Abstract
Urinary incontinence is a common problem in both men and women. In this review article we address treatment of the various forms of incontinence with conservative treatments, medical therapy, devices and surgery. The US Preventive Services Task Force, The Cochrane Database of Systematic Reviews, and PubMed were reviewed for articles focusing on urinary incontinence. Conservative therapy with education, fluid and food management, weight loss, timed voiding and pelvic floor physical therapy are all simple office-based treatments for incontinence. Medical therapy for incontinence currently is only available for urgency incontinence in the form of anticholinergic medication. Condom catheters, penile clamps, urethral inserts and pessaries can be helpful in specific situations. Surgical therapies vary depending on the type of incontinence, but are typically offered if conservative measures fail.
Keywords: Adrenergic beta-3 Receptor Agonists, bladder retraining, lower urinary tract symptoms, muscarinic antagonists, overactive urinary bladder, pelvic floor physical therapy, pessaries, stress urinary incontinence
Introduction
Urinary incontinence (UI) is a common problem. Urgency incontinence, stress incontinence and mixed incontinence are the most prevalent forms of incontinence and can be treated in a variety of ways. In this review office-based treatments with conservative therapy, medication and devices will be covered in detail and the various surgical therapies will be summarized. Part 1 of this review article addressed the various types of incontinence as well as how to diagnose them [Cameron et al. 2013].
Methods
The following data sources were reviewed: The US Preventive Services Task Force, The Cochrane Database of Systematic Reviews, and PubMed. A MEDLINE search was conducted using the key words of incontinence plus: anticholinergic, antimuscarinic, artificial urinary sphincter, bladder irritants, bladder retraining, clinical practice guidelines, device, duloxetine, estrogen, female sling, imipramine, knack maneuver, male sling, mixed incontinence, pelvic floor physical therapy, penile clamp, pessary, pharmacotherapy, stress, urethral bulking agent, urge, urge suppression, and weight loss. SORT criteria [Ebell et al. 2004] were utilized to grade the quality of evi-dence.
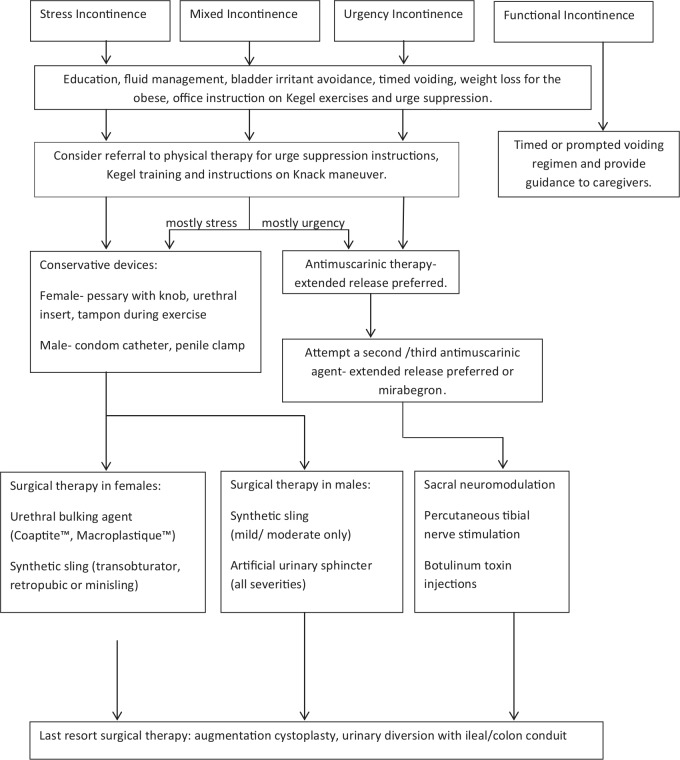
Conservative treatment
Fluid/food irritant management (stress and urgency incontinence) (level of evidence C)
Various food components have been implicated in increasing the incidence of urgency urinary incontinence (UUI); however, there exists little data on the effectiveness of eliminating these foods. These substances include: citrus fruits and juices; carbonated beverages, especially those containing artificial sweeteners; chocolate (which contains theobromine, a methylxanthine similar to caffeine); caffeinated coffee and tea; and excessive amounts of water [Abrams et al. 2010]. Green tea, however, seems to be protective [Hirayama and Lee, 2011].
Caffeine consumption has been linked to the universal notion of increased urinary frequency, yet its direct effect as a potential bladder irritant is poorly understood. It is a natural diuretic which has a direct stimulatory effect on bladder smooth muscle. A prospective cohort study of over 65,000 women aged 37–79 years in the Nurses’ Health Study phases I and II over a 4-year period found a modestly increased risk of UI at least twice weekly in women with the highest (greater than 450 mg) versus the lowest (less than 150 mg) daily intake, with an attributable risk of 25% [Jura et al. 2011].
Timed voiding for functional incontinence (stress and urgency incontinence) (level of evidence C)
One Cochrane review evaluated the impact and effect of timed voiding for functional incontinence. Timed voiding is a behavioral strategy that allows a voiding schedule commensurate with personal habits, and does not attempt to increase voiding times or holding. Nine trials examining 674 elderly patients (mostly women) compared prompted voiding to unprompted voiding. Overall, the authors concluded limited evidence as to whether either form improves incontinence, but posited that prompted voiding increased self-initiated voiding and decreased incontinent episodes in the short term [Eustice et al. 2000]. It is unknown whether such effects would be sustained over a long period of prompted voiding, or if incontinence would recur after stopping the prompting. Another Cochrane Review examined the use of a fixed interval of voiding (timed voiding) for the management of UI in 2 trials consisting of 298 elderly women with reduced cognition and impaired mobility who resided in aged care homes [Ostaszkiewicz et al. 2004]. This review concluded that there was insufficient evidence for using timed voiding for the management of UI, but cited low risk of potential harm.
