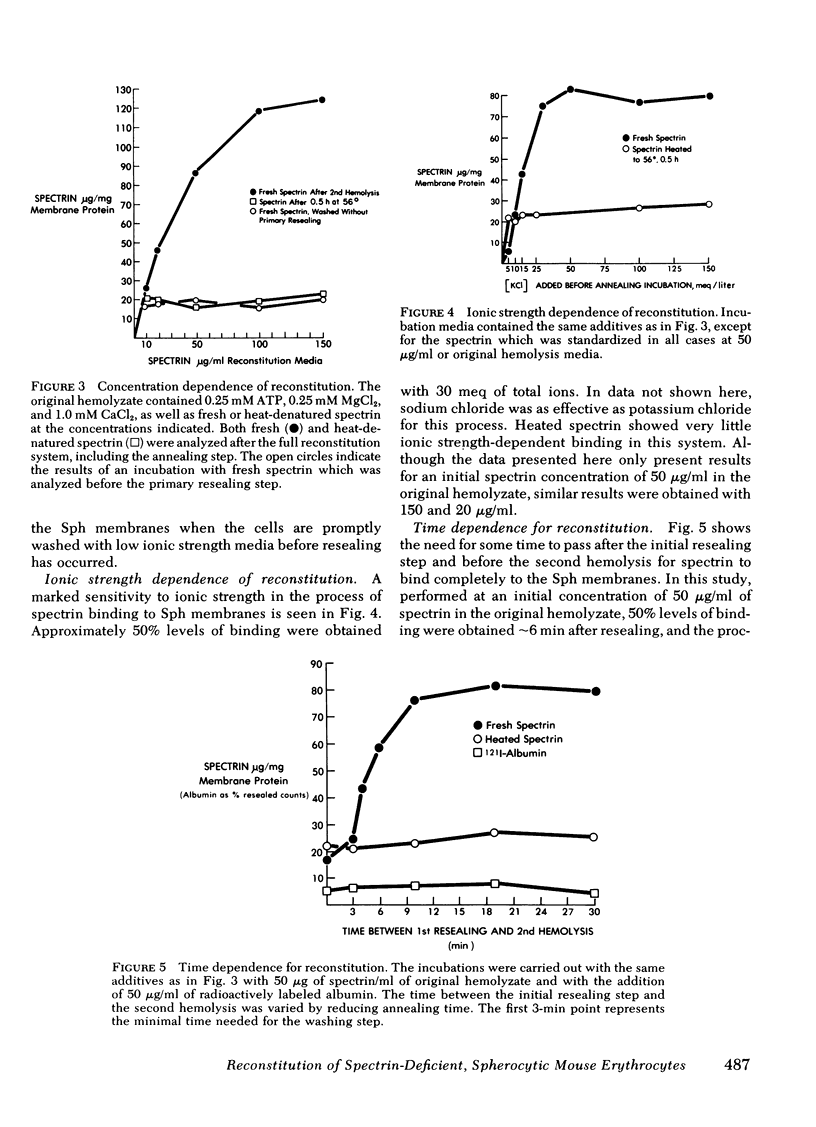
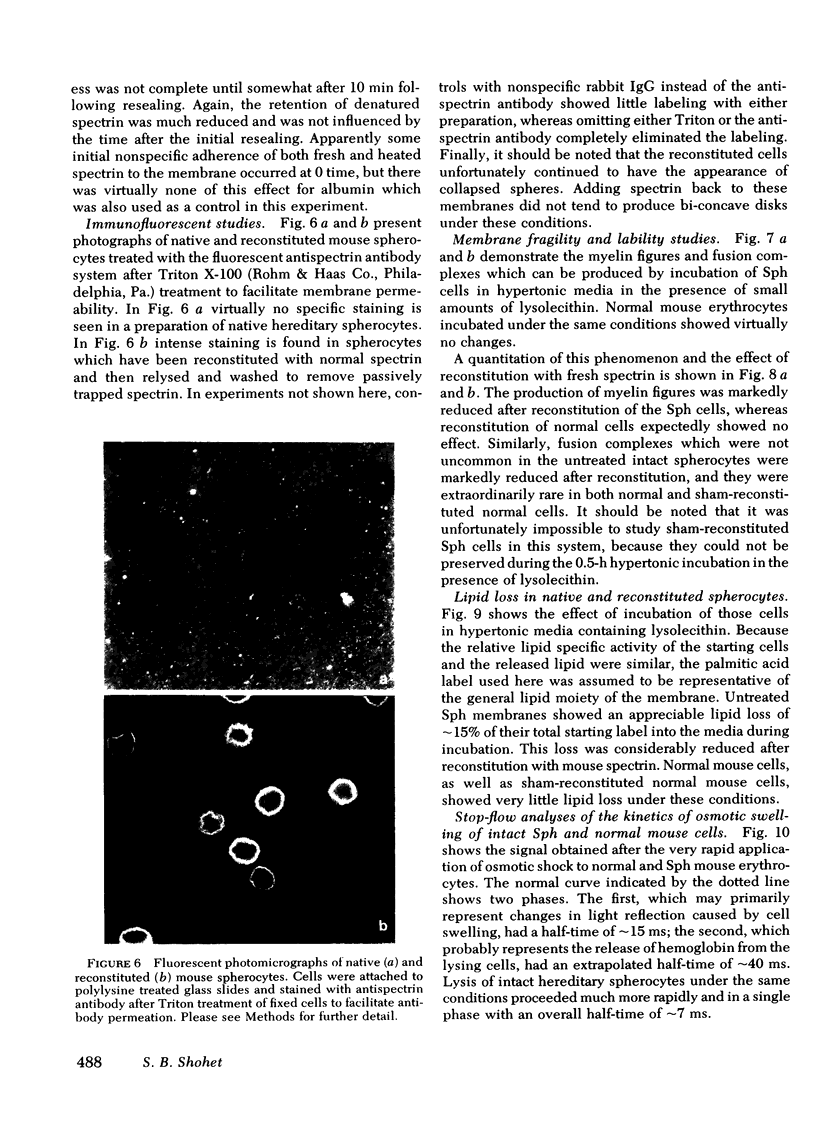
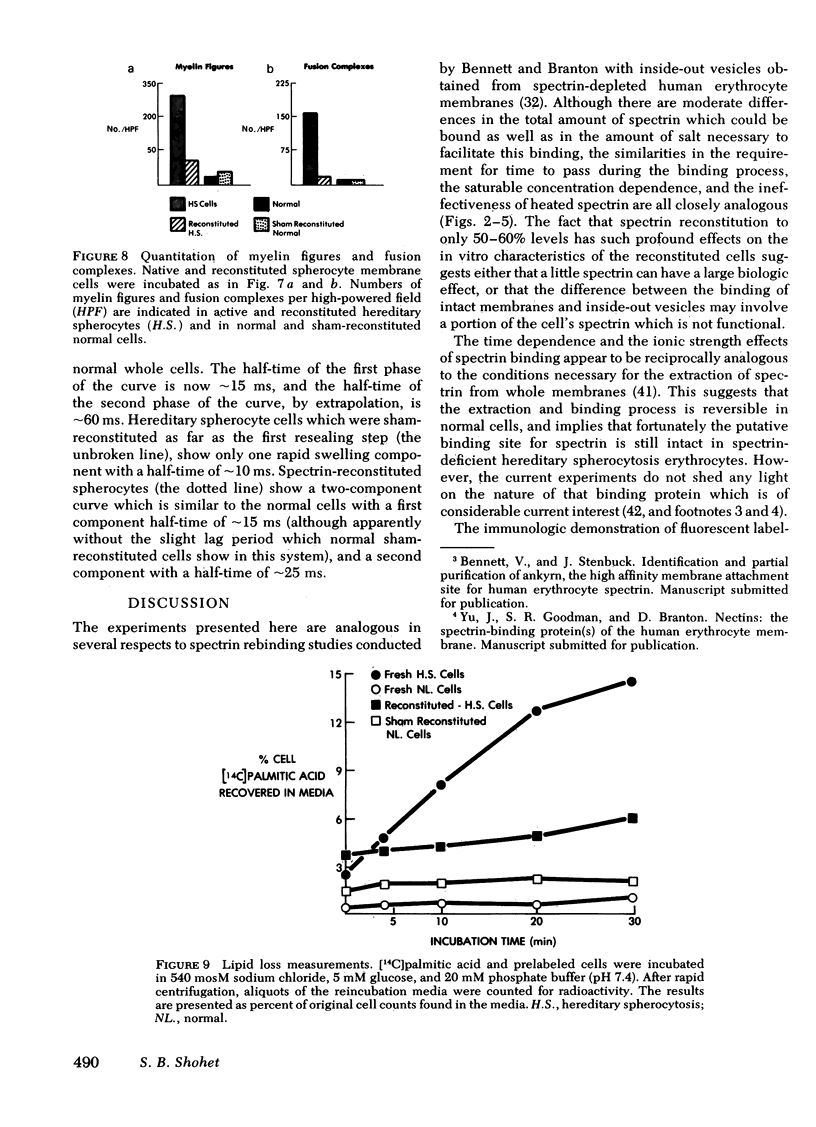
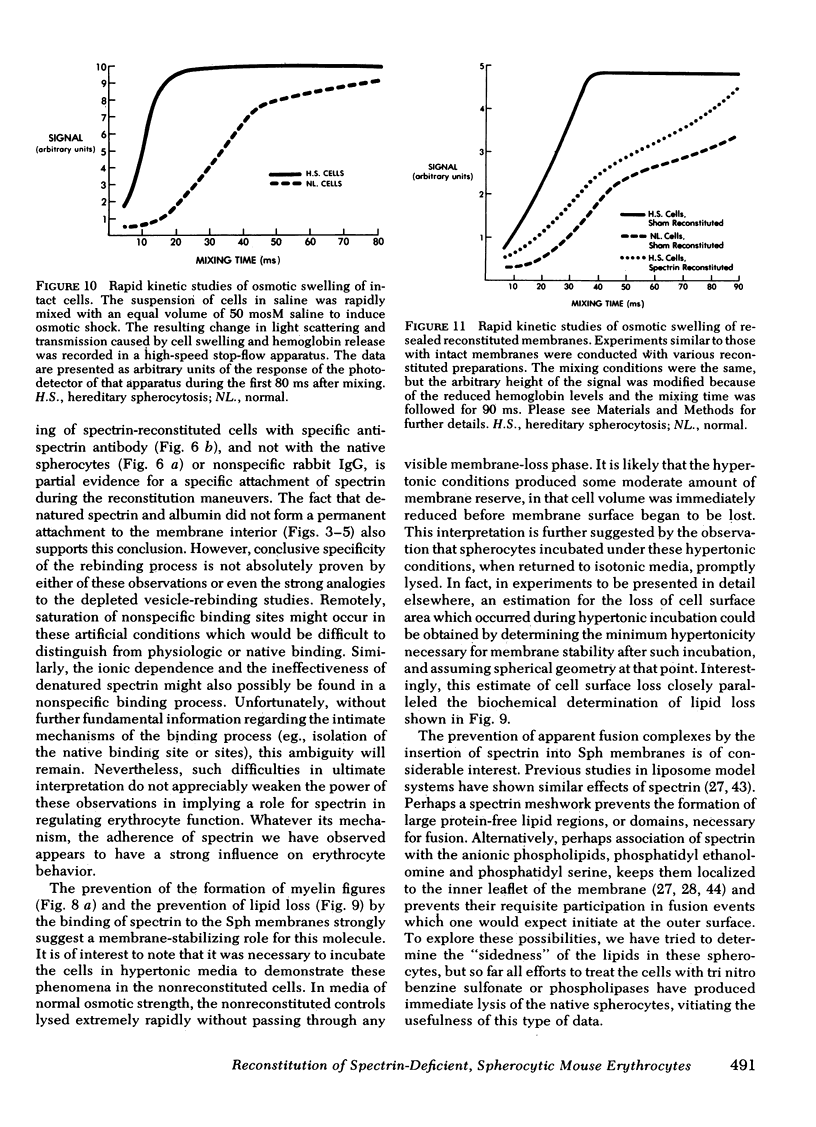
Abstract
To study directly the role of spectrin in erythrocyte membrane function, we have designed a reconstituted membrane system using erythrocyte membranes from spectrin-deficient mice and purified spectrin from normal mice. The normal spectrin is inserted into the spectrin-deficient spherocytes by exchange hemolysis. Thereafter, raising the ionic strength and temperature reseals the cells and, with time, facilitates binding of the spectrin to the spectrin-deficient membranes. The binding is apparently specific as shown by its dependence upon the concentration of undenatured spectrin and the concentration of salt used, as well as by the immunofluorescent appearance of the reconstituted cells after treatment with specific antispectrin antibody. In terms of in vitro cellular behavior, the reconstituted preparations show marked changes in comparison to the untreated spherocytes. In particular, membrane stability, as measured by the reduction of myelin figure formation and lipid loss, is considerably enhanced. In addition, membrane fusion, which occurs readily with the untreated spherocytes, is virtually eliminated. Finally, the osmotic behavior of the native spherocytes is appreciably altered, such that the early phase of osmotically induced swelling, as measured in a high-speed stop-flow apparatus, is delayed and modified. Taken together, these findings indicate specific roles for spectrin in the stabilization of the erythrocyte membrane, in the limitation of membrane fusion, and in the modulation of the membrane's response to osmotic stress.
