Abstract
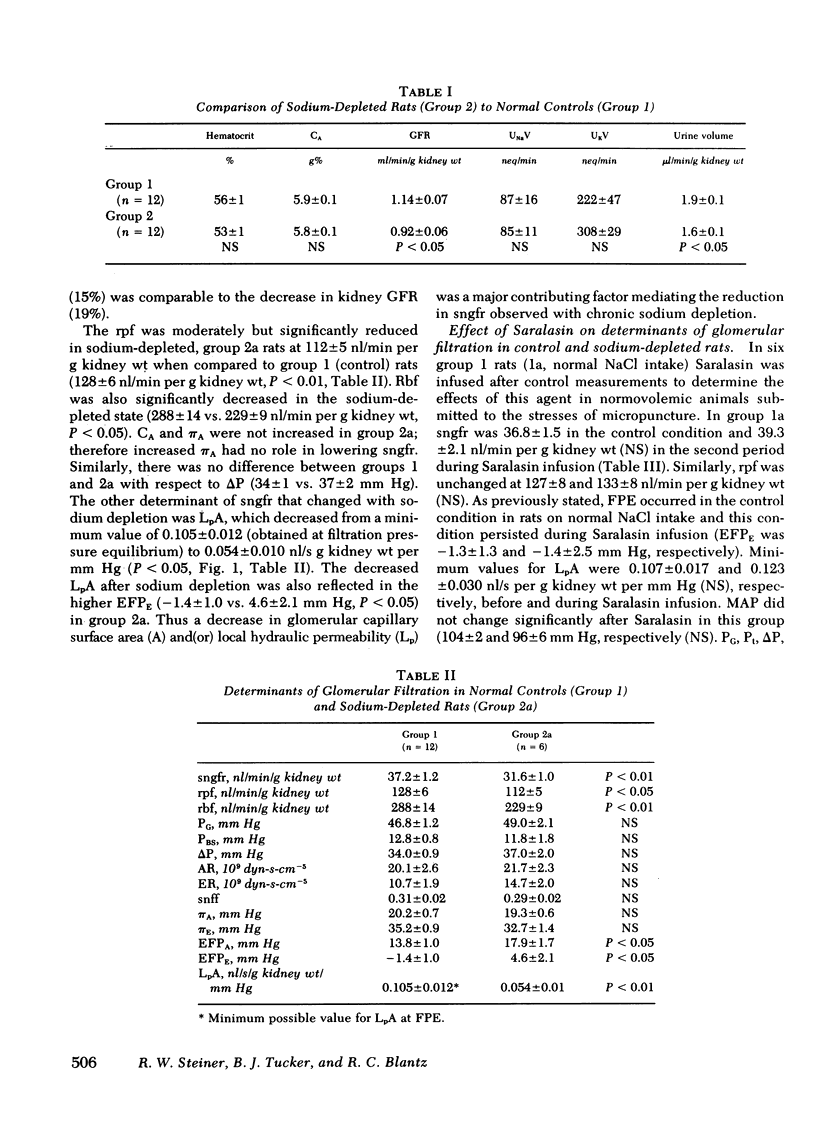
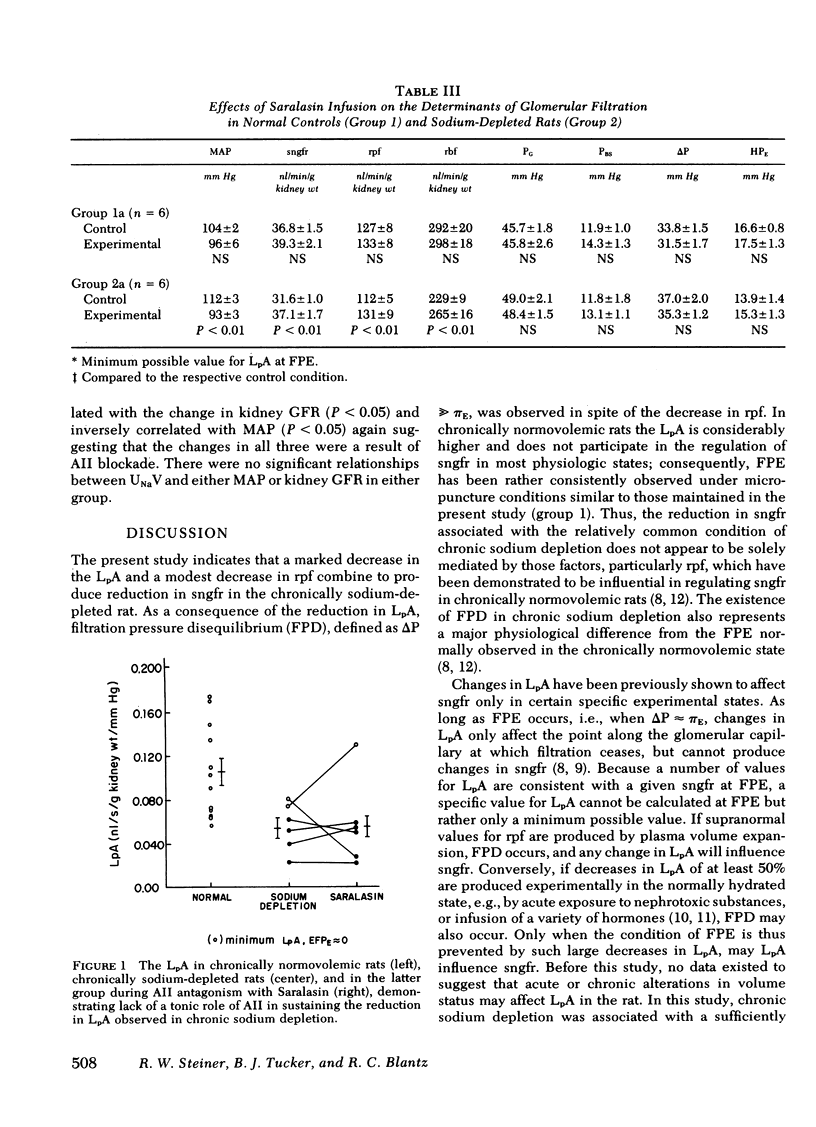
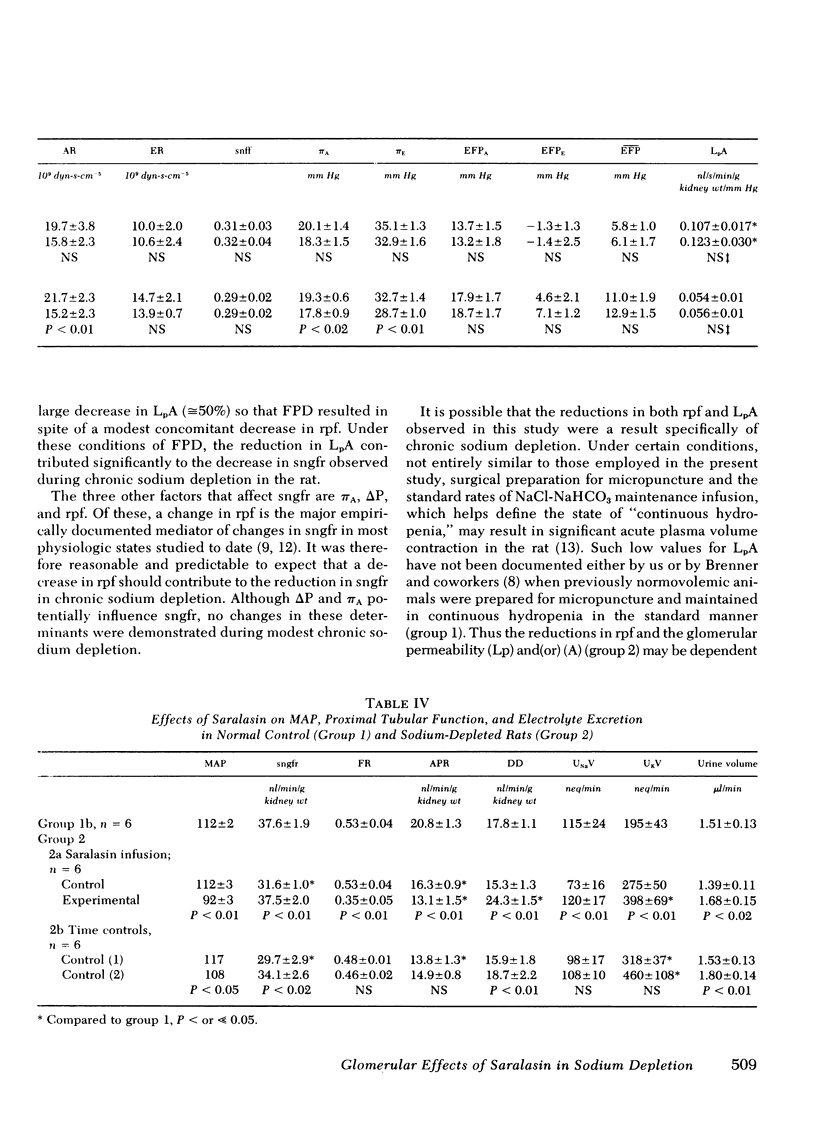
In chronic sodium depletion the glomerular filtration rate may be reduced, and alterations in proximal tubular function may contribute to the maintenance of antinatriuresis. Measurements were made by micropuncture technique in superficial nephrons of the Munich-Wistar rat of (a) the determinants of glomerular filtration rate, (b) peritubular capillary hydrostatic and oncotic pressure, and (c) proximal tubular fractional and absolute reabsorption in both a control group (group 1, n = 12) and a group of chronically sodium-depleted rats (group 2, n = 12). Single nephron filtration rate (sngfr) was 37.2±1.2 in group 1 and 31.6±1.0 nl/min/g kidney wt (P < 0.05) in group 2. Of the factors potentially responsible for the observed