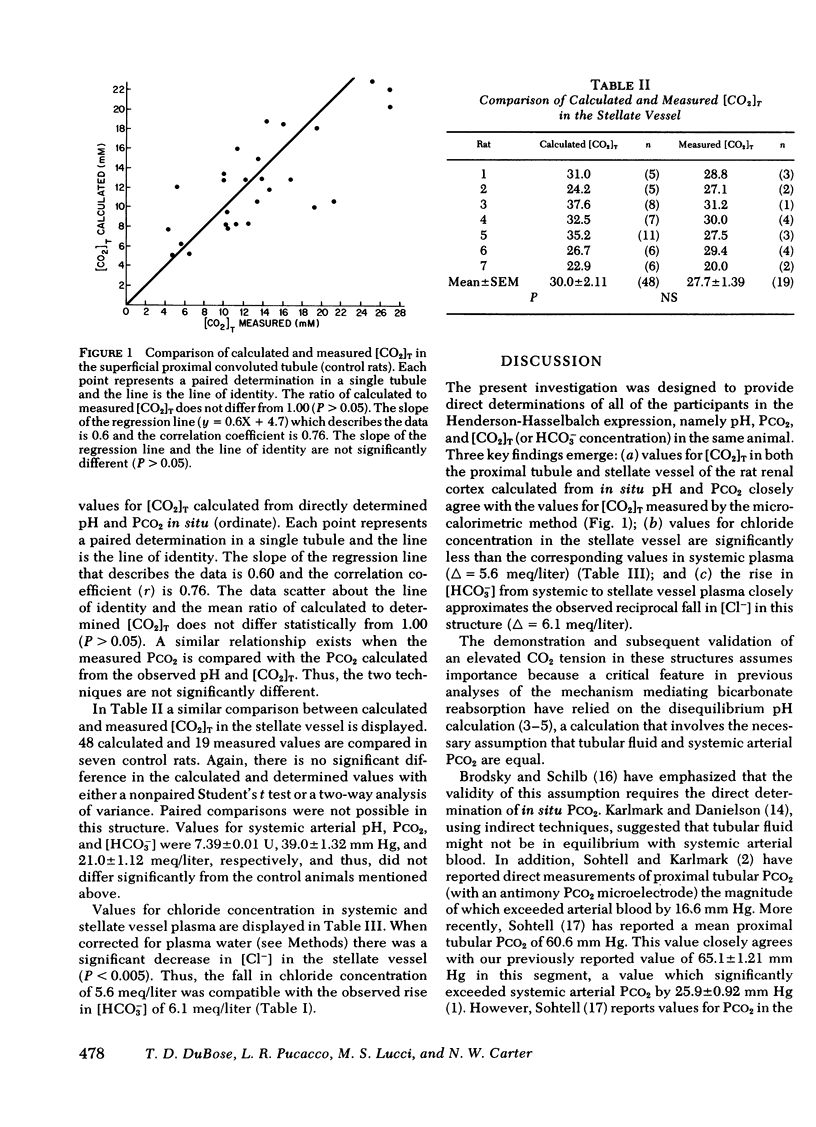
Abstract
Previous studies evaluating the mechanism of renal HCO-3 reabsorption have assumed equilibrium between systemic arterial blood and tubular fluid PCO2. We have recently reported that the PCO2 in proximal and distal tubular fluid as well as the stellate vessel significantly exceeded arterial PCO2 by 25.9 +/- 0.92 mm Hg. The purpose of this study was to determine directly, for the first time, pH, PCO1, and total CO2 concentration in the accessible structures of the rat renal cortex with both microelectrodes and microcalorimetry. In addition, the concentrations of chloride and total CO2 were compared in the stellate vessel. The data demonstrate that: (a) values for total [CO2] in both the proximal tubule and stellate vessel calculated from in situ determination of pH and PCO2 closely agree with the measured values for total [CO2]: (b) values for chloride concentration in the stellate vessel are significantly less than the corresponding values in systemic plasma (delta[Cl-] = 5.6 meq/liter); and (c) the rise in [HCO-3] from systemic to stellate vessel plasma closely approximates the observed reciprocal fall in [Cl-] in this structure.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherton J. C. Comparison of chloride concentration and osmolality in proximal tubular fluid, peritubular capillary plasma and systemic plasma in the rat. J Physiol. 1977 Dec;273(3):765–773. doi: 10.1113/jphysiol.1977.sp012122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRESLER E. H. The problem of the volume component of body fluid homeostasis. Am J Med Sci. 1956 Jul;232(1):93–104. doi: 10.1097/00000441-195607000-00014. [DOI] [PubMed] [Google Scholar]
- Bidani A., Crandall E. D., Forster R. E. Analysis of postcapillary pH changes in blood in vivo after gas exchange. J Appl Physiol Respir Environ Exerc Physiol. 1978 May;44(5):770–781. doi: 10.1152/jappl.1978.44.5.770. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Galla J. H. Influence of postglomerular hematocrit and protein concentration on rat nephron fluid transfer. Am J Physiol. 1971 Jan;220(1):148–161. doi: 10.1152/ajplegacy.1971.220.1.148. [DOI] [PubMed] [Google Scholar]
- Caflisch C. R., Pucacco L. R., Carter N. W. Manufacture and utilization of antimony pH electrodes. Kidney Int. 1978 Aug;14(2):126–141. doi: 10.1038/ki.1978.100. [DOI] [PubMed] [Google Scholar]
- DuBose T. D., Jr, Pucacco L. R., Seldin D. W., Carter N. W. Direct determination of PCO2 in the rat renal cortex. J Clin Invest. 1978 Aug;62(2):338–348. doi: 10.1172/JCI109134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBose T. D., Jr, Seldin D. W., Kokko J. P. Segmental chloride reabsorption in the rat nephron as a function of load. Am J Physiol. 1978 Feb;234(2):F97–105. doi: 10.1152/ajprenal.1978.234.2.F97. [DOI] [PubMed] [Google Scholar]
- Filho E. M., Malnic G. H in cortical peritubular capillaries of rat kidney. Pflugers Arch. 1976 Jun 22;363(3):211–217. doi: 10.1007/BF00594603. [DOI] [PubMed] [Google Scholar]
- Forster R. E., Crandall E. D. Time course of exchanges between red cells and extracellular fluid during CO2 uptake. J Appl Physiol. 1975 Apr;38(4):710–718. doi: 10.1152/jappl.1975.38.4.710. [DOI] [PubMed] [Google Scholar]
- Frömter E., Gessner K. Free-flow potential profile along rat kidney proximal tubule. Pflugers Arch. 1974;351(1):69–83. doi: 10.1007/BF00603512. [DOI] [PubMed] [Google Scholar]
- Giebisch G., Malnic G. Studies on the mechanism of tubular acidification. Physiologist. 1976 Nov;19(4):511–524. [PubMed] [Google Scholar]