Weight loss (stress incontinence) (level of evidence B)
Obesity is a strong independent risk factor for the development of both stress and urgency incontinence [Subak et al. 2009a], yet the mechanisms linking these two conditions are poorly understood. It has been postulated that abdominal obesity and increased intra-abdominal pressure exert greater pressure upon pelvic viscera. Therefore, weight loss in the obese should improve the frequency of stress urinary incontinence (SUI) episodes. Also achieving a healthy weight has multiple other health benefits to patients.
One randomized trial studied 338 obese women median age 53 years, with 10 or more episodes of UI per week who were assigned to an intensive 6-month weight-loss program consisting of dietary restrictions, exercise, and behavior modification (226 women), or given an education program aimed at weight loss strategies (112 women) [Subak et al. 2009b]. Observed weight loss was 8% in the intervention group compared with 1.6% in the control group, while decreased frequency of stress incontinence episodes were 47% and 28%, respectively, although weight loss did not decrease urgency incontinence. Weight loss has also been shown to decrease stress and urgency incontinence episodes in women with type II diabetes [Phelan et al. 2012] and in women who underwent bariatric surgery [Roberson et al. 2010; Burgio et al. 2007].
Pelvic floor physical therapy (stress and urgency incontinence)
Women with stress or mixed incontinence can significantly reduce urine loss via cough through volitional contractions of pelvic floor muscles both before and during a cough (level of evidence C) [Miller et al. 1998]. Such timing of a pelvic muscle contraction with the moment of expected leakage (the Knack maneuver) has been shown to minimize incontinence when performed correctly. The term ‘knack’ in this case implies the ‘trick or skill’ of stopping anticipated leakage without permanently changing the actual physiology of the pelvic musculature [Miller et al. 2008].
A systematic review found moderate evidence from four randomized, controlled trials (RCTs) supporting higher continence rates after institution of pelvic floor muscle training (PFMT) (level of evidence B) [Shamliyan et al. 2008]. In this review seven trials examined PFMT, eight trials examined the effects of PFMT combined with bladder training and four combined PFMT with biofeedback. All demonstrated significant reductions in UI with PFMT, but it was found to be more beneficial when combined with bladder training. There is inconsistent low-level evidence to support use of magnetic or electrical stimulation in improving UI (level of evidence C) [Bergman et al. 2004]. This data supports the utilization of PFMT in improving UI in women, as benefits outweigh potential adverse risks. Skilled physical therapists are required to maximize effectiveness, along with appropriate patient engagement and follow up.
Weighted vaginal cones are commonly used to help women train their pelvic floor muscles to improve UI. Typically increasingly smaller cones are inserted into the vagina and the woman contracts her pelvic floor to prevent them from sliding out. A Cochrane review evaluated 15 trials that found that cones were superior to no active treatment, but there was little evidence of difference between cones and PFMT or electrostimulation (level of evidence C) [Herbison et al. 2002].
Bladder retraining (urgency incontinence) (level of evidence C)
Bladder training is a behavioral therapeutic strategy that encourages patients with urgency incontinence to increase the amount of time between emptying the bladder, thereby increasing the amount of fluid the bladder can hold. It also can potentially diminish leakage and the sense of urinary urgency. Bladder training requires that the patient follow a fixed voiding schedule, regardless of whether the patient senses the urge to void.
A Cochrane review examined trials comparing bladder training with no training and although they described only a limited number of outcomes, the concept of bladder training was slightly favored despite absence of statistical support [Wallace et al. 2004] (see the appendix for an example of bladder training instructions).
Medical therapy (urgency incontinence)
Oral medications for urgency incontinence include antimuscarinic (anticholinergic) agents (level of evidence A) that competitively inhibit muscarinic receptors and most recently selective β3-adrenergic receptor agonists (level of evidence B). Only antimuscarinics have a grade A recommendation from the International Consultation on Incontinence based on level 1 evidence [Abrams et al. 2010]. The most commonly used anticholinergic agents are oxybutynin and tolterodine. In a head-to-head comparison, oxybutynin was slightly more effective but had higher incidence of side effects than tolterodine [Harvey et al. 2001]. Newer anticholinergics (e.g. solifenacin, darifenacin, trospium) may have more specificity for M3 receptors (present in detrusor muscles) and less penetration of the blood–brain barrier; however, actual clinical advantage is minimal compared with the long-acting forms of oxybutynin and tolterodine [Hay-Smith et al. 2005].
The two most common side effects of antimuscarinics are constipation and dry mouth. Owing to these side effects, discontinuation rates can be high. A recent study found that the median time for overall discontinuation was 4.8 months, with a 6-month unadjusted cumulative incidence of 59% with the lowest discontinuation rate among the extended-release anticholinergics. Since these tend to be better tolerated they are generally recommended over the immediate-release counterparts [Gopal et al. 2008]. All anticholinergics can worsen cognitive impairment in the elderly and those with dementia. Because these groups are those most often affected by UI, care should be taken to start these medications at low doses and to avoid short-acting oxybutynin, which has been shown to cause this side effect more often than its long-acting counterpart or tolterodine [Gopal et al. 2008] (Table 1). Older anticholinergics such as scopolamine and hyoscyamine are not indicated for the treatment of urge incontinence due to their high side effects and lower efficacy. Anticholinergics are contraindicated in patients with narrow angle-closure glaucoma or significant urinary obstruction (residual urine over 200 cm3).
Table 1.
