Abstract
Nikolaus Friedreich (1825-1882) presented clinical findings in 6 patients with a severe hereditary disorder of the nervous system and secured full autopsies in 4 of them. He was fascinated by the spinal cord lesions in the siblings of two unrelated families, and in the first 3 of his 5 long articles stressed the destruction of the dorsal columns. He recognized the relatively minor symmetrical lesions of the anterolateral fasciculi but did not separate dorsal spinocerebellar tracts (Flechsig’s bundles) and corticospinal tracts. While he studied the dorsal spinal roots in great detail and established their principal abnormality, namely, axonal thinning without axonal loss, he reported dorsal root ganglia as entirely normal. He made an insightful description of atrophic neurons in the gracile nuclei (clavae) but overlooked the invariable atrophy of the dentate nuclei. He followed the families over a period of 14 years, but acknowledged the hereditary nature of the disease only very late. He proposed a developmental defect for the medulla oblongata, retaining his interpretation that the spinal lesion was inflammatory. This review honors Friedreich for his insight into a “new” disease in the late 19th century and updates his neuropathological findings. It is remarkable that Friedreich also described the abnormal hearts in the disease that now bears his name since hypertrophic cardiomyopathy is now recognized as the main cause of death in Friedreich’s ataxia.
Keywords: Friedreich’s ataxia, dorsal columns, dorsal roots, hereditary ataxia, spinal cord
Historical introduction
Nikolaus Friedreich (1825-1882) wrote 5 long articles on a seemingly new spinal disease (Friedreich 1863a; 1863b; 1863c; 1876; 1877), but it is the relatively short postscriptum to the fourth paper (Friedreich, 1877) that shows his insight into what is now known as “Friedreich’s ataxia”. Friedreich did not set out to discuss the hereditary nature of this disease but lamented that despite the “great abundance” of case material in Heidelberg little attention had been paid to disorders of the spinal cord (Friedreich 1863a). He also stated that physicians experienced great hindrances in the removal of the spinal cord, and that the microscope was indispensible in the proper diagnosis of spinal cord disease. He expressed his intention to separate several diseases of the spinal cord from tabes dorsalis (Friedreich 1863a). Friedreich’s clinical descriptions were elaborate and in very complex German sentence structure. He was aware of kyphoscoliosis and heart disease though he believed that the 4 patients in whom he could secure autopsies had succumbed to “abdominal typhus”, presumably a severe terminal febrile illness that in retrospect was unrelated to typhoid fever. In his third publication, Friedreich (1863c) sought to accomplish his original goal of improved characterization of diseases of the spinal cord by comparing his clinical and neuropathological observations with those by other authors. It was not until 1876 or 13 years after his first 3 papers that the term “hereditary” appears in the title of the article (Friedreich 1876). His first and second patients (family 1) were brother and sister; and patients 3-6 (family 2) also were siblings (3 sisters, one brother). Their clinical histories included some information on the parents and unaffected siblings (Friedreich, 1863b). The parents did not have comparable illnesses though Friedreich saw fit to report alcoholism of the father in the second family and that coitus leading to conception of the four affected sibs had occurred during the father’s drunkenness. Discounting this incursion into the personal lives of parents and children, autosomal recessive transmission is evident. Friedreich (1876) wrote about having a total of 9 patients with comparable illnesses, but the hereditary nature seems to be true only for the original six.
Friedreich received little recognition during his life time for these insightful descriptions of “Friedreich’s ataxia”. Rudolf Virchow did not mention Friedreich’s work on spinal cord degeneration in his 1882 obituary (Virchow, 1882) though an anonymous obituary in the same year (Anonymous, 1882) referred to these publications as “perhaps his most important work”. This anonymous obituary also contains a description of Friedreich’s rapid academic progress and 59 publications. Friedreich came from a family of physicians. Father and grandfather were professors of medicine. It is of interest to neurologists, neurosurgeons, and neuropathologists that Friedreich qualified as a university lecturer at the age of 28 by his thesis translated as “Contribution to the science of tumors of the cranial cavity”. Several historical accounts of Friedreich’s life are available. A relatively recent review of Friedreich’s contributions by Andermann (1976) includes a photograph and a reproduction of the 1877 illustrations of the affected spinal cord (Friedreich, 1877).