reduction in sngfr, there was no change in systemic oncotic pressure or the transglomerular hydrostatic pressure gradient. Sngfr was lower in group 2 because of both a reduced single nephron plasma flow (rpf) (128±6 vs. 112±5 nl/min per g kidney wt, P < 0.05) and additionally to a decrease in the glomerular permeability coefficient, LpA, from a minimum value of 0.105±0.012 in group 1 to 0.054±0.01 nl/s per g kidney wt per mm Hg (P < 0.01) after chronic sodium depletion. There was no difference in fractional proximal tubular reabsorption between group 1 and group 2. Absolute proximal reabsorption (APR) was reduced from 20.8±1.3 in group 1 to 16.3±0.9 nl/min per g kidney wt in group 2.
The role of angiotensin II (AII) in maintaining glomerular and proximal tubular adaptations to chronic sodium depletion was assessed in subsets of groups 1 and 2 by the infusion of the AII antagonist Saralasin at a rate of 1 μg/kg per min. In group 1 rats, Saralasin had no effect on sngfr, rpf, or LpA, because animals remained at filtration pressure equilibrium. In group 2 rats, AII blockade was associated with an increase in sngfr from 31.6±1.0 to 37.1±1.7 nl/min per g kidney wt (P < 0.01). Rpf increased during Saralasin infusion solely as a result of a decrease in afferent arteriolar resistance from 21.7±2.3 to 15.2±2.3 109 dyn-s-cm−5 (P < 0.01). Saralasin infusion did not affect the reduced LpA in group 2, as LpA remained 0.056±0.02 nl/s per g kidney wt per mm Hg and rats remained disequilibrated. In spite of the increase in sngfr in group 2, AII antagonism further decreased APR to 13.1±1.5 (P < 0.01). Distal delivery therefore, increased from a control value of 15.3±1.3 to 24.3±1.5 nl/min per g kidney wt (P < 0.01).