Medical therapy for overactive bladder.
| Name (brand name) | Formulation | Starting dose | Maximum dose |
|---|---|---|---|
| Oxybutynin (Ditropan) | Oral IR | 2.5 mg, 2–3 times a day | 5 mg, 4 times a day |
| (Gelnique) | Oral ER | 5 mg, once daily | 30 mg, once daily |
| (Oxytrol) | GelPatch | 1 sachet of 10% gel or 3 pumps of 3% gel daily | 1 sachet or 3 pumps daily |
| 1 patch every 3-4 days | 1 patch at a time (approved for OTC use Fall 2013) | ||
| Tolterodine (Detrol): Reduce by half in renal/hepatic diseases | Oral IR | 1 mg, twice daily | 2 mg, twice daily |
| Oral ER | 2 mg, once daily | 4 mg, once daily | |
| Fesoterodine (Toviaz): Reduce by half in renal/hepatic diseases | Oral ER | 4 mg, once daily | 8 mg, once daily |
| Solifenacin (Vesicare): Reduce by half in renal/hepatic diseases | Oral ER | 5 mg, once daily | 10 mg, once daily |
| Darifenacin (Enablex): Reduce by half in hepatic diseases | Oral ER | 7.5 mg, once daily | 15 mg, once daily |
| Trospium (Sanctura): In renal impairment, reduce IR by half; ER not recommended | Oral IR | 20 mg, once daily | 20 mg, twice daily |
| Oral ER | 60 mg, once daily | 60 mg, once daily | |
| Mirabegron (Myrbetriq) | Oral ER | 25 mg, once daily | 50 mg, once daily |
ER, extended release; IR, immediate release; OTC, over the counter
Mirabegron (Myrbetriq) is currently the only clinically utilized β3-adrenergic receptor agonist and was FDA approved in 2012 for the treatment of overactive bladder. It has been shown to reduce urgency, frequency and urgency incontinence episodes similarly to tolterodine. Given their different receptor profiles, it does not share the same side effects as the antimuscarinic medications, but there are concerns about its effect on the cardiovascular system. Its primary side effects are a mild increase in blood pressure, nasopharyngitis, headache, UTI and it is a moderate CYP2D6 inhibitor [Andersson et al. 2013].
Currently, no medications are approved by FDA for the treatment of stress incontinence. Estrogen had been used widely in the past to treat stress incontinence in women, due to its theoretical ability to improve urethral closure through increased urethral vascularity/thickness and sensitized α-adrenergic receptors in the bladder neck [Weiss, 2005]. However, a Cochrane review of 31 published studies failed to show objective improvement in SUI and actually may have shown worsening incontinence with oral agents [Cody et al. 2009]. Thus, due to the lack of evidence of its effectiveness and increased awareness of the harms of hormone therapy [Rossouw et al. 2002], oral estrogen should be avoided as a treatment of stress incontinence. Topical intravaginal estrogen in the same Cochrane review [Cody et al. 2009] was mildly effective for decreasing frequency and urgency (level of evidence C).
Duloxetine is a serotonin–norepinephrine reuptake inhibitor currently approved by the FDA for the treatment of depression. It failed FDA approval for the treatment of stress incontinence due to poor efficacy and increased suicidal ideation and suicide in depressed patients. In RCTs, duloxetine reduced UI episodes by 54–64%, compared with 41% reduction in the control group [Dmochowski et al. 2003]. Given the risk and poor efficacy it cannot be recommended. Tricyclic antidepressants, most notably imipramine, have been used off label for the treatment of UI. Imipramine facilitates urine storage by two mechanisms: decreasing bladder contractility through an antispasmodic effect on the detrusor muscle, and increasing outlet resistance through an alpha-adrenergic effect on the bladder neck. It has traditionally been used for the treatment of nocturnal enuresis in children. Older animal studies and low-quality human trials suggested a benefit in stress and urge incontinence, but high-quality evidence is lacking. Currently, the use of imipramine for management of stress and urge incontinence lacks strong clinical evidence and cannot be recommended [Hunsballe and Djurhuus, 2001].
Incontinence management with devices
Despite conservative and medical therapy some patients remain incontinent but do not desire surgical therapy or are not suitable candidates. In addition, some patients desire symptom relief while they are waiting for surgery and devices can significantly help these individuals.
Absorbent pads (urgency and stress incontinence) (level of evidence B)
Absorbent incontinence products can be used for any type of UI. They are the obvious choice for many patients and are often employed before seeking medical advice. The expense to a patient of this form of treatment cannot be underestimated. Recent studies estimate the annual costs of incontinence treatment in the US to be between US$16.5 billion and US$19.5 billion with around 9% of this cost being for absorbent products [Hu et al. 2004; Wilson et al. 2001].
For patients it is of the utmost importance to their quality of life that these products are inconspicuous and dependable [Fader et al. 2010; Teunissen et al. 2009]. Among adults with incontinence 60 years and older living in the community, 87% of women utilize pads to manage their incontinence, with 50% using menstrual type pads rather than incontinence specific pads. In clinical trials women with more severe incontinence significantly prefer disposable ‘pull-up’ style to other designs and none of the washable designs were deemed acceptable [Fader et al. 2010]. In contrast, only 15% of incontinent men in the community use any absorbent product and they tend to use absorbent pads designed for women or self-constructed reusable pads made of towels [Teunissen et al. 2009]. Men commonly find pads ‘babyish’ or ‘feminine’ which may explain their reluctance to use these products [Fader et al. 2010]. In clinical trials, men prefer regular disposable diapers and disposable T-shaped diapers over pads with respect to urine leakage and staying in place during the day. Men are the only group for whom washable diapers have been found to be an acceptable choice, particularly at night [Fader et al. 2008].