The French school of neurologists is responsible for recognizing the importance of Friedreich’s work, and the landmark article by Pierre Marie (1893) separated this hereditary disease from other inherited cerebellar ataxias, based on clinical and pathological observations. The diagnosis of “Friedreich’s ataxia” has endured. In contrast, the dominant ataxias underwent an extended evolution from numerous eponyms and the descriptive “olivopontocerebellar atrophy” to the current terminology of “spinocerebellar ataxia”.
This review looks back at Friedreich’s accounts of the neuropathology of the disease and his impressions on the etiology. It seeks to match by modern neuroanatomical techniques what he observed. Friedreich was a clinician and prescribed several remedies that were popular at the time (silver nitrate; liver oil; nux vomica [containing strychnine]; and iron iodate). He concluded wistfully that none of them worked. To this day, no effective treatment of Friedreich’s ataxia has emerged.
Friedreich’s description of the neuropathology
Friedreich was fascinated by the lesions of the spinal cord and devoted large portions of his neuropathological description to the gross and microscopic appearance of dorsal and anterolateral columns (Friedreich, 1863a, b; Friedreich 1876; 1877). Figure 1 recalls his observations (Friedreich, 1877): reduced diameter of the spinal cord at all levels (fig. 1a); thinning of all dorsal roots (fig. 1a); and gray discoloration and gelatinous appearance of dorsal and anterolateral columns on cross sections (fig. 1b). He used carmine, hematoxylin, iodine violet, and gold chloride to stain microscopic sections (fig. 1c). Figure 1c is a copy of Friedreich’s figure 1d in Table IV of volume 70 (1877) of Virchow’s Archiv für pathologische Anatomie und Physiologie und für klinische Medizin, displaying cross sections of the spinal cord at all levels. At the thoracic level, the entire dorsal column showed degeneration. At the cervical level, the lesion was more prominent in the gracile fasciculus of Goll. Friedreich also illustrated the wedge-shaped degeneration of the anterolateral columns (fig. 1c; [Friedreich, 1877]) that is recalled by an immunohistochemical stain of myelin basic protein in fig. 1d. The degeneration of the anterolateral columns was absent in the lower lumbar and sacral spinal cord (figs. 1f and g in Friedreich [1877]). Friedreich’s illustration of the thoracic spinal cord did not show the typical bulges of Clarke’s column that are normally visible (fig. 1d). It is not certain that this hand-drawn illustration intended to show atrophy of the dorsal nuclei. Friedreich was fully aware, however, of the existence of Clarke’s column (Clarke, 1851) and reported neuronal loss in all four cases as part of his microscopic descriptions (recalled in fig. 2). Friedreich’s articles make little mention of the clinicoanatomic correlation of the degenerating dorsal and anterolateral columns because knowledge of fiber tracts and their parent neurons in spinal gray matter was still evolving. Paul Flechsig whose name became closely associated with the dorsal spinocerebellar tract (“Flechsig’s fasciculus” or “bundle”) was 22 years junior to Friedreich, and his book on the long tracts of the human brain and spinal cord was not published until 1876 (Flechsig, 1876). In his last article, the postscriptum, Friedreich (1877) cited Flechsig (1876) but only because he used gold chloride to stain nerve fibers. He provided no legends or magnification bars to his illustrations (Friedreich, 1877), but the text suggests that the drawings were made from gold chloride-stained sections (fig. 1c).
Figure 1. The spinal cord in Friedreich’s ataxia.