Full text
PDF


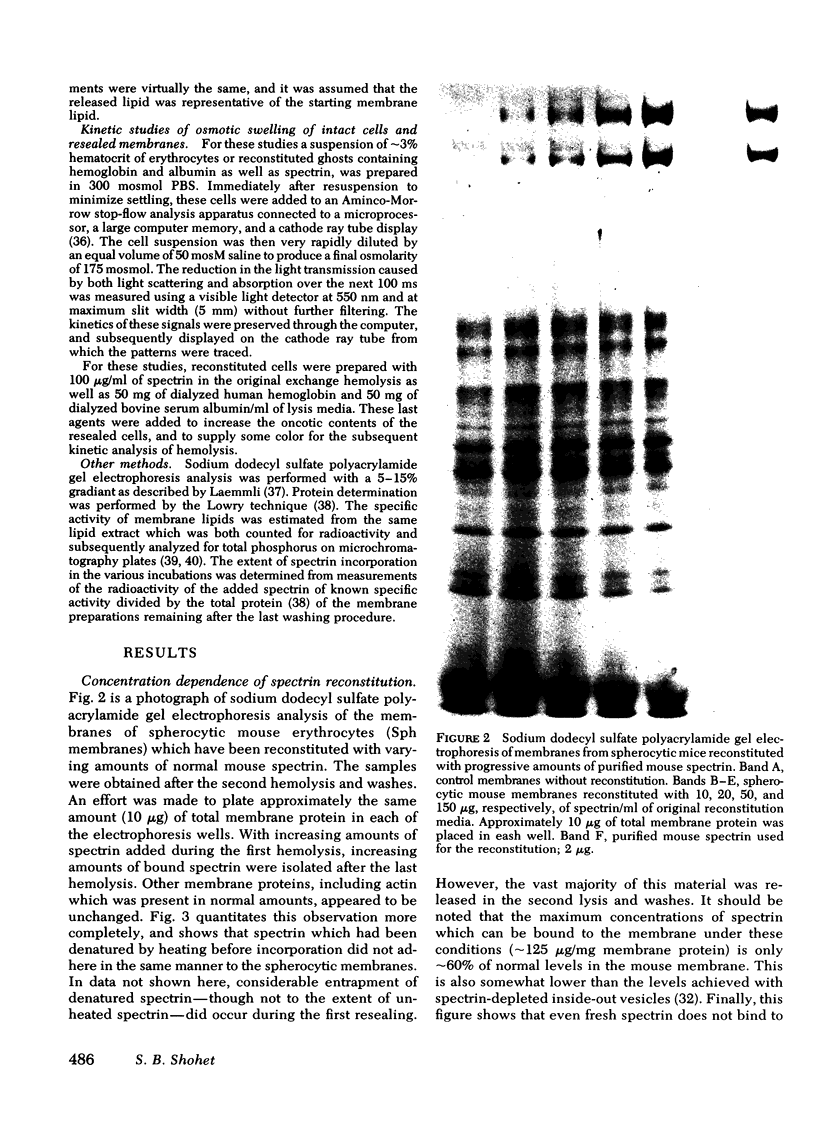




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Fairbanks G. Phosphorylation of endogenous substrates by erythrocyte membrane protein kinases. I. A monovalent cation-stimulated reaction. Biochemistry. 1974 Dec 31;13(27):5507–5514. doi: 10.1021/bi00724a009. [DOI] [PubMed] [Google Scholar]
- Bennett V., Branton D. Selective association of spectrin with the cytoplasmic surface of human erythrocyte plasma membranes. Quantitative determination with purified (32P)spectrin. J Biol Chem. 1977 Apr 25;252(8):2753–2763. [PubMed] [Google Scholar]
- Beutler E., Guinto E., Johnson C. Human red cells protein kinase in normal subjects and patients with hereditary spherocytosis, sickle cell disease, and autoimmune hemolytic anemia. Blood. 1976 Dec;48(6):887–898. [PubMed] [Google Scholar]
- Birchmeier W., Singer S. J. On the mechanism of ATP-induced shape changes in human erythrocyte membranes. II. The role of ATP. J Cell Biol. 1977 Jun;73(3):647–659. doi: 10.1083/jcb.73.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin P., Galand C. Erythrocyte membrane phosphorylation in hereditary spherocytosis. Biomedicine. 1977 Jan 31;27(1):34–36. [PubMed] [Google Scholar]
- Clark M. R., Shohet S. B. Hybrid erythrocytes for membrane studies in sickle cell disease. Blood. 1976 Jan;47(1):121–131. [PubMed] [Google Scholar]
- Clarke M. Isolation and characterization of a water-soluble protein from bovine erythrocyte membranes. Biochem Biophys Res Commun. 1971 Nov;45(4):1063–1070. doi: 10.1016/0006-291x(71)90445-1. [DOI] [PubMed] [Google Scholar]
- Graham C., Avruch J., Fairbanks G. Phosphoprotein phosphatase of the human erythrocyte. Biochem Biophys Res Commun. 1976 Sep 20;72(2):701–708. doi: 10.1016/s0006-291x(76)80096-4. [DOI] [PubMed] [Google Scholar]
- Greenquist A. C., Shohet S. B., Bernstein S. E. Marked reduction of spectrinin hereditary spherocytosis in the common house mouse. Blood. 1978 Jun;51(6):1149–1155. [PubMed] [Google Scholar]
- Greenquist A. C., Shohet S. B. Defective protein phosphorylation in membranes of hereditary spherocytosis erythrocytes. FEBS Lett. 1974 Nov 1;48(1):133–135. doi: 10.1016/0014-5793(74)81080-x. [DOI] [PubMed] [Google Scholar]
- Greenquist A. C., Shohet S. B. Phosphorylation in erythrocyte membranes from abnormally shaped cells. Blood. 1976 Dec;48(6):877–886. [PubMed] [Google Scholar]
- Haest C. W., Plasa G., Kamp D., Deuticke B. Spectrin as a stabilizer of the phospholipid asymmetry in the human erythrocyte membrane. Biochim Biophys Acta. 1978 May 4;509(1):21–32. doi: 10.1016/0005-2736(78)90004-4. [DOI] [PubMed] [Google Scholar]
- Hiller G., Weber K. Spectrin is absent in various tissue culture cells. Nature. 1977 Mar 10;266(5598):181–183. doi: 10.1038/266181a0. [DOI] [PubMed] [Google Scholar]