Incontinence-associated dermatitis occurs when stool or urine are in contact with skin. It ranges from redness, swelling, oozing, crusting and scaling, to loss of skin integrity and infection. Secondary infection with candida is also commonly seen. To prevent these complications, patients need to perform a structured skin care cleansing protocol after each incontinence episode with a dedicated perineal cleanser (liquid, emulsion, foam or towelette) and not with regular bar soap or antibacterial handwash since these are drying. This cleansing should be followed by a moisturizer such as glycerine, lanolin or mineral-oil based products. Moisture barriers are the next component in skin breakdown prevention and act as a shield to protect the skin from irritants and moisture. These can be incorporated with cleansers or moisturizers. Common ingredients include petrolatum dimethicone, lanolin or zinc oxide and are available over the counter. Candidiasis typically presents as a maculopapular rash with satellite lesions. It should be treated with a topical antifungal combined with a barrier cream or with an initial layer of antifungal followed by barrier cream [Nix and Haugen, 2010].
Female devices (stress incontinence)
Women have two main device options in treating stress incontinence: urethral inserts (level of evidence C) and continence pessaries (level of evidence B). These devices are not effective for pure urgency incontinence since they both increase urethral outlet resistance. Urethral inserts (Femsoft®; Rochester Medical Corp.) are short silicone single use tubes that are placed in the urethra by the patient and are held in place by a mineral-oil-filled bulbous sheath that is at the bladder neck (Figure 1). Each time a woman voids she removes the device and replaces a new one. In a two-year follow up of 150 women there were only minor adverse events such as urinary tract infections (UTI) (31.3%) and mild urethral trauma (6.7%) [Sirls et al. 2002]. These devices have not achieved widespread use, likely given the long-term urethral irritation and availability of less invasive products. Continence pessaries are rings or dishes placed vaginally with a knob or prongs that face the urethra to promote continence (Figure 2). They are an excellent choice particularly in a woman with concomitant prolapse for whom the pessary can improve both conditions. Common complications include vaginal discharge, new onset of difficulty voiding, spontaneous expulsion and more rarely vaginal erosion in the forgotten or neglected pessary [Trowbridge and Fenner, 2007]. One RCT with 446 women that compared an incontinence pessary, behavioral therapy, or both, found that pessaries alone had the least improvement at 3 months (40% much or very much better) compared with behavioral therapy with or without pessary (49%). At 12 months, however, there were no differences between groups with overall 32% of patients being much or very much better, likely due to the loss of efficacy of behavior therapy if it is not continued [Richter et al. 2010]. Pessaries do require a provider to properly fit the device and many women require more than one fitting to achieve comfort, but the majority of women can be easily fitted and learn how to remove the device themselves. An ideal situation is when a woman can place her own pessary for times when she anticipates urine leakage, such as an evening out dancing or during exercise. Some women with very mild incontinence use menstrual tampons in a similar fashion since these also can slightly increase urethral outlet pressure. In women with significant pelvic organ prolapse, placement of a pessary can improve urgency and voiding difficulty that are due to the prolapse [Trowbridge and Fenner, 2007].
Figure 1.
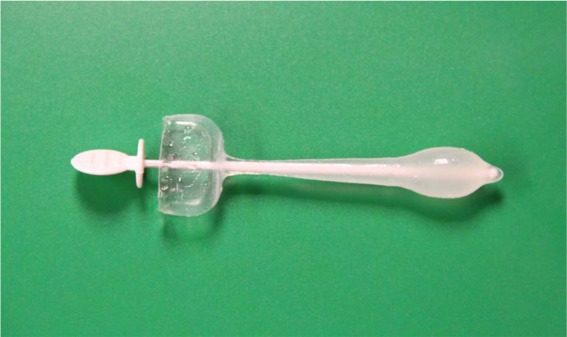
Female urethral insert. Mineral-oil-filled bulbous sheath holds device in place (on right) after white stylet is removed.
Figure 2.

Ring pessaries with support with a knob (left) and without (right).
Male devices (stress and urgency incontinence)
Men have more options than women since the penis can serve as an attachment for external collection devices and can be compressed by clamps to achieve continence. Condom catheters are probably the best known external collection devices (level of evidence B) (Figure 3). They are much safer than indwelling catheters with respect to risk of UTI, patient comfort and even death [Saint et al. 2006]. The primary concerns with modern one-piece self-adhesive roll-on condom catheters include ease of putting on the sheath, and the risk of the sheath falling off, leakage, skin redness or discomfort [Pemberton et al. 2006]. These sheaths require proper sizing and good instruction on application, as well as a quality adhesive to be effective. There are many products available and most are latex free. Patients tend to prefer one brand over another, so in clinical practice it is practical to fit a man with several brands and allow him to choose the one best suited to his needs. Unfortunately, some patients are never able to achieve a good fit, with the device frequently falling off often due to poor application, obesity, high levels of physical activity or a retractile penis. Another similar option is a body worn urinal attached with a body strap that is not adherent to the penis. The other male-specific option is a penile clamp which is a device applied to the pendulous part of the penis preventing urine leakage (level of evidence B) (Figure 3). These are best used for pure stress incontinence. These clamps carry the risk of penile edema, urethral erosion, pain, and skin breakdown unless the penis is inspected properly on a daily basis. The three most common clamps were compared in a crossover study. The Cunningham clamp had better continence than the U-Tex clamp and the C3, which was preferred by patients, but it lowered penile blood flow on Doppler the most [Moore et al. 2004]. In cognitively intact men who can remove the device if it causes pain redness or other complications, it is an excellent discreet option.
Figure 3.

Adhesive condom catheter (top) and penile clamp (bottom).