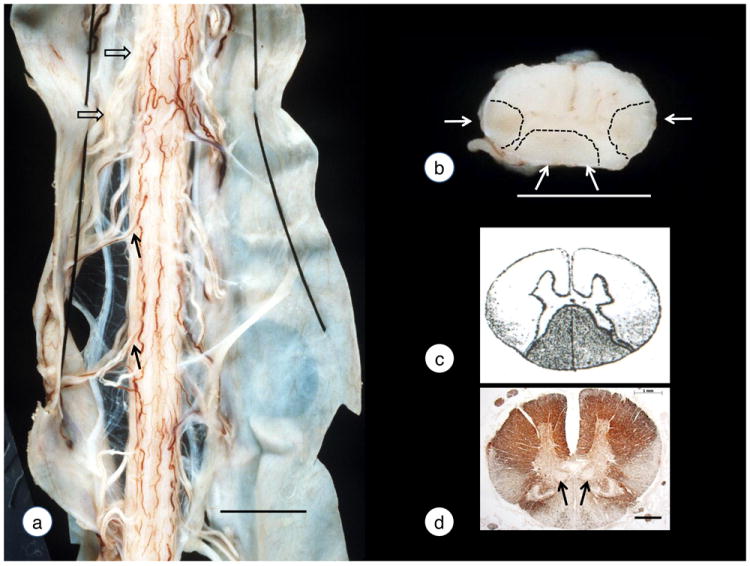
(a)-(b) gross specimens; (c) reproduction of Friedreich’s drawing of the thoracic spinal cord. (d) immunohistochemistry of myelin basic protein. The photograph of the dorsal aspect of the spinal cord (a) shows reduction of the transverse diameter to 6-7 mm (normal: over 10 mm) and thinning of dorsal roots (solid arrows). Anterior roots (open arrows) are normal. A transverse slice of the thoracic spinal cord (b) reveals an overall reduction in size and gray discoloration of the dorsal and anterolateral columns (white arrows and outlined by interrupted lines). Stippling in Friedreich’s 1877 drawing of the thoracic spinal (c) indicates degeneration in the dorsal and posterior anterolateral columns (probably gold chloride stain). A modern immunohistochemical stain of myelin basic protein confirms fiber depletion in the same locations (d). Friedreich (1877) stressed the wedge-like loss of fibers in the anterolateral columns but did not realize that the imperceptible transition from the base to the tip affected two independent long tracts of the spinal cord, namely, the dorsal spinocerebellar and corticospinal tracts. The arrows in (d) indicate the dorsal nuclei in Clarke’s column (see also fig. 2). Friedreich’s drawing (c) did not illustrate the dorsal nuclei though they should have been visible at this magnification (see [d]). Note that Friedreich (1877) rendered his drawings of the spinal cord with the anterior fissure facing upward. For historical accuracy, this style is retained here. This practice was not universal, and Clarke (1851) drew spinal cord sections in the currently familiar way. The “upside-down” display of the spinal cord in Friedreich’s ataxia continued into the early 20th century (Lambrior, 1911), and the “switch” may have begun with Mott’s (1907) paper. Bars: (a) 10 mm; (b) 5 mm; (d) 1 mm.
Figure 2. Dorsal nucleus of Clarke’s column in Friedreich’s ataxia.
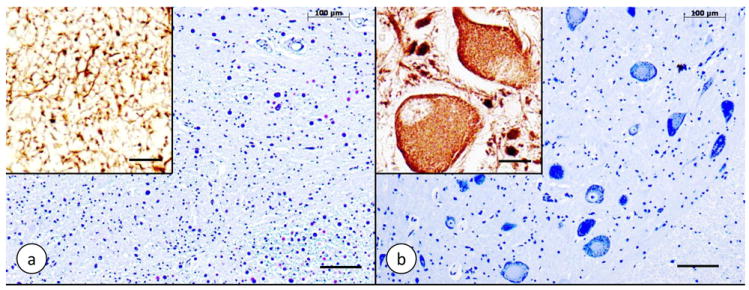
(a) Friedreich’s ataxia; (b) normal control. (a) and (b) Cresyl Violet; insets: immunohistochemistry of class-III-β-tubulin. The normal dorsal nucleus displays large round nerve cells with peripherally located chromatin (b). The nerve cells have a few coarse dendrites (inset in [b]). In Friedreich’s ataxia (a), large neurons are absent. Class-III-β-tubulin reaction product is present in only a few neuritic processes that may represent the remaining afferent fibers (a, inset). Bars: (a) and (b), 100 μm; insets, 20 μm.