- JOE M., TEASDALE J. M., MILLER J. R. A new mutation (sph) causing neonatal jaundice in the house mouse. Can J Genet Cytol. 1962 Jun;4:219–225. doi: 10.1139/g62-026. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Karnovsky M. J. Irreversible deformation of the spectrin-actin lattice in irreversibly sickled cells. J Clin Invest. 1976 Oct;58(4):955–963. doi: 10.1172/JCI108549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Ukena T. E. Diminished spectrin extraction from ATP-depleted human erythrocytes. Evidence relating spectrin to changes in erythrocyte shape and deformability. J Clin Invest. 1978 Mar;61(3):815–827. doi: 10.1172/JCI108996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T. Isolation of spectrin from erythrocyte membranes. Methods Enzymol. 1974;32:275–277. doi: 10.1016/0076-6879(74)32028-9. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Palade G. E. Inactivation of adenosine triphosphatase and disruption of red cell membrances by trypsin: protective effect of adenosine triphosphate. Proc Natl Acad Sci U S A. 1967 Sep;58(3):991–995. doi: 10.1073/pnas.58.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto N., Yawata Y., Jacob H. S. Association of decreased membrane protein phosphorylation with red blood cell spherocytosis. Blood. 1977 Feb;49(2):233–239. [PubMed] [Google Scholar]
- Mombers C., van Dijck P. W., van Deenen L. L., de Gier J., Verkleij A. J. The interaction of spectrin - actin and synthetic phospholipids. Biochim Biophys Acta. 1977 Oct 17;470(2):152–160. doi: 10.1016/0005-2736(77)90096-7. [DOI] [PubMed] [Google Scholar]
- Nakashima K., Beutler E. Effect of anti-spectrin antibody and ATP on deformability of resealed erythrocyte membranes. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3823–3825. doi: 10.1073/pnas.75.8.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Marchesi V. T., Singer S. J. The localization of spectrin on the inner surface of human red blood cell membranes by ferritin-conjugated antibodies. J Cell Biol. 1971 Oct;51(1):265–272. doi: 10.1083/jcb.51.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Painter R. G. Anionic sites of human erythrocyte membranes. II. Antispectrin-induced transmembrane aggregation of the binding sites for positively charged colloidal particles. J Cell Biol. 1973 Nov;59(2 Pt 1):395–406. doi: 10.1083/jcb.59.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R. G., Sheetz M., Singer S. J. Detection and ultrastructural localization of human smooth muscle myosin-like molecules in human non-muscle cells by specific antibodies. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1359–1363. doi: 10.1073/pnas.72.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palek J., Liu S. C., Snyder L. M. Metabolic dependence of protein arrangement in human erythrocyte membranes. I. Analysis of spectrin-rich complexes in ATP-depleted red cells. Blood. 1978 Mar;51(3):385–395. [PubMed] [Google Scholar]