Surgical therapy
Urethral bulking agents (stress incontinence) (level of evidence B)
SUI is often a surgically treated condition since pelvic floor physical therapy and weight loss rarely result in a cure. In female patients the least-invasive approach is to use urethral injectable agents. These therapies are typically done in the clinic and involve injecting a synthetic bulking agent via a cystoscope into the urethra effectively bulking up the urethra improving coaptation. Collagen, which was the standard agent, is no longer marketed commercially. Several agents have been employed over the years with many no longer being recommended due to particle migration, local abscess reaction, or ineffectiveness. Currently, the two agents in use that have been best studied are silicon microparticles (Macroplastique®) and calcium hydroxyapatite (Coaptite®). Both have been shown to be nonantigenic, nonmigratory, and have very few side effects [Cameron and Haraway, 2011]. The most common side effects are UTI and transient retention. Unfortunately, cure is uncommon (30%) and most women only see an improvement in their incontinence [Dmochowski et al. 2010]. Bulking agents are typically offered to women who are not surgical candidates due to comorbidities or women who do not want invasive surgery [Cameron and Haraway, 2011]. They are not recommended for male SUI since they have not been well studied, have low rates of achieving dryness (4–20%) [Herschorn et al. 2010] and are not FDA approved for this purpose.
Female sling surgery (stress incontinence)
The synthetic midurethral sling can be performed in the outpatient setting and has an extensive safety profile with over 15 years of data. There are two well-studied approaches: the retropubic (TVT™, SPARC™, etc.) and transobturator (TOT™, Obtryx™, etc.) (level of evidence A) with long-term cure rates of 84% [Dmochowski et al. 2010] for the retropubic approach and shorter-term similar success for transobturator [Latthe et al. 2010]. Although similar in success they have different complication profiles with the retropubic approach having a greater risk of bladder injury or bleeding and the obturator approach having a greater risk of severe thigh pain and vaginal injury [Latthe et al. 2010] since the tape is placed through the adductor muscles.
Mini slings placed via only a vaginal incision (level of evidence B) were devised to prevent these complications and to increase ease of placement. Comparisons thus far have shown them to have inferior effectiveness [Kennelly et al. 2010] in early trials but superiority in others [Sivaslioglu et al. 2012]. Complications are similar to the transobturator sling.
Unlike vaginal prolapse repairs with mesh kits that have received significant press due to the risk of complicated vaginal erosion [Food and Drug Administration, 2012], midurethral slings have a very low volume of mesh and vaginal extrusions (7%) [Dmochowski et al. 2010] can typically be treated with a minor revision and excision of the offending part of the sling.
Treatment of severe incontinence with intrinsic sphincter deficiency or women who have failed midurethral slings is controversial.
Male incontinence surgery (stress incontinence)
Mesh slings are also available in men to treat mild to moderate stress incontinence. The Advance™ is a transobturator mesh sling (level of evidence B) [Herschorn et al. 2010] and the Virtue™ is a quadratic sling with transobturator and prepubic arms (level of evidence C). Cure rates are highly dependent on preoperative incontinence level and typically range between 34% and 67% for Advance™ [Cornu et al. 2011]; however, only intermediate-term results are available at this time. Complications of these procedures are uncommon (mesh infection in 0–6%, urethral injury in 0–2%, and scrotal numbness or pain in 16–72%), but resolves in almost all men by 3 months [Herschorn et al. 2010].
The artificial urinary sphincter (AUS) is the gold standard treatment for male stress incontinence, with a long-term postprocedure continence rate of 69–90% (level of evidence B). It involves implantation of an inflatable cuff around the bulbar urethra connected to a scrotal control pump with a fluid reservoir in the lower abdomen. It requires dexterity and understanding of the mechanism since the patient must squeeze the scrotal pump to be able to urinate. Many men do not favor this approach, since they cannot simply void like with the male slings, however this is the only effective therapy for severe incontinence. Complications of infection, erosions and urethral atrophy do occur and the device can malfunction over time and need replacing [Herschorn et al. 2010].
Surgery for refractory urgency incontinence
In both men and women urgency incontinence is considered refractory when the patient has failed both behavioral modifications and two or three different antimuscarinic drugs [Herbison and Arnold, 2009]. There are currently two neuromodulation devices for refractory UUI: posterior tibial nerve stimulator (Urgent PC Neuromodulation System™, Uroplasty, Inc., Minnetonka, MN) approved by the FDA in 2011(level of evidence C); and implantable sacral neuromodulation devices (Interstim™, Medtronic, Minneapolis, MN) FDA approved in 1997 (level of evidence B). The PTNS therapy is administered in the office weekly in 30-minute sessions for 12 weeks via a small needle inserted posterior to the lateral malleolus of the ankle connected to an electrical stimulation device. It has been shown to improve urgency, frequency, and urgency incontinence [Peters et al. 2010]. The treatment cycle is repeated once symptoms begin to recur. Complications are minor and typically due to irritation from the needle; however, with the need for retreatment many patients are eager for a more durable response. The sacral neuromodulation device [Herbison and Arnold, 2009] is implanted in two steps to assess effectiveness prior to actual implantation of the neuromodulator in a small pocket in the buttock adipose tissue. Once implanted, patients are given a personal programming device that allows minor adjustments to the settings and allows it to be turned off as needed. The major drawbacks of this device are the need for replacement of the device when the battery is drained (3–5 years) and device complications such as migration of the lead, pain from the device and lead breakage which can typically be corrected by device revision, but are quite common occurring in 30% of devices [White et al. 2009]. Onabotulinumtoxin A (Botox) bladder injections via cystoscope are FDA approved for individuals with neurogenic bladder and in 2012 were approved for patients without neurological disease who have urgency incontinence (level of evidence B). In the phase III randomized, placebo-controlled trial of 100U onabotulinumtoxin A (Botox) compared with placebo injected cystoscopically in the bladder the frequency of urge incontinence episodes were reduced by 2.7 per day with 23% of patients becoming dry in the botulinum toxin arm [Nitti et al. 2012]. The most common side effect is urinary retention (5.4%), but is otherwise well tolerated [Fowler et al. 2012]. The injections can be performed in the office, or under sedation depending on patient and provider preference. Last-resort surgical therapies for urgency incontinence include detrusor myomectomy, bladder augmentation with either small bowel or colon or urinary diversion with an ileal conduit in the case of completely untreatable conditions. These surgeries, given their morbidity, are rare in the absence of severe bladder disease.