Friedreich devoted a single sentence to spinal ganglia and declared neurons and “connective tissue” entirely normal (Friedreich, 1877). This statement is surprising because the origin of dorsal root axons from spinal ganglia was known. Friedreich presented a detailed report of dorsal and ventral roots (Friedreich, 1863b), including their intramedullary portions (Friedreich, 1877). It is of great interest that he detected no axonal interruptions, only thinning, which is very much the current concept of the dorsal root lesion (Koeppen et al, 2009). He commented on the absence of “fatty degeneration”, presumably implying lack of degenerating myelin. Figure 3 recalls the typical lesion of dorsal root ganglia in Friedreich’s ataxia, the diminutive axons in dorsal roots, and the intact axons of ventral roots. It is peculiar that the degree of myelination in dorsal roots is actually higher than normal (Koeppen et al, 2009).
Figure 3. Dorsal root ganglia and spinal roots in Friedreich’s ataxia.
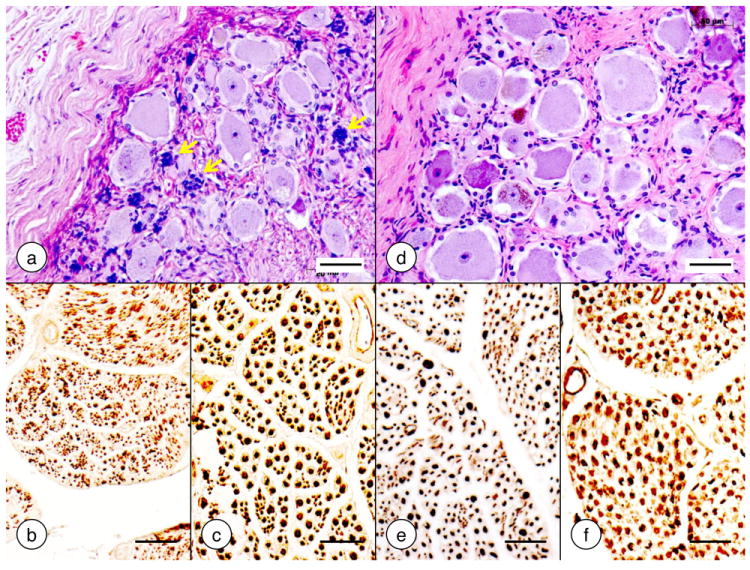
(a)-(c) Friedreich’s ataxia; (d)-(f) normal control. (b) and (e) dorsal roots; (c) and (f) ventral roots. (a) and (d) hematoxylin and eosin stain; (b)-(c) and (e)-(f) immunohistochemistry of phosphorylated neurofilament protein in axons. In Friedreich’s ataxia, the dorsal root ganglion shows smaller than normal neuronal sizes (a). The hypercellularity is due to proliferating satellite cells. These cells also form residual nodules (yellow arrows), indicating the sites of destroyed neurons. Several nerve cells are surrounded by more than one layer of satellite cells (a). In the normal control, satellite cells form a single delicate layer around neurons (d). In Friedreich’s ataxia, the dorsal root shows innumerable thin axons while thick axons are absent (b). Figure 3e represents a normal dorsal root for comparison. The ventral root in Friedreich’s ataxia is normal (c). The scattered islands of thin axons may or may not be significant. Figure 3f displays a normal ventral root for comparison. Bars, 50 μm.
