/j.bbrc.2012.05.056. [DOI] [PubMed] [Google Scholar]
- Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, Schwartz B, Simantov R, Kelley S. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev. 2006;5 (10:835–844. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- Wu JM, Chen Y, Chen JC, Lin TY, Tseng SH. Tetrandrine induces apoptosis and growth suppression of colon cancer cells in mice. Cancer Lett. 2010;287 (2:187–195. doi: 10.1016/j.canlet.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Zhang J, Gold KA, Kim E. Sorafenib in non-small cell lung cancer. Expert Opin Investig Drugs. 2012;21 (9:1417–1426. doi: 10.1517/13543784.2012.699039. [DOI] [PubMed] [Google Scholar]
- Zhang W, Konopleva M, Ruvolo VR, McQueen T, Evans RL, Bornmann WG, McCubrey J, Cortes J, Andreeff M. Sorafenib induces apoptosis of AML cells via Bim-mediated activation of the intrinsic apoptotic pathway. Leukemia. 2008a;22 (4:808–818. doi: 10.1038/sj.leu.2405098. [DOI] [PubMed] [Google Scholar]
- Zhang W, Konopleva M, Shi YX, McQueen T, Harris D, Ling X, Estrov Z, Quintas-Cardama A, Small D, Cortes J, Andreeff M. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008b;100 (3:184–198. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813 (11:1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Ziegler DS, Keating J, Kesari S, Fast EM, Zawel L, Ramakrishna N, Barnes J, Kieran MW, Veldhuijzen van Zanten SE, Kung AL. A small-molecule IAP inhibitor overcomes resistance to cytotoxic therapies in malignant gliomas in vitro and in vivo. Neuro-oncology. 2011;13 (8:820–829. doi: 10.1093/neuonc/nor066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757 (5–6:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.