Abstract
1,1′-Diethyl-2,2′-cyanine iodide (decynium22; D22) is a potent blocker of the organic cation family of transporters (EMT/OCT) known to move endogenous monoamines like dopamine and norepinephrine across cell membranes. Decynium22 is a cation with a relatively high affinity for all members of the OCT family in both human and rat cells. The mechanism through which decynium22 blocks OCT transporters is poorly understood. We tested the hypothesis that denynium22 may compete with monoamines utilizing OCT to permeate the cells. Using the ability of D22 to aggregate and produce fluorescence at 570 nm, we measured D22 uptake in cultured astrocytes. The rate of D22 uptake was strongly depressed by acid pH and by elevated external K+. The rate of uptake was similar to that displayed by 4-(4-(dimethylamino)-styryl)-N-methylpyridinium (ASP+), a well established substrate for OCT and high-affinity Na+-dependent monoamine transporters. These data were supported by measurement of electrogenic uptake using whole cell voltage clamp recording. Decynium22 depressed norepinephrine, but not glutamate uptake. These data are also consistent with the described OCT transporter characteristics. Taken together, our results suggest that decynium22 accumulation might be a useful instrument to study monoamine transport in the brain, and particularly in astrocytes, where they may play a prominent role in monoamine uptake during brain dysfunction related to monoamines (like Parkinson disease)and drug addiction.
Index Words: astrocytes, OCT transporter, decynium22, ASP+, fluorescence
INTRODUCTION
Monoamines play important roles in several disorders such as Parkinson’s disease, Alzheimer’s disease and drug addiction. There are several families of monoamine transporters in the CNS: (i) transporters with high affinity but low capacity (like DAT, NET) and (ii) transporters with low affinity but high capacity (like organic cation transporters; OCTs) (1; 2; 3). Non-neuronal uptake through OCTs was found in glia and glial cells outnumber neurons about ten times in the brain. This suggests a prominent role of glial cells in the regulation of extracellular monoamines.
The members of the organic cation transporter (OCT) family can move endogenous monoamines across cell membranes (4; 5). 1,1′-Diethyl-2,2′-cyanine iodide (decynium22) is an organic cation widely used as a blocker for OCT transporters since it has a very high affinity for all members of this family in both human and rat cells (6; 7). Importantly, it has little effect on high affinity monoamine transporters such as the dopamine transporter (DAT) and norepinephrine transporter (NET) (8; 6). This makes decynium22 an important tool to distinguish between the transporter families. This substance has been shown to block uptake of 1-Methyl-4-phenylpyridinium (MPP), a prototypical substrate for specific non-neuronal transporter system (uptake 2) mediated by the OCT3 transporter in cultured rat astrocytes (9).
The mechanisms through which the quinoline derivatives, decynium22 and quinine, block the OCT are poorly understood. It has been shown that quinine is a competitive blocker for the rat OCT2 transporter from the cytoplasmic side (10), but the same authors have found that quinine is a noncompetitive blocker for the rat OCT1/OCT2 transporter from the extracellular side (11). This prompted the question of whether quinoline derivatives can enter the cell. Arndt et al., (11) measured radiolabeled-quinine uptake in Xenopus oocytes and established that it does go inside the cell, but found no corresponding substrate-coupled current. As the OCT transport is known to be electrogenic, they proposed that the quinine molecule can be transported into the cell in an uncharged form by simple diffusion. The same paper claims that decynium22 also is a noncompetitive blocker for OCT transporters from outside of the cell, but Schömig et al., (8), the group who first introduced decynium22 as potent blocker of OCT family transporters, established that decynium22 is a competitive blocker. The possibility that decynium22 may be captured by the cells has not been evaluated.
The fluorescence of this dye in monomeric form is practically absent, however it forms molecular aggregates (J-aggregates) characterized by very strong superradiant emission with a maximum near 570–580 nm. (12; 13; 14). Therefore, if decynium22 is captured by the cells it may form J-aggregates that can be measured as fluorescence in astrocytes. Thus, we tested the hypothesis that decynium22 may compete with monoamines using OCT and permeate glial cells. We found that, indeed, incubation with decynium22 caused a time dependent increase of fluorescence in astrocytes. Pharmacological characterization of decynium22 uptake in astrocytes suggested that uptake was through OCT transporters. Preliminary data were reported in abstract form (15).
METHODS
Materials Used
1,1′-Diethyl-2,2′-cyanine iodide (decyinium22); 4-(4-(dimethylamino)-styryl)-N-methylpyridinium (ASP+) and all other substances used were purchased from Sigma Chemical Co. (St. Louis, MO).