- Karlmark B., Danielson B. G. Titratable acid, PCO2, bicarbonate and ammonium ions along the rat proximal tubule. Acta Physiol Scand. 1974 Jun;91(2):243–258. doi: 10.1111/j.1748-1716.1974.tb05681.x. [DOI] [PubMed] [Google Scholar]
- Kunau R. T., Jr The influence of the carbonic anhydrase inhibitor, benzolamide (CL-11,366), on the reabsorption of chloride, sodium, and bicarbonate in the proximal tubule of the rat. J Clin Invest. 1972 Feb;51(2):294–306. doi: 10.1172/JCI106814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnic G., De Mello Aires M., Giebisch G. Micropuncture study of renal tubular hydrogen ion transport in the rat. Am J Physiol. 1972 Jan;222(1):147–158. doi: 10.1152/ajplegacy.1972.222.1.147. [DOI] [PubMed] [Google Scholar]
- Malnic G., Steinmetz P. R. Transport processes in urinary acidification. Kidney Int. 1976 Feb;9(2):172–188. doi: 10.1038/ki.1976.19. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Pucacco L. R., Carter N. W. A glass-membrane pH microelectrode. Anal Biochem. 1976 Jun;73(2):501–512. doi: 10.1016/0003-2697(76)90200-1. [DOI] [PubMed] [Google Scholar]
- Puschett J. B., Zurbach P. E. Re-evaluation of microelectrode methodology for the in vitro determination of pH and bicarbonate concentration. Kidney Int. 1974 Aug;6(2):81–91. doi: 10.1038/ki.1974.83. [DOI] [PubMed] [Google Scholar]
- RECTOR F. C., Jr, CARTER N. W., SELDIN D. W. THE MECHANISM OF BICARBONATE REABSORPTION IN THE PROXIMAL AND DISTAL TUBULES OF THE KIDNEY. J Clin Invest. 1965 Feb;44:278–290. doi: 10.1172/JCI105142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. H., Finn J. T., Vaughan G., Steinmetz P. R. Distribution of metabolic CO2 and the transported ion species in acidification by turtle bladder. Am J Physiol. 1974 Feb;226(2):283–289. doi: 10.1152/ajplegacy.1974.226.2.283. [DOI] [PubMed] [Google Scholar]
- Schwartz J. H., Steinmetz P. R. CO2 requirements for H+ secretion by the isolated turtle bladder. Am J Physiol. 1971 Jun;220(6):2051–2057. doi: 10.1152/ajplegacy.1971.220.6.2051. [DOI] [PubMed] [Google Scholar]
- Sohtell M., Karlmark B. In vivo micropuncture PCO2 measurements. Pflugers Arch. 1976 May 12;363(2):179–180. doi: 10.1007/BF01062288. [DOI] [PubMed] [Google Scholar]
- Sohtell M. PCO2 of the proximal tubular fluid and the efferent arteriolar blood in the rat kidney. Acta Physiol Scand. 1979 Feb;105(2):137–145. doi: 10.1111/j.1748-1716.1979.tb06325.x. [DOI] [PubMed] [Google Scholar]
- Steinhausen M. Further information on the cortical countercurrent system in rat kidney. Yale J Biol Med. 1972 Jun-Aug;45(3-4):451–456. [PMC free article] [PubMed] [Google Scholar]
- Vieira F. L., Malnic G. Hydrogen ion secretion by rat renal cortical tubules as studied by an antimony microelectrode. Am J Physiol. 1968 Apr;214(4):710–718. doi: 10.1152/ajplegacy.1968.214.4.710. [DOI] [PubMed] [Google Scholar]
- Vurek G. G., Warnock D. G., Corsey R. Measurement of picomole amounts of carbon dioxide by calorimetry. Anal Chem. 1975 Apr;47(4):765–767. doi: 10.1021/ac60354a024. [DOI] [PubMed] [Google Scholar]
- Weinstein S. W., Szyjewicz J. Early postglomerular plasma concentrations of chloride, sodium, and inulin in the rat kidney. Am J Physiol. 1976 Sep;231(3):822–831. doi: 10.1152/ajplegacy.1976.231.3.822. [DOI] [PubMed] [Google Scholar]
- Weinstein S. W., Szyjewicz J. Superficial nephron tubular-vascular relationships in the rat kidney. Am J Physiol. 1978 Mar;234(3):F207–F214. doi: 10.1152/ajprenal.1978.234.3.F207. [DOI] [PubMed] [Google Scholar]