Conclusion and practice recommendations
Conservative therapy with education, fluid and food management, weight loss, timed voiding and pelvic floor physical therapy are all simple office-based treatments of incontinence. Medical therapy for incontinence currently is only available for urgency incontinence in the form of anticholinergic medication. Condom catheters, penile clamps, urethral inserts and pessaries can be helpful in specific situations. Surgical therapies vary depending on the type of incontinence, but are typically offered if conservative measures fail. A flow chart of therapeutic options can be followed to best direct patient care, as shown in Figure 4.
Figure 4.
Therapeutic options to direct patient care.
Appendix
An example of bladder training includes the following steps:
Empty your bladder as soon as you get up in the morning.
Void at the specific times you have discussed with your healthcare provider.
Wait until your next scheduled time before you void again, and empty your bladder even if you feel no urge to urinate.
Follow the schedule during waking hours only. At night, go to the bathroom only if you awaken and find it necessary.
When you feel the urge to urinate before the next designated time, use ‘urge suppression’ techniques or try relaxation techniques such as deep breathing.
If the urge is suppressed, adhere to the schedule. If you cannot suppress the urge, wait 5 minutes then slowly make your way to the bathroom.
After urinating, re-establish the schedule. Repeat this process every time an urge is felt.
When you have accomplished your initial goal, gradually increase the time between emptying your bladder by 15-minute intervals.
Increase the time between each urination until you reach a 3- to 4-hour voiding interval.
It should take between 6 and 12 weeks to accomplish your ultimate goal. Do not be discouraged by setbacks. You may find you have good days and bad days. As you continue bladder retraining, you will start to notice more and more good days, so keep practicing.
Footnotes
Conflict of interest statement: The authors have no conflicts of interest to declare.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Contributor Information
Anne P. Cameron, University of Michigan Department of Urology, 3875 Taubman Center, 1500 East Medical Center Drive, Ann Arbor, MI 48109-5330, USA
Masahito Jimbo, University of Michigan Departments of Family Medicine and Urology, Ann Arbor, MI, USA.
Joel J. Heidelbaugh, University of Michigan Departments of Family Medicine and Urology, Ann Arbor, MI, USA
References
- Abrams P., Andersson K., Birder L., Brubaker L., Cardozo L., Chapple C., et al. for the Members of Committees and Fourth International Consultation on Incontinence (2010) Fourth International Consultation on Incontinence Recommendations of the International Scientific Committee: Evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinence. Neurourol Urodyn 29: 213–240 DOI: 10.1002/nau.20870 [DOI] [PubMed] [Google Scholar]
- Andersson K., Martin N., Nitti V. (2013) Selective beta3-adrenoceptor agonists in the treatment of the overactive bladder. J Urol, in press. DOI: 10.1016/j.juro.2013.02.104 [DOI] [PubMed] [Google Scholar]
- Cameron A. P., Heidelbaugh J. B., Masahito J. (2013). Diagnosis and office based treatment of urinary incontinence in adults: part one diagnosis and testing. Therapeutic Advances in Urology. DOI: 10.1177/1756287213489720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J., Robertson J., Elia G. (2004) Effects of a magnetic field on pelvic floor muscle function in women with stress urinary incontinence. Altern Ther Health Med 10: 70–72 [PubMed] [Google Scholar]
- Burgio K., Richter H., Clements R., Redden D., Goode P. (2007) Changes in urinary and fecal incontinence symptoms with weight loss surgery in morbidly obese women. Obstet Gynecol 110: 1034–1040 DOI: 10.1097/01.AOG.0000285483.22898.9c [DOI] [PubMed] [Google Scholar]
- Cameron A., Haraway A. (2011) The treatment of female stress urinary incontinence: an evidence-based review. Open Access J Urol 3: 109–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody J., Richardson K., Moehrer B., Hextall A., Glazener C. (2009) Oestrogen therapy for urinary incontinence in post-menopausal women. Cochrane Database Syst Rev 4: CD001405 DOI: 10.1002/14651858.CD001405.pub2 [DOI] [PubMed] [Google Scholar]
- Cornu J., Sebe P., Ciofu C., Peyrat L., Cussenot O., Haab F. (2011) Mid-term evaluation of the transobturator male sling for post-prostatectomy incontinence: focus on prognostic factors. BJU Int 108: 236–240 DOI: 10.1111/j.1464-410X.2010.09765.x [DOI] [PubMed] [Google Scholar]
- Dmochowski R., Blaivas J., Gormley E., Juma S., Karram M., Lightner D., et al. for the Female Stress Urinary Incontinence Update Panel of the American Urological Association Education and Research, Inc (2010) Update of AUA guideline on the surgical management of female stress urinary incontinence. J Urol 183: 1906–1914 DOI: 10.1016/j.juro.2010.02.2369 [DOI] [PubMed] [Google Scholar]