Astrocyte primary cultures
Primary cultures of astrocytes were prepared from the neocortex of 1–2 day old rats. Brains were removed after decapitation and the meninges stripped away to minimize fibroblast contamination. The forebrain cortices were collected and dissociated using the stomacher blender method. The cell suspension was then allowed to filter by gravity only through a #60 sieve and then through a #100 sieve. After centrifugation, the cells were suspended in modified Eagle’s medium (containing 30 mM glucose, 2 mM glutamine, 1 mM pyruvate and 10% fetal bovine serum, 100 units/ml of penicillin/streptomycin) and plated on uncoated 75 cm2 flasks at a density of 100,000 cells/cm2. The medium was exchanged with fresh culture medium about every 5 days. At confluence (about 12–14 days), the mixed glial cultures were treated with 50 mM leucine methylester (pH 7.4) for 60 min to effectively kill microglia. Cultures were then allowed to recover for at least one day in growth medium prior to reseeding. Astrocytes were dissociated by trypsinization and reseeded onto coverglasses at least two days prior to the imaging experiments. The purity of the astrocyte cultures was greater than 95% as assessed by immunocytochemical staining for glial fibrillary acidic protein (GFAP; data not shown). The “Principles of laboratory animal care” (NIH publication No. 85–23, revised 1985) were followed.
Microscopy and image analysis
On the day of the experiment, a coverglass with astrocytes was mounted in a closed imaging chamber (Warner Inst, UT). After the chamber was connected to the perfusion system, cells were immediately mounted on an Olympus 1×70 microscope with Lambda DG4 computer-driven excitation system, and the microscope was focused on the center of the monolayer of cells. During the measurement, cells remained in a closed chamber and could be perfused with experimental solutions. The external buffer was composed of (in mM): 145 NaCl, 2 KCl, 2.5 CaCl2, 2.5 MgCl2, 10 HEPES(Na), pH 7.4. In some experiments, KCl was substituted by NaCl, the appropriate monoamine substrates and inhibitors were added or the pH was adjustedto 6.4 or 8.4.
Background autofluorescence was established by collecting images prior to the addition of 1μM decynium22 or 2μM of ASP+. Time-series fluorescent images were recorded using a 40× oil immersion objective. The excitation was 480/30× nm; the emitted light was filtered with a 495- to 575-nm band pass filter (mean 535 nm). The gain (contrast) and offset (brightness) for the DCC camera tube was set to avoid detector saturation at the concentration used in these experiments (1 μM of decynium22 and 2μM of ASP+). The acquisition rate was 1 frame every 10 sec to prevent photobleaching of decynium22 and ASP+ sequestered inside the cell, with exposure of 100 or 200 msec.
The fluorescent images were processed using MetaMorph imaging software (Universal Imaging Corp., Downington, PA). Fluorescence accumulation was established by measuring the average pixel intensity of fluorescent images of a cell identified from the differential interference contrast (DIC) image. Decynium22 or ASP+ accumulation were defined as the fluorescence of the cells minus background fluorescence by automatic background subtraction (standard feature of MetaMorph). In the current experiments, background fluorescence was negligible.
Whole cell recordings
Membrane currents were measured with the single electrode whole-cell patch-clamp technique. Cells were visualized using a Nikon 300 inverted microscope (Nikon, Japan). Two Narishige hydraulic micromanipulators (Narishige, MMW-203, Japan) were used for (1) voltage-clamp recording, and (2) positioning a micropipette with 30 – 50 μm tip diameter for application of test solutions. A five valve system for fast drug application (MS Concentration Clamp; VS-2001, Vibraspec, PA), controlled by a second computer, was connected to the outlets.
Electrodes from hard glass (GC-150-10 glass tubing, Clark Electromedical Instruments, England) were pulled in four steps using a Sutter P-97 puller (Novato, CA). After filling with intracellular solution (ICS) containing (in mM): KCl 141, MgCl2 1, CaCl2 1, EGTA 10, HEPES 10, Na2ATP 3, pH adjusted to 7.2 with NaOH/HCl, they had resistances of 4–6 MΩ; after cell penetration, the access resistance was 10–15 MΩ, compensated by at least 75%. The extracellular solution (ECS) contained (in mM): NaCl 138, CaCl2 2, MgCl2 1.9, and HEPES 10. High frequencies (>1 kHz) were cut off, using an Axopatch-200B amplifier and a CV-203BU headstage, and digitized at 5 kHz through a DigiData 1200A interface (Axon Instruments, USA). The pClamp 7 and 9 (Axon Instrument, USA) software packages were used for data acquisition and analysis.