Friedreich made several statements about peripheral nerves. In 1863 (Friedreich, 1863), he wrote that the sciatic nerve displayed “simple atrophy of all axons” but concluded in 1877 that peripheral nerves and muscles of upper and lower extremities were normal (Friedreich, 1877). He made no mention of purely sensory nerves, and it is unlikely that he examined the sural nerve. Figure 4 recalls the severe sensory neuropathy that is now a critical part of the neuropathology in Friedreich’s ataxia (Morral et al, 2010). The main lesion is the paucity of myelinated fibers (figs. 4a and b). Large myelinated axons are entirely absent (fig. 4b). Myelin debris is generally absent though slow progression of the neuropathy is evident by comparison of myelinated fiber density in sural nerve biopsies of very young patients with Friedreich’s ataxia (Ouvrier et al, 1982) and autopsy samples (Morral et al, 2010). The best available interpretation of the pathogenesis of sensory neuropathy in Friedreich’s ataxia is a combination of inefficient myelination and a superimposed slowly progressive axonopathy (Morral et al, 2010). While sensory nerves and dorsal roots in Friedreich’s ataxia share the lack of large myelinated fibers, there is a difference that still awaits an explanation: the degree of myelination in dorsal roots is actually higher than normal (Koeppen et al, 2009).
Figure 4. The sural nerve in Friedreich’s ataxia.
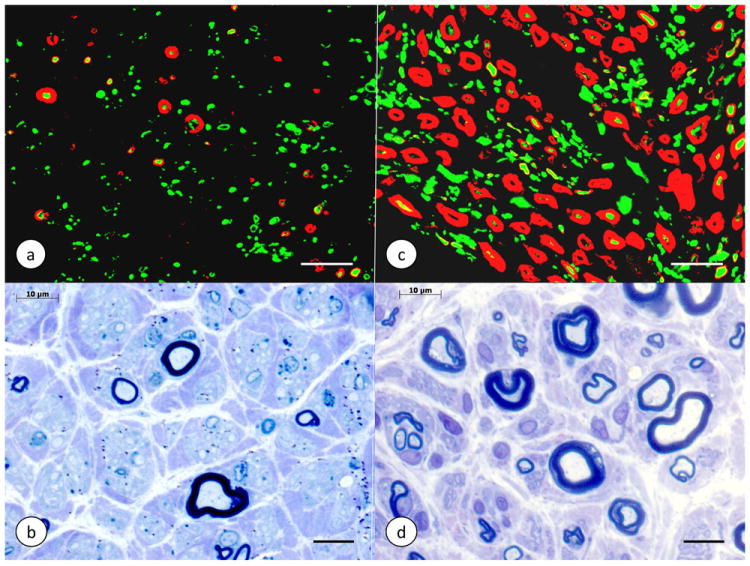
(a)-(b) Friedreich’s ataxia; (c)-(d) normal control. (a) and (c) double-label immunofluorescence of P0 protein in myelin (Cy3, red) and phosphorylated neurofilament protein in axons (Alexa 488, green); (b) and (d) Toluidine Blue-stained semithin section of plastic-embedded nerve. The sural nerve in Friedreich’s ataxia (a, b) shows a great paucity of myelin sheaths. The few persistent myelinated fibers are thin. In (b), only one relatively large myelinated axon is visible. A visual comparison of axons in Friedreich’s ataxia (a) and the normal control (c) also confirms the overall reduction of axonal diameters (Morral et al., 2010). Specimen in (b) (autopsy) by courtesy of Dr. Benjamin B. Gelman, Galveston, TX, USA; specimen in (d) (biopsy) by courtesy of Dr. Jiang Qian, Albany, N.Y., USA. Bars: (a) and (c), 20 μm; (b) and (d), 10 μm.
The description of an attenuated medulla oblongata must be recognized as a most insightful contribution by Friedreich (Friedreich, 1877). We may dismiss his report of an atrophic hypoglossal nucleus. Recognizing that atrophy of the clavae (gracile nuclei) was due to extended dorsal column degeneration anticipated the concept of transsynaptic degeneration by many years. Friedreich also described degenerating fibers in the corpora restiformia (inferior cerebellar peduncles) though he did not conclude that fiber depletion was related to the wedge-like degeneration in the anterolateral spinal cord (Friedreich, 1877). Figure 5 recalls Friedreich’s description of small nerve cells in the gracile nucleus. It is of interest that he observed that all axons in the brain stem seemed abnormally thin.
Figure 5. The gracile nucleus in Friedreich’s ataxia.