- Dmochowski R., Miklos J., Norton P., Zinner N., Yalcin I., Bump R. for the Duloxetine Urinary Incontinence Study Group (2003) Duloxetine versus placebo for the treatment of North American women with stress urinary incontinence. J Urol 170: 1259–1263 DOI: 10.1097/01.ju.0000080708.87092.cc [DOI] [PubMed] [Google Scholar]
- Ebell M., Siwek J., Weiss B., Woolf S., Susman J., Ewigman B., et al. (2004) Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Am Board Fam Pract 17: 59–67 [DOI] [PubMed] [Google Scholar]
- Eustice S., Roe B., Paterson J. (2000) Prompted voiding for the management of urinary incontinence in adults. Cochrane Database Syst Rev 2: CD002113 DOI: 10.1002/14651858.CD002113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader M., Bliss D., Cottenden A., Moore K., Norton C. (2010) Continence products: research priorities to improve the lives of people with urinary and/or fecal leakage. Neurourol Urodyn 29: 640–644 DOI: 10.1002/nau.20918 [DOI] [PubMed] [Google Scholar]
- Fader M., Cottenden A., Getliffe K. (2008) Absorbent products for moderate-heavy urinary and/or faecal incontinence in women and men. Cochrane Database Syst Rev 4: CD007408 DOI: 10.1002/14651858.CD007408 [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (2011) Urogynecologic Surgical Mesh: Update on the Safety and Effectiveness of Transvaginal Placement for Pelvic Organ Prolapse. Food and Drug Administration [Google Scholar]
- Fowler C., Auerbach S., Ginsberg D., Hale D., Radziszewski P., Rechberger T., et al. (2012) OnabotulinumtoxinA improves health-related quality of life in patients with urinary incontinence due to idiopathic overactive bladder: a 36-week, double-blind, placebo-controlled, randomized, dose-ranging trial. Eur Urol 62: 148–157 DOI: 10.1016/j.eururo.2012.03.005 [DOI] [PubMed] [Google Scholar]
- Gopal M., Haynes K., Bellamy S., Arya L. (2008) Discontinuation rates of anticholinergic medications used for the treatment of lower urinary tract symptoms. Obstet Gynecol 112: 1311–1318 DOI: 10.1097/AOG.0b013e31818e8aa4 [DOI] [PubMed] [Google Scholar]
- Harvey M., Baker K., Wells G. (2001) Tolterodine versus oxybutynin in the treatment of urge urinary incontinence: a meta-analysis. Am J Obstet Gynecol 185: 56–61 DOI: 10.1067/mob.2001.116371 [DOI] [PubMed] [Google Scholar]
- Hay-Smith J., Herbison P., Ellis G., Morris A. (2005) Which anticholinergic drug for overactive bladder symptoms in adults. Cochrane Database Syst Rev 3: CD005429 DOI: 10.1002/14651858.CD005429 [DOI] [PubMed] [Google Scholar]
- Herbison G., Arnold E. (2009) Sacral neuromodulation with implanted devices for urinary storage and voiding dysfunction in adults. Cochrane Database Syst Rev 2: CD004202 DOI: 10.1002/14651858.CD004202.pub2 [DOI] [PubMed] [Google Scholar]
- Herbison P., Plevnik S., Mantle J. (2002) Weighted vaginal cones for urinary incontinence. Cochrane Database Syst Rev 1: CD002114 DOI: 10.1002/14651858.CD002114 [DOI] [PubMed] [Google Scholar]
- Herschorn S., Bruschini H., Comiter C., Grise P., Hanus T., Kirschner-Hermanns R., et al. for the Committee of the International Consultation on Incontinence (2010) Surgical treatment of stress incontinence in men. Neurourol Urodyn 29: 179–190 DOI: 10.1002/nau.20844 [DOI] [PubMed] [Google Scholar]
- Hirayama F., Lee A. (2011) Green tea drinking is inversely associated with urinary incontinence in middle-aged and older women. Neurourol Urodyn 30: 1262–1265 DOI: 10.1002/nau.20987 [DOI] [PubMed] [Google Scholar]
- Hu T., Wagner T., Bentkover J., Leblanc K., Zhou S., Hunt T. (2004) Costs of urinary incontinence and overactive bladder in the United States: a comparative study. Urology 63: 461–465 [DOI] [PubMed] [Google Scholar]
- Hunsballe J., Djurhuus J. (2001) Clinical options for imipramine in the management of urinary incontinence. Urol Res 29: 118–125 [DOI] [PubMed] [Google Scholar]
- Jura Y., Townsend M., Curhan G., Resnick N., Grodstein F. (2011) Caffeine intake, and the risk of stress, urgency and mixed urinary incontinence. J Urol 185: 1775–1780 DOI: 10.1016/j.juro.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennelly M., Moore R., Nguyen J., Lukban J., Siegel S. (2010) Prospective evaluation of a single incision sling for stress urinary incontinence. J Urol 184: 604–609 DOI: 10.1016/j.juro.2010.04.003 [DOI] [PubMed] [Google Scholar]
- Latthe P., Singh P., Foon R., Toozs-Hobson P. (2010) Two routes of transobturator tape procedures in stress urinary incontinence: a meta-analysis with direct and indirect comparison of randomized trials. BJU Int 106: 68–76 DOI: 10.1111/j.1464-410X.2009.09051.x [DOI] [PubMed] [Google Scholar]
- Miller J., Ashton-Miller J., DeLancey J. (1998) A pelvic muscle precontraction can reduce cough-related urine loss in selected women with mild SUI.J Am Geriatr Soc 46: 870–874 [DOI] [PubMed] [Google Scholar]
- Miller J., Sampselle C., Ashton-Miller J., Hong G., DeLancey J. (2008) Clarification and confirmation of the Knack maneuver: the effect of volitional pelvic floor muscle contraction to preempt expected stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct 19: 773–782 DOI: 10.1007/s00192-007-0525-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K., Schieman S., Ackerman T., Dzus H., Metcalfe J., Voaklander D. (2004) Assessing comfort, safety, and patient satisfaction with three commonly used penile compression devices. Urology 63: 150–154 [DOI] [PubMed] [Google Scholar]