Statistics
GraphPad Prism was used to present and analyze imaging data. Linear regressions and an unpaired t-test were used to show the difference in the slope of accumulation. Calculation of correlation coefficients (Pearson r) was used to show similarity between accumulation of ASP+ and decynium22. Confidence levels of 0.95 were used in this study.
RESULTS
Due to the expected low fluorescence yield (estimated to be 0.001 (16), we did not anticipate substantial fluorescence of decynium22. Initially, we planned experiments using decynium22 as a non-fluorescent substance to block accumulation of 4-(4-(dimethylamino)-styryl)-N-methylpyridinium (ASP+), a well established substrate for OCT (17) and high-affinity Na+-dependent monoamine transporters (18), inside cultured astrocytes. Surprisingly, we discovered that after being perfused with a solution containing 1μM decynium22, astrocytes accumulated the fluorescence of decynium22 (Fig. 1a). One possible way to explain the strong fluorescence of decynium22 can be aggregation of the dye inside the astrocytes. Whilethe fluorescence of this dye in monomeric form is very weak, it is known to form molecular aggregates (J-aggregates) characterized by very strong superradiant emission with a maximum near 570–580 nm (12; 13; 14). The parameters we used (see Methods) are suitable for the excitation of fluorescence of J-aggregates of decynium22 as 480 nm falls near a short wavelength peak of the excitation spectrum of these aggregates.
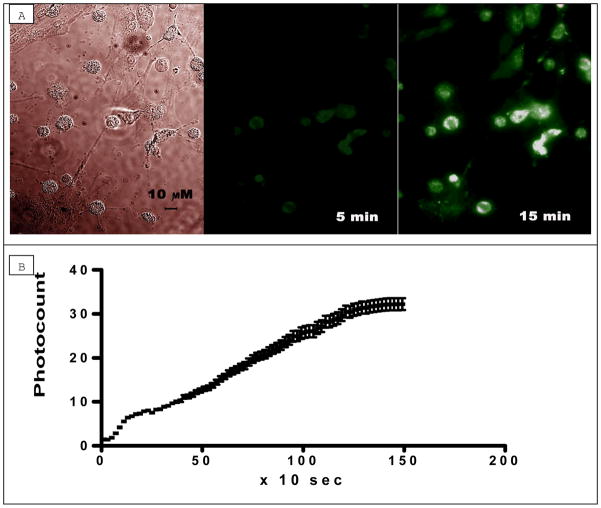
Figure 1. Accumulation of 1,1′-diethyl-2,2′-cyanine iodide (decynium22 or D22) in cultured cortical astrocytes during perfusion with buffer containing 1 μM of D22.
a. From left to right: Differential Interference Contrast (DIC) image of cultured astrocytes (40x Oil immersion); fluorescence after 5 min of perfusion with D22; fluorescence after 15 min of perfusion with D22.
b. Graph showing mean (± S.D.) fluorescence with time after application of Decynium22 (1 μM) to cultured cortical astrocytes (n = 14 astrocytes).
The accumulation curve of decynium22 can be divided into three parts: very fast initial growth, then a linear part and, finally, saturation. In the majority (95%) of cells, accumulation of 1 μM decynium22 was nearly linear during at least 15 minutes (i.e., without saturation) (Fig. 1b). The accumulation timescale was different for different astrocytes, some had no accumulation of decynium22, and some had relatively fast accumulation that leads to the brighter final image of these cells (Fig. 1a, 15 min of accumulation). Very few cells showed fast saturation.
In order to determine the influence of membrane potential on decynium22 accumulation, we examined the effect of increased external K+ on decynium22 accumulation using HEPES buffer with Na+ substituted by K+. Increasing external K+ depolarizes the membrane potential of astrocytes. Application of 1 μM decynium22 in the following buffer (in mM): 145 KCl, 2 KCl, 2.5 CaCl2, 2.5 MgCl2, 10 HEPES(Na) significantly reduced decynium22 uptake (p<0.05), thus abolishing the accumulation (Fig. 2a). The slope in normal buffer was 0.5534 ± 0.02075, and in high K+ buffer it was 0.008723 ± 0.04400, while after wash-out it became 0.5535 ± 0.03214 (n=16). Note that the slope in high K+ was about 1/60th of normal. As the ratio of slopes reflects the speed of accumulation, this means that the speed of decynium22 accumulation in high K+ was about 60 times lower than in normal conditions.
Figure 2. Effect of high [K+]o on accumulation of ASP+ (a fluorescent analog of MPP) and decynium 22 (D22) in cultured astrocytes.