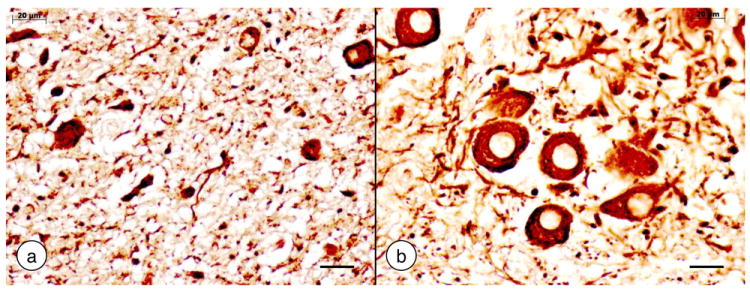
(a) Friedreich’s ataxia; (b) normal control. Immunohistochemistry of non-phosphorylated neurofilament protein. Only a few small neurons remain in the gracile nucleus of Friedreich’s ataxia (a). The normal gracile nucleus (b) displays large round and oval nerve cells. Bars: 20 μm.
Friedreich reported no abnormalities of the forebrain though he emphasized thin slicing and detailed examination (Friedreich, 1877). It would appear that he did not systematically study the cerebral cortex under the microscope. The existence of large pyramidal cells now known as Betz cells was known since 1874 (Betz, 1874a, b). Mott (1907) made camera lucida drawings of the entire motor cortex, and atrophy of these large upper motor neurons is now considered invariable (Oppenheimer, 1979). It is surprising that Friedreich missed the grossly visible collapse of the dentate nucleus. Mott (1907) may have been the first to draw attention to the lesion of the dentate nucleus in Friedreich’s ataxia, which is almost invariably present (review in Koeppen et al [2011]). Figure 6 illustrates atrophy of the dentate nucleus in Friedreich’s ataxia as it is understood at this time (Koeppen et al, 2011).
Figure 6. The dentate nucleus in Friedreich’s ataxia.
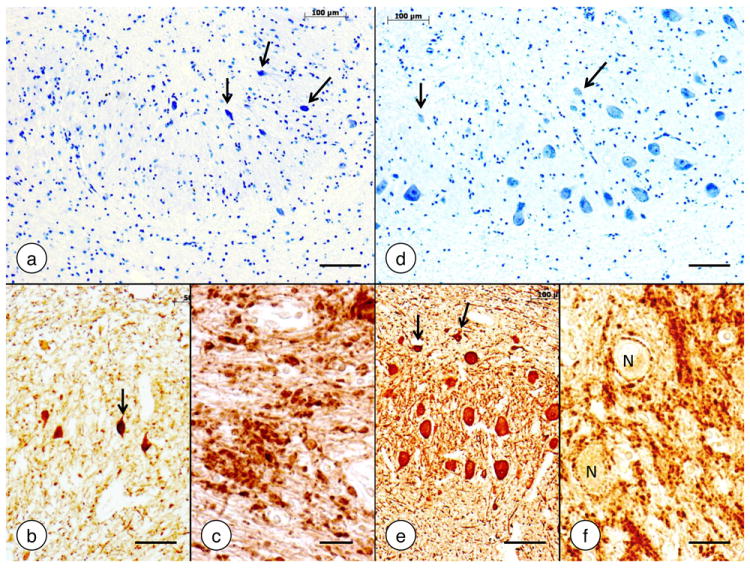
(a)-(c) Friedreich’s ataxia; (d)-(f) normal control. (a) and (d) Cresyl Violet; (b) and (e) immunohistochemistry of neuron-specific enolase; (c) and (f) immunohistochemistry of synaptophysin. In Friedreich’s ataxia, the large neurons of the dentate nucleus have disappeared (a). The small nerve cells (a, arrows) do not represent atrophic large neurons. Small neurons are also present in the normal dentate nucleus (d, arrows). They display strong reaction product with anti-neuron specific enolase (b and e, arrows). The synaptophysin stain in Friedreich’s ataxia (c) shows coarse clusters of modified corticonuclear terminals termed grumose degeneration. In the normal dentate nucleus (f), synaptophysin reaction product labels much smaller axosomatic and axodendritic terminals and generates negative images of normal neurons (N). Bars: (a-b), (d-e), 100 μm; (c) and (f), 20 μm.