- Pinder J. C., Bray D., Gratzer W. B. Actin polymerisation induced by spectrin. Nature. 1975 Dec 25;258(5537):765–766. doi: 10.1038/258765a0. [DOI] [PubMed] [Google Scholar]
- SNYDER F., STEPHENS N. Quantitative carbon-14 and tritium assay of thin-layer chromatography plates. Anal Biochem. 1962 Aug;4:128–131. doi: 10.1016/0003-2697(62)90029-5. [DOI] [PubMed] [Google Scholar]
- Schenkein I., Levy M., Uhr J. W. The use of glucose oxidase as a generator of H 2 O 2 in the enzymatic radioiodination of components of cell surfaces. Cell Immunol. 1972 Nov;5(3):490–493. doi: 10.1016/0008-8749(72)90076-7. [DOI] [PubMed] [Google Scholar]
- Sha'afi R. I., Rich G. T., Sidel V. W., Bossert W., Solomon A. K. The effect of the unstirred layer on human red cell water permeability. J Gen Physiol. 1967 May;50(5):1377–1399. doi: 10.1085/jgp.50.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Singer S. J. On the mechanism of ATP-induced shape changes in human erythrocyte membranes. I. The role of the spectrin complex. J Cell Biol. 1977 Jun;73(3):638–646. doi: 10.1083/jcb.73.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohet S. B., Nathan D. G., Karnovsky M. L. Stages in the incorporation of fatty acids into red blood cells. J Clin Invest. 1968 May;47(5):1096–1108. doi: 10.1172/JCI105799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva P. P., Nicolson G. L. Freeze-etch localization of concanavalin A receptors to the membrane intercalated particles of human erythrocyte ghost membranes. Biochim Biophys Acta. 1974 Sep 23;363(3):311–319. doi: 10.1016/0005-2736(74)90071-6. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Detmers P. Actin in erythrocyte ghosts and its association with spectrin. Evidence for a nonfilamentous form of these two molecules in situ. J Cell Biol. 1975 Sep;66(3):508–520. doi: 10.1083/jcb.66.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidekamm E., Brdiczka B., Wildermuth M. Phospholipid composition of human erythrocyte spectrin. Mol Biol Rep. 1978 Feb 28;4(1):25–28. doi: 10.1007/BF00775176. [DOI] [PubMed] [Google Scholar]
- Wolfe L. C., Lux S. E. Membrane protein phosphorylation of intact normal and hereditary spherocytic erythrocytes. J Biol Chem. 1978 May 10;253(9):3336–3342. [PubMed] [Google Scholar]
- Yu J., Fischman D. A., Steck T. L. Selective solubilization of proteins and phospholipids from red blood cell membranes by nonionic detergents. J Supramol Struct. 1973;1(3):233–248. doi: 10.1002/jss.400010308. [DOI] [PubMed] [Google Scholar]