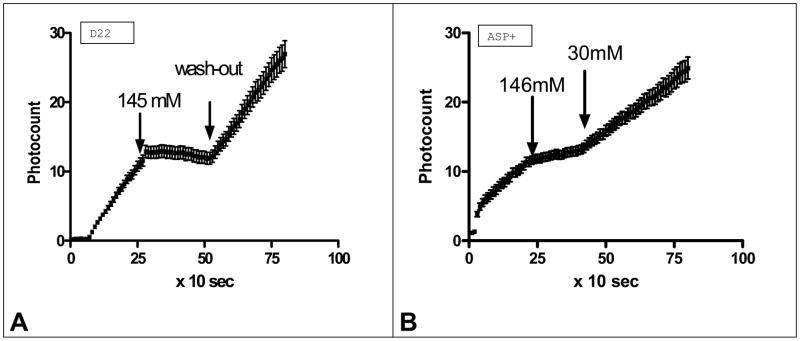
a. Effects of application of buffer containing different K+ concentrations and containing 1 μM of decynium22 on accumulation of D22 fluorescence in astrocytes (n=16).
b. Effects of application of buffer containing different K+ concentrations and containing 2 μM of ASP+ on accumulation of ASP+ fluorescence in astrocytes (n=17).
Note: The speed of D22 as well as ASP+ accumulations were significantly reduced in high K+ concentration and returned to normal after wash-out.
Previously, we found (data not shown) that cultured astrocytes can accumulate the well established fluorescent OCT substrate 4-(4-(dimethylamino)-styryl)-N-methylpyridinium (ASP+; (17)). We found that ASP+ accumulation was also dependent on external K+ concentration (Fig. 2b). Therefore, we calculated the correlation coefficient between the ASP+ and decynium22 accumulation curves in normal external K+ to determine if they have similar shape. The correlation between both processes was found significant with the possibility of no similarity being less then 0.001 (Correlation coefficient (Pearson r) = 0.9540). This similarity suggests an equivalent mechanism of transport of these two substances.
We next determined the pH dependence of decynium22 (Fig. 3a) and ASP+ (Fig. 3b) accumulation inside these cells, by incubating astrocytes with either 1 μM decynium22 or 2 μM ASP+ in buffers of different pH (pH = 6.4, 7.4 and 8.4). If decynium22 were being transported into astrocytes in the same manner as ASP+, we expected that the rate of accumulation would increase in alkaline pH and decrease in acid pH. The slopes of accumulation of decynium22 in pH 7.4, 8.4 and 6.4 were 0.07170 ± 0.004729, 0.1952 ± 0.01916 and −0.03220 ± 0.01409 (n=8), respectively (Fig. 3a). Some loss of fluorescence was seen at pH 6.4 accounting for the negative slope. The slopes were all statistically different from each other. The accumulation speed of decynium22 was 2.72 times greater in pH 8.4 than in pH 7.4.
Figure 3. Effect of pH on accumulation of ASP+ (a fluorescent analog of MPP) and decynium 22 (D22) in cultured astrocytes.
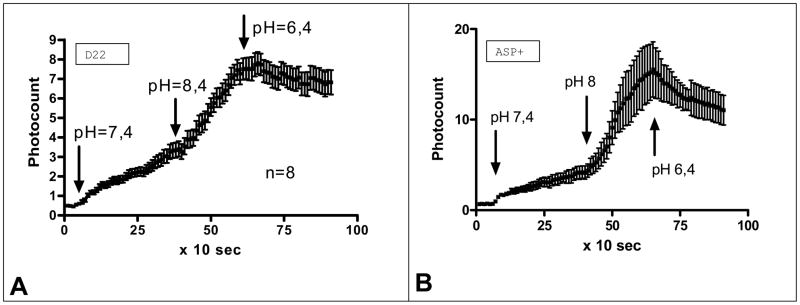
a. Effects of application of buffer with different pH (pH=7.4; pH=8.4 and pH=6.4, subsequently) containing 1 μM of decynium22 on accumulation of D22 fluorescence in astrocytes (n=8).
b. Effects of application of buffer with different pH (pH=7.4; pH=8.4 and pH=6.4, subsequently) containing 2 μM of ASP+ on accumulation of ASP fluorescence in astrocytes (n=4). Note the striking similarity of pH dependence of accumulation of these dyes which suggests a similar pathway of transport.
Similarly, the slopes of accumulation of ASP+ in pH 7.4, 8.4 and 6.4 were 0.08387 ± 0.004401, 0.5318 ± 0.03500 and −0.1473 ± 0.02123 (n=15), respectively (Fig. 3b). A comparison of pH influence on ASP+ and decynium22 accumulation in cultured astrocytes found them to be very similar (Fig. 3a and b). Correlation of both processes was significant, with the possibility of their bearing no similarity being less then 0.001 (Correlation coefficient (Pearson r) = 0.9319). This similarity as well suggests an equivalent mechanism of transportof both decynium22 and ASP+.