- Nitti V., Dmochowski R., Herschorn S., Sand P., Thompson C., Nardo C., et al. for the EMBARK Study Group (2012) OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3 randomized placebo-controlled trial. J Urol, in press. DOI: 10.1016/j.juro.2012.12.022 [DOI] [PubMed] [Google Scholar]
- Nix D., Haugen V. (2010) Prevention and management of incontinence-associated dermatitis. Drugs Aging 27: 491–496 DOI: 10.2165/11315950-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Ostaszkiewicz J., Johnston L., Roe B. (2004) Timed voiding for the management of urinary incontinence in adults. Cochrane Database Syst Rev 1: CD002802 DOI: 10.1002/14651858.CD002802.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton P., Brooks A., Eriksen C., Frost S., Graham S., Greenman L., et al. (2006) A comparative study of two types of urinary sheath. Nurs Times 102: 36–41 [PubMed] [Google Scholar]
- Peters K., Carrico D., Perez-Marrero R., Khan A., Wooldridge L., Davis G., et al. (2010) Randomized trial of percutaneous tibial nerve stimulation versus Sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol 183: 1438–1443 DOI: 10.1016/j.juro.2009.12.036 [DOI] [PubMed] [Google Scholar]
- Phelan S., Kanaya A., Subak L., Hogan P., Espeland M., Wing R., et al. for the Look AHEAD Research Group (2012) Weight loss prevents urinary incontinence in women with type 2 diabetes: results from the Look AHEAD trial. J Urol 187: 939–944 DOI: 10.1016/j.juro.2011.10.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter H., Burgio K., Brubaker L., Nygaard I., Ye W., Weidner A., et al. for the Pelvic Floor Disorders Network (2010) Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol 115: 609–617 DOI: 10.1097/AOG.0b013e3181d055d4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson E., Gould J., Wald A. (2010) Urinary and fecal incontinence after bariatric surgery. Dig Dis Sci 55: 2606–2613 DOI: 10.1007/s10620-010-1190-9 [DOI] [PubMed] [Google Scholar]
- Rossouw J., Anderson G., Prentice R., LaCroix A., Kooperberg C., Stefanick M., et al. for the Writing Group for the Women’s Health Initiative Investigators (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288: 321–333 [DOI] [PubMed] [Google Scholar]
- Saint S., Kaufman S., Rogers M., Baker P., Ossenkop K., Lipsky B. (2006) Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc 54: 1055–1061 DOI: 10.1111/j.1532-5415.2006.00785.x [DOI] [PubMed] [Google Scholar]
- Shamliyan T., Kane R., Wyman J., Wilt T. (2008) Systematic review: randomized, controlled trials of nonsurgical treatments for urinary incontinence in women. Ann Intern Med 148: 459–473 [DOI] [PubMed] [Google Scholar]
- Sirls L., Foote J., Kaufman J., Lightner D., Miller J., Moseley W., et al. (2002) Long-term results of the FemSoft urethral insert for the management of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 13: 88–95; discussion 95. [DOI] [PubMed] [Google Scholar]
- Sivaslioglu A., Unlubilgin E., Aydogmus S., Keskin L., Dolen I. (2012) A prospective randomized controlled trial of the transobturator tape and tissue fixation mini-sling in patients with stress urinary incontinence: 5-year results. J Urol 188: 194–199 [DOI] [PubMed] [Google Scholar]
- Subak L., Richter H., Hunskaar S. (2009a) Obesity and urinary incontinence: epidemiology and clinical research update. J Urol 182(6 Suppl.): S2–S7 DOI: 10.1016/j.juro.2009.08.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subak L., Wing R., West D., Franklin F., Vittinghoff E., Creasman J., et al. for the PRIDE Investigators (2009b) Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med 360: 481–490 DOI: 10.1056/NEJMoa0806375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen T., Lagro-Janssen A. (2009) Sex differences in the use of absorbent (incontinence) pads in independently living elderly people: do men receive less care? Int J Clin Pract 63: 869–873 DOI: 10.1111/j.1742-1241.2008.01975.x [DOI] [PubMed] [Google Scholar]
- Trowbridge E., Fenner D. (2007) Practicalities and pitfalls of pessaries in older women. Clin Obstet Gynecol 50: 709–719 DOI: 10.1097/GRF.0b013e3180d0a4ce [DOI] [PubMed] [Google Scholar]
- Wallace S., Roe B., Williams K., Palmer M. (2004) Bladder training for urinary incontinence in adults. Cochrane Database Syst Rev 1: CD001308 DOI: 10.1002/14651858.CD001308.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. (2005) Selecting medications for the treatment of urinary incontinence. Am Fam Physician 71: 315–322 [PubMed] [Google Scholar]
- White W., Mobley J., III, Doggweiler R., Dobmeyer-Dittrich C., Klein F. (2009) Incidence and predictors of complications with sacral neuromodulation. Urology 73: 731–735 DOI: 10.1016/j.urology.2008.11.047 [DOI] [PubMed] [Google Scholar]
- Wilson L., Brown J., Shin G., Luc K., Subak L. (2001) Annual direct cost of urinary incontinence. Obstet Gynaecol 98: 398–406 [DOI] [PubMed] [Google Scholar]