Friedreich’s thoughts on etiology and pathogenesis
In his second article, Friedreich (1863b) discussed the etiology of the disorder and mentioned hereditary “predisposition”. Fourteen years later (Friedreich, 1877), he connected the obvious heredity with “inhibited development” of the medulla oblongata. It is peculiar that despite this extraordinary insight, he persisted in his original claim that the disease process in the spinal cord was a chronic spinal leptomeningitis (Friedreich, 1877). He thought that the inflammation began on the surface overlying the dorsal columns and progressed deep into the spinal white matter and then across the gray matter of the dorsal horns to the anterolateral columns. He may have been influenced by the prevalence of tabes dorsalis at the time, and the gait disorder in Friedreich’s ataxia does resemble tabetic gait. Tabes dorsalis and Friedreich’s ataxia share severe proprioceptive sensory loss in the legs. Friedreich was impressed by the abundance of corpora amylacea in the atrophic areas of the spinal cord and connected atrophy of Clarke’s column in his third case with co-existing spinal cord cavitation. The clear space in the spinal cord must now be interpreted as an irrelevant hydromyelia. Corpora amylacea occur in many atrophic diseases of the brain and are also of no importance to Friedreich’s ataxia.
A word of praise for Nikolaus Friedreich
Friedreich’s career was cut short by his death at 52 from an aneurysm of the thoracic aorta. In his obituary, Virchow (1882) wrote that Friedreich had made this diagnosis on himself, causing him much anxiety. It instilled in him an urgency to accomplish his work. What is Friedreich’s critical contribution? At first, his descriptions were case reports, but his tenacity in pursuit of an answer over 14 years led to the recognition of a new disease. He had to work with very limited technology and, in retrospect, immature neuroanatomical knowledge. Ignoring some of his idiosyncrasies and oversights, Friedreich must receive praise for using his skills in clinical medicine, anatomy, and pathology to characterize a disease that had not been appreciated before his time. Friedreich must also be credited for his gross and microscopic descriptions of the heart. The gross description is quite elegant and remains accurate to this day. In translation, he stated that “the musculature of the left ventricle…rather thicker than normal though somewhat flabby and withered; on a slice, it shows itself as significantly anemic and peculiarly patchy, marbled, in appearance" (Friedreich 1863a, p. 406). He also stated, incorrectly, that “the microscope shows a colossal fatty degeneration of the musculature of the left ventricle, so that none of the cross-striations of the elements can be detected anywhere” (Friedreich 1863a, p. 396). It is likely that he interpreted extensive myocardial fibrosis as degenerating cardiomyocytes. Most patients with Friedreich’s ataxia die from hypertrophic cardiomyopathy but the recognition that heart disease is an integral part of the pathological phenotype did not come until 1946 (Russell, 1946).
Acknowledgments
The author receives financial support from National Institutes of Health (grant No. R01 NS069454); Friedreich’s Ataxia Research Alliance; National Ataxia Foundation; and Neurochemical Research, Inc. The neuropathological laboratory is maintained by the Veterans Affairs Medical Center in Albany, N.Y. 12208 USA.
Footnotes
The author has no conflict of interest to declare.