In support of this, we found that some astrocytes accumulate ASP+, whereas others do not and an equivalent distribution of decynium22 accumulation was observed in these cells (Fig. 4), i.e. cells without uptake of ASP+ also have no accumulation of decynium22 (Fig. 4 white arrowheads). This is most likely due to a lack of transporters for ASP+ and decynium22 present in their membrane. This finding strengthens the idea that ASP+ and decynium22 are transported into the cell by the same mechanism. For the purposes of this study, we first perfused the cells with 2 μM ASP+ containing buffer, recording its accumulation by astrocyte culture for 5 min (a time where little to no saturation of fluorescence was observed). We then washed out free ASP+ from the preparation for 1 min with the buffer alone, and subtracted the background fluorescence image (this subtraction also subtracted the ASP+ fluorescence accumulated inside the cells). Following that, we added decynium22 (1μM) and recorded its accumulation for 5 min in the standard manner (Fig. 4).
Figure 4. ASP+ and Decynium22 accumulation in astrocytes.
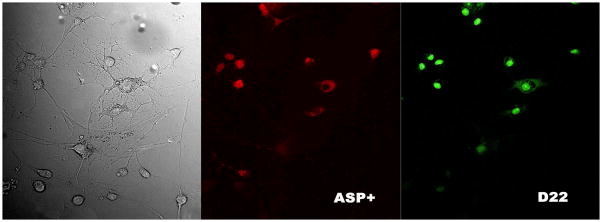
Decynium22 (right panel; green cells) was accumulated by the same cells that had previously accumulated ASP+ (middle panel; red cells). From left to right: 40x oil immersion image of cultured astrocytes in bright field; accumulation of ASP+ fluorescence in the same astrocytes population (middle panel, red), accumulation of D22 fluorescence (right panel, green). Please note, that cells, that have not accumulated ASP+ also have no accumulation of decynuim22 (D22, and vise versa, cells that accumulate one of these dyes, also accumulated the other one). Note: that ASP+ and D22 probably share the same transporter pathway.
In patch-clamp experiments, we directly applied decynium22 (10μM) to the astrocyte membrane in whole-cell voltage-clamp mode with a holding potential of 80 mV. In these conditions, astrocytes generated inward membrane currents of about 20–30 pA amplitude (Fig. 5a). Generation of the current means decynium22 is probably a substrate for the transporter. The current amplitude for the 10μM decynium22 was similar to the current generated by the application of 250 μM norepinephrine (NE) (Fig. 5b) or 250 μM of prazosine (data not shown) known substrates of the OCT transporters (1; 4;6; 7).
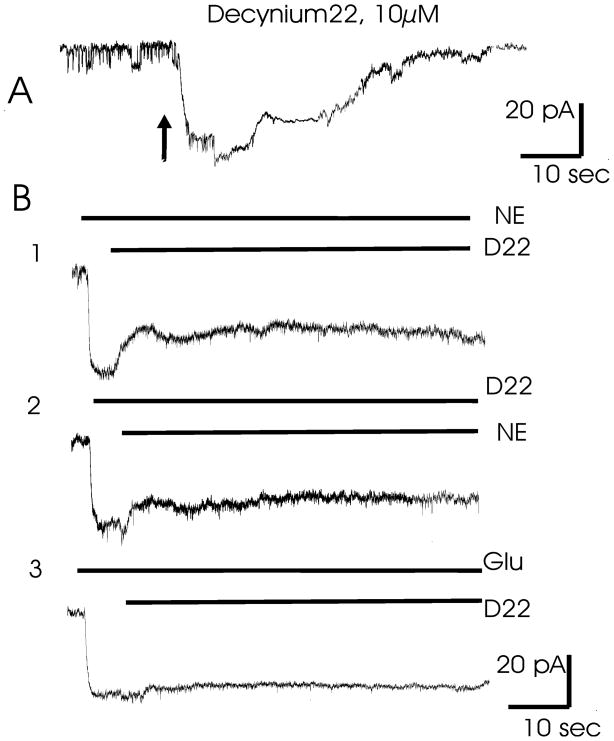
Figure 5. Membrane currents in cultured astrocytes as a response to the application of decynium22 and other substrates.
A. Brief application of 10 μM of D22 to the astrocyte membrane leads to generation of inward current.
B1. Application of 200μM NE elicited inward current that was partially blocked by additional application of 10μM of D22.
B2. Application of 10μM of D22 elicited inward current that was partially depressed by 200μM of NE.
B3. Application of 100 μM of glutamate elicited inward current that was not blocked by 10 μM of D22.
Note: that D22 and NE probably share the same transporter pathway, while D22 and Glu do not.
To demonstrate aninteraction between decyinium22 and norepinephrine, either norepinephrine (250 μM) or decynium22 (10 μM) were applied 3–6 sec prior to co-application of these substances. The resulting recordings demonstrated that these substances interact, producing mutual inhibition. Application of norepinephrine induced inward current (Fig. 5b1) that was partially blocked by decynium22 application. Similarly, application of decynium22 induced inward current that was partially blocked by NE (Fig. 5b2). The fact that current amplitude after co-application is reduced confirmed that these substrates probably share the same permeation pathway. Conversely, co-application of glutamate (250 μM) and decynium22 (Fig. 5b3) do not show any interaction.
DISCUSSSION
Our imaging experiments show that decynium22 is transported inside cultured astrocytes. This process was studied by measuring the time-dependent accumulation of decynium22 fluorescence inside the cells. In its monomeric form decyinium22 is very weakly fluorescent, whereas in aggregation this substance has very strong superradiant emission with a maximum near 570–580 nm (12; 13; 14). Recently, it was found that J-aggregation of cyanine dyes can be templated by DNA (19), and it is possible that some other biological molecules may also promote aggregation. Therefore, it is possible that decynium22 can form J-aggregates inside the cells.
Although the pathway for decynium22 entry into the cell has not been definitively determined, decynium22 competes with monoamines (Fig.5) and is accumulated similar as ASP+ in the same target cells (Fig.4). In addition, pharmacological characteristics of ASP+ and D22 uptakes are identical (Figs. 2,3). Therefore, previously noted that D22 is a potent blocker of OCT-type transporters could be wrong. Our experiments simply explain that these transporters a likely pathway for both decynium22 as well as for ASP+ and probably other bioamines. Decynium22 has a very high affinity to OCT transporters in a variety of species (20; 1; 21; 22; 6) and OCT transporters have been clearly detected in astrocytes. By partial sequencing, it was found that a splice variant for OCT1 identical to the monoamine Uptake 2 system, with only partial sequence identity to hOCT is present in glioma cells (5). Recently, it was also established that Uptake 2 in cortical astrocytes is mediated by the OCT3 transporter (9). The same group of authors has demonstrated by RT-PCR that astrocytes express OCT1, OCT2 and OCT3 mRNA (3).
Our data support the idea that decynium22 entry into astrocytes may be through OCT type transporters. Our patch clamp experiments have shown that decynium22 transport is electrogenic, as external application of decynium22 elicited the inward ion current in clamped astrocytes (Fig.5). The current elicited by decynium22 was antagonized by norepinephrine (NE), a known substrate for the OCT transporter and vice versa with decynium22 antagonizing norepinephrine-induced current (Fig.5B1, B2). A reciprocal relationship between norepinephrine and decynium22 can be explained as competition for the transporter where decynium22 and norepinephrine share the same transporter pathway. Indeed, D22 is specific and is not competing with glutamate transport (Fig. 5B3) where glutamate elicited current was not antagonized by decynium22 suggesting that decynium22 does not go through the well characterized glutamate transporter of astrocytes.
OCT transporters have been suggested to be driven by the external/internal K+ gradient that determines the membrane potential of cells (5;23; 9;6). Our results show that decynium22 uptake in cultured astrocytes is reduced in elevated external K+, suggesting that uptake may be through an OCT type transporter (Fig. 2). Furthermore, we found that the rate of decynium22 uptake by astrocytes was pH sensitive(Fig. 3)and increased in alkaline solutions (pH=8.4), while inhibited in acidic pH (6.4). These data are also consistent with the described OCT transporter characteristics, as these transporters show significant cis inhibition of uptake when the external pH is reduced (H+ increased) (21). It is known that decynium22 aggregates better in alkaline than in acidic solutions, and in strongly acidic solutions the dye does not fluoresce at all (13; 14). However, in the pH range of the present study(pH 6.4 – 8.4) there is no appreciable difference in aggregation and fluorescence, therefore, fluorescence accumulation changes in the present study can be attributed to changes in uptake velocity. Furthermore, these pH experiments can differentiate between decynium22 uptake through plasma membrane monoamine transporters (PMAT) recently cloned from human brain (24) that have similar characteristics as OCT transporters and OCT transporters themselves. PMAT transporters are greatly stimulated by acid pH (25), whereas OCT transporters are inhibited (21) as also shows Figure3. Recently and finally, it was confirmed that PMAT transporters are not present in astrocytes (26).