References
- Andermann F. Nicolaus Friedreich and degenerative atrophy of the posterior columns of the spinal cord. Can J Neurol Sci. 1976;3:275–277. doi: 10.1017/s0317167100025452. [DOI] [PubMed] [Google Scholar]
- Anomymous. Nicolaus Friedreich. Zeitschr Klin Med. 1882;5:281–284. [Google Scholar]
- Betz Anatomischer Nachweis zweier Gehirncentra (Anatomical demonstration of two brain centers) Centralbl Medizin Wissensch. 1874a;12:578–580. [Google Scholar]
- Betz Anatomischer Nachweis zweier Gehirncentra (Anatomical demonstration of two brain centers) Centralbl Medizin Wissensch. 1874b;12:595–599. [Google Scholar]
- Clarke JL. Researches into the structure of the spinal chord. Phil. Trans Roy Soc Lond. 1851;141:607–621. [Google Scholar]
- Flechsig P. Die Leitungsbahnen im Gehirn und Rückenmark des Menschens auf Grund entwicklungsgeschichtlicher Untersuchungen (The conductive tracts in brain and spinal cord of humans based on developmental investigations) W. Engelmann; Leipzig: 1876. [Google Scholar]
- Friedreich N. Ueber degenerative Atrophie der spinalen Hinterstränge (On degenerative atrophy of the spinal dorsal columns) Virchows Arch Pathol Anat Physiol Klin Med. 1863a;26:391–419. [Google Scholar]
- Friedreich N. Ueber degenerative Atrophie der spinalen Hinterstränge (On degenerative atrophy of the spinal dorsal columns) Virchows Arch Pathol Anat Physiol Klin Med. 1863b;26:433–459. [Google Scholar]
- Friedreich N. Ueber degenerative Atrophie der spinalen Hinterstränge (On degenerative atrophy of the spinal dorsal columns) Virchows Arch Pathol Anat Physiol Klin Med. 1863c;27:1–26. [Google Scholar]
- Friedreich N. Ueber Ataxie mit besonderer Berücksichtigung der hereditären Formen (About ataxia with special consideration of the hereditary forms) Virchows Arch Pathol Anat Physiol Klin Med. 1876;68:145–245. [Google Scholar]
- Friedreich N. Ueber Ataxie mit besonderer Berücksichtigung der hereditären Formen. Nachtrag (About ataxia with special consideration of the hereditary forms. Postscriptum) Virchows Arch Pathol Anat Physiol Klin Med. 1877;70:140–152. [Google Scholar]
- Koeppen AH, Morral JA, Davis AN, Qian J, Petrocine SV, Knutson MD, Gibson WM, Cusack MJ, Li D. The dorsal root ganglion in Friedreich’s ataxia. Acta Neuropathol. 2009;118:763–776. doi: 10.1007/s00401-009-0589-x. [DOI] [PubMed] [Google Scholar]
- Koeppen AH, Davis AN, Morral JA. The cerebellar component of Friedreich’s ataxia. Acta Neuropathol. 2011;122:323–330. doi: 10.1007/s00401-011-0844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrior AA. Un cas de maladie de Friedreich avec autopsie (A case of Friedreich’s disease with autopsy) Rev Neurol. 1911;21:525–540. [Google Scholar]
- Marie P. Sur l’hérédo-ataxie cérébelleuse (On cerebellar heredoataxia) Sem Med. 1893;13:444–447. [Google Scholar]
- Morral JA, Davis AN, Qian J, Gelman BB, Koeppen AH. Pathology and pathogenesis of sensory neuropathy in Friedreich’s ataxia. Acta Neuropathol. 2010;120:97–108. doi: 10.1007/s00401-010-0675-0. [DOI] [PubMed] [Google Scholar]
- Mott FW. Case of Friedreich’s disease, with autopsy and systematic microscopical examination of the nervous system. Arch Neurol Psychiat (Lond) 1907;3:180–200. [Google Scholar]
- Oppenheimer DR. Brain lesions in Friedreich’s ataxia. Can J Neurol Sci. 1979;6:173–176. doi: 10.1017/s0317167100119596. [DOI] [PubMed] [Google Scholar]
- Ouvrier RA, McLeod JG, Conchin TE. Friedreich’s ataxia. Early detection and progression of peripheral nerve abnormalities. J Neurol Sci. 1982;55:137–145. doi: 10.1016/0022-510x(82)90095-8. [DOI] [PubMed] [Google Scholar]
- Russell DS. Myocarditis in Friedreich’s ataxia. J Pathol Bacteriol. 1946;4:91–102. doi: 10.1002/path.1700580414. [DOI] [PubMed] [Google Scholar]
- Virchow R. Zur Erinnerung an Nicolaus Friedreich (In Memoriam of Nicolaus Friedreich) Virchows Arch Pathol Anat Physiol Klin Med. 90:215–220. [Google Scholar]