In summary, in support of decynium22 entering the cell through an OCT transporter, we have demonstrated significant similarity of decynium22 uptake and 4-(4-(dimethylamino)-styryl)-N-methylpyridinium (ASP+) uptake in astrocytes (Figs. 1–4). ASP+ is a known substrate for OCT-type transporters (17) because of the chemical similarity of this fluorescent dye to 1-Methyl-4-phenylpyridinium (MPP) (18). There was a high correlation (about 0.95) between the accumulation of decynium22 and ASP+ in astrocyte, as well as very similar effects of pH and K+ ion gradient on ASP+ and decynium22 uptake. Furthermore, we have shown that only astrocytes that had previously accumulated ASP+ then accumulated decynium22. This suggests that the same transporters are involved in dye accumulation.
Decynium22 is transported into astrocytes in a pH and K+ sensitive manner where it displays a strong fluorescence probably due to aggregation within the cell. The regulation of uptake speed, and similarity with ASP+ uptake suggest that OCT transporters may mediate decynium22 accumulation. Decynium22 fluorescence can be a useful instrument to study monoamine transporters in the brain.
Acknowledgments
The authors wish to thank Ms. Natalia Skachkova and Paola Lopez Pieraldi for their fine technical assistance. This work was supported by NIH-NINDS and NCRR SNRP-NS39408, NIH-MBRS-SO6-GM50695, NIH-RCMI-G12RR03035, NIH-SNRP-NS39405 and NIH-MBRS-S06-GM08224.
References
- 1.Gründemann D, Gorboulev V, Gambaryan S, Veyhl M, Koepsell H. Drug excretion mediated by a new prototype of polyspecific transporter. Nature. 1994;372(6506):549–52. doi: 10.1038/372549a0. [DOI] [PubMed] [Google Scholar]
- 2.Wu X, Kekuda R, Huang W, Fei Y-J, Leibach FH, Chen J, Conway SJ, Ganapathy V. Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake2) and evidence for the expression of the transporter in the brain. J Biol Chem. 1998;273:32776–32786. doi: 10.1074/jbc.273.49.32776. [DOI] [PubMed] [Google Scholar]
- 3.Inazu M, Takeda H, Matsumiya T. Molecular and functional characterization of an Na+-independent choline transporter in rat astrocytes. J Neurochem. 2005;94:1427–1437. doi: 10.1111/j.1471-4159.2005.03299.x. [DOI] [PubMed] [Google Scholar]
- 4.Gründemann D, Liebich G, Kiefer N, Koster S, Schömig E. Selective substrates for non-neuronal monoamine transporters. Mol Pharmacol. 1999;56:1–10. doi: 10.1124/mol.56.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Busch AE, Karbach U, Miska D, Gorboulev V, Akhoundova A, Volk C, Arndt P, Ulzheimer JC, Sonders MS, Baumann C, Waldegger S, Lang F, Koepsell H. Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Mol Pharmac. 1998;54:342–352. doi: 10.1124/mol.54.2.342. [DOI] [PubMed] [Google Scholar]
- 6.Hayer-Zillgen M, Bruss M, Bonisch H. Expression and pharmacological profile of the human organic cation transporters hOCT1, hOCT2 and hOCT3. Br J Pharmacol. 2002;136:829–836. doi: 10.1038/sj.bjp.0704785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gründemann D, Koschker A-C, Haag C, Honold C, Zimmermann T, Schömig E. Activation of the extraneuronal monoamine transporter (EMT) from rat expressed in 293 cells. Br J Pharmacol. 2002;137:910–918. doi: 10.1038/sj.bjp.0704926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schömig E, Babin-Ebell J, Russ H. 1,1′-Diethyl-2,2′-cyanine (decynium22) potently inhibits the renal transport of organic cations. Arch Pharm. 1993;347:379–383. doi: 10.1007/BF00165387. [DOI] [PubMed] [Google Scholar]
- 9.Inazu M, Takeda H, Matsumiya T. Expression and functional characterization of the extraneuronal monoamine transporter in normal human astrocytes. J Neurochem. 2003;84:43–52. doi: 10.1046/j.1471-4159.2003.01566.x. [DOI] [PubMed] [Google Scholar]
- 10.Budiman T, Bamberg E, Koepsell H, Nagel G. Mechanism of electrogenic cation transport by the cloned organic cation transporter 2 from rat. J Biol Chem. 2000;275:29413–29420. doi: 10.1074/jbc.M004645200. [DOI] [PubMed] [Google Scholar]
- 11.Arndt P, Volk C, Gorboulev V, Budiman T, Popp C, Ulzheimer-Teuber I, Akhoundova A, Koppatz S, Bamberg E, Nagel G, Koepsell H. Interaction of cations, anions, and weak base quinine with quinine with rat renal cation transporter rOCT2 compared with rOCT1. Am J Physiol Renal Physiol. 2001;281:454–468. doi: 10.1152/ajprenal.2001.281.3.F454. [DOI] [PubMed] [Google Scholar]
- 12.Jelley EE. Spectral absorption and fluorescence of dyes in the molecular state. Nature. 1936;138:1009–1010. [Google Scholar]
- 13.Stiel H, Daehne S, Teuchner K. J-aggregates of pseudoisocyanine in solution: New data from nonlinear spectroscopy. J Lumines. 1988;39:351–357. [Google Scholar]
- 14.Struganova IA. Dynamics of formation of 1,1′-diethyl-2,2′-cyanine iodide J-aggregates in solution. J Physical Chem A. 2000;104:9670–9674. [Google Scholar]
- 15.Inyushin MY, Kucheryavykh YV, Kucheryavykh LY, Struganova IA, Eaton MJ, Skatchkov SN, Jimenez-Rivera CA, Sanabria P. Program No. 489.19. 2006 Neuroscience Meeting Planner. Atlanta, GA: Society for Neuroscience; 2006. Membrane potential and pH-dependent accumulation of decynium22 (1,1′-diethyl-2,2′-cyanine iodide) fluorescence in astrocytes. Online. [Google Scholar]
- 16.Tredwell CJ, Keary CM. Picosecond time resolved fluorescence lifetimes of the polymethine and related dyes. Chem Phys. 1979;43:307–316. [Google Scholar]
- 17.Çetinkaya I, Ciarimboli G, Yalçinkaya G, Mehrens T, Velic A, Hirsch JR, Gorboulev V, Koepsell H, Schlatter E. Regulation of human organic cation transporter hOCT2 by PKA, PI3K, and calmodulin-dependent kinases. Am J Physiol Renal Physiol. 2003;284:F293–F302. doi: 10.1152/ajprenal.00251.2002. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz JW, Blakely RD, DeFelice LJ. Binding and transport in norepinephrine transporters: real-time, spatially resolved analysis in single cells using a fluorescent substrate. J Biol Chem. 2003;278:9768–9777. doi: 10.1074/jbc.M209824200. [DOI] [PubMed] [Google Scholar]
- 19.Hannah KC, Armitage BA. DNA-templated assembly of helical cyanine dye aggregates: A supramolecular chain polymerization. Acc Chem Res. 2004;37:845–853. doi: 10.1021/ar030257c. [DOI] [PubMed] [Google Scholar]
- 20.Russ H, Sonna J, Keppler K, Baunach S, Schömig E. Cyanine-related compounds: a novel class of potent inhibitors of extraneuronal noradrenaline transport. Naunyn Schmiedebergs Arch Pharmacol. 1993;348:458–465. doi: 10.1007/BF00173203. [DOI] [PubMed] [Google Scholar]
- 21.Gründemann D, Babin-Ebell J, Martel F, Ording N, Schmidt A, Schömig E. Primary structure and functional expression of the apical organic cation transporter from kidney epithelial LLC-PK1 cells. J Biol Chem. 1997;272:10408–10413. doi: 10.1074/jbc.272.16.10408. [DOI] [PubMed] [Google Scholar]
- 22.Koepsell H, Gorboulev V, Arndt P. Molecular pharmacology of organic cation transporters in kidney. J Membr Biol. 1999;167:103–117. doi: 10.1007/s002329900475. [DOI] [PubMed] [Google Scholar]
- 23.Urakami Y, Okuda M, Masuda S, Saito H, Inui KI. Functional characteristics and membrane localization of rat multispecific organic cation transporters, OCT1 and OCT2, mediating tubular secretion of cationic drugs. J Pharmacol Exp Ther. 1998;287(2):800–805. [PubMed] [Google Scholar]
- 24.Engel K, Zhou M, Wang J. Identification and characterization of a novel monoamine transporter in the human brain. J Biol Chem. 2004;279:50042–50049. doi: 10.1074/jbc.M407913200. [DOI] [PubMed] [Google Scholar]
- 25.Xia L, Engel K, Zhou M, Wang J. Membrane localization and pH-dependent transport of a newly cloned organic cation transporter (PMAT) in kidney cells. Am J Physiol Renal Physiol. 2007;292:F362–F690. doi: 10.1152/ajprenal.00302.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dahlin A, Xia L, Kong W, Hevner R, Wang J. Expression and immunolocalization of the plasma membrane monoamine transporter in the brain. Neuroscience. 2007;146(3):1193–211. doi: 10.1016/j.neuroscience.2007.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]