In conclusion, both a decrease in LpA and a reduction in rpf were major factors mediating the decrease in glomerular filtration rate observed in chronic sodium depletion. Saralasin infusion revealed a significant effect of AII on rpf and afferent arteriolar resistance in chronic sodium depletion, but no effect of AII on either efferent arteriolar resistance or the decrease in LpA could be demonstrated. Saralasin had no effect in rats that were not chronically sodium depleted. In group 2 rats AII antagonism reduced APR even though sngfr increased, suggesting an influence of AII on proximal reabsorption. The marked changes observed during Saralasin infusion in the chronically sodium-depleted rat reveal important modifying effects of endogenously generated AII on both the glomerulus and proximal tubule.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylis C., Deen W. M., Myers B. D., Brenner B. M. Effects of some vasodilator drugs on transcapillary fluid exchange in renal cortex. Am J Physiol. 1976 Apr;230(4):1148–1158. doi: 10.1152/ajplegacy.1976.230.4.1148. [DOI] [PubMed] [Google Scholar]
- Blantz R. C. Dynamics of glomerular ultrafiltration in the rat. Fed Proc. 1977 Nov;36(12):2602–2608. [PubMed] [Google Scholar]
- Blantz R. C. Effect of mannitol on glomerular ultrafiltration in the hydropenic rat. J Clin Invest. 1974 Nov;54(5):1135–1143. doi: 10.1172/JCI107857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blantz R. C., Konnen K. S., Tucker B. J. Angiotensin II effects upon the glomerular microcirculation and ultrafiltration coefficient of the rat. J Clin Invest. 1976 Feb;57(2):419–434. doi: 10.1172/JCI108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Falchuk K. H., Keimowitz R. I., Berliner R. W. The relationship between peritubular capillary protein concentration and fluid reabsorption by the renal proximal tubule. J Clin Invest. 1969 Aug;48(8):1519–1531. doi: 10.1172/JCI106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Daugharty T. M., Deen W. M., Robertson C. R. Dynamics of glomerular ultrafiltration in the rat. II. Plasma-flow dependence of GFR. Am J Physiol. 1972 Nov;223(5):1184–1190. doi: 10.1152/ajplegacy.1972.223.5.1184. [DOI] [PubMed] [Google Scholar]
- Hall J. E., Guyton A. C., Jackson T. E., Coleman T. G., Lohmeier T. E., Trippodo N. C. Control of glomerular filtration rate by renin-angiotensin system. Am J Physiol. 1977 Nov;233(5):F366–F372. doi: 10.1152/ajprenal.1977.233.5.F366. [DOI] [PubMed] [Google Scholar]
- Hall J. E., Guyton A. C., Trippodo N. C., Lohmeier T. E., McCaa R. E., Cowley A. W., Jr Intrarenal control of electrolyte excretion by angiotensin II. Am J Physiol. 1977 Jun;232(6):F538–F544. doi: 10.1152/ajprenal.1977.232.6.F538. [DOI] [PubMed] [Google Scholar]
- Harris P. J., Young J. A. Dose-dependent stimulation and inhibition of proximal tubular sodium reabsorption by angiotensin II in the rat kidney. Pflugers Arch. 1977 Jan 17;367(3):295–297. doi: 10.1007/BF00581370. [DOI] [PubMed] [Google Scholar]
- LANGFORD H. G. TUBULAR ACTION OF ANGIOTENSIN. Can Med Assoc J. 1964 Jan 25;90:332–333. [PMC free article] [PubMed] [Google Scholar]
- Lohmeier T. E., Cowley A. W., Jr, Trippodo N. C., Hall J. E., Guyton A. C. Effects of endogenous angiotensin II on renal sodium excretion and renal hemodynamics. Am J Physiol. 1977 Nov;233(5):F388–F395. doi: 10.1152/ajprenal.1977.233.5.F388. [DOI] [PubMed] [Google Scholar]
- Lowitz H. D., Stumpe K. O., Ochwadt B. Micropuncture study of the action of angiotensin-II on tubular sodium and water reabsorption in the rat. Nephron. 1969;6(3):173–187. doi: 10.1159/000179727. [DOI] [PubMed] [Google Scholar]
- Maddox D. A., Price D. C., Rector F. C., Jr Effects of surgery on plasma volume and salt and water excretion in rats. Am J Physiol. 1977 Dec;233(6):F600–F606. doi: 10.1152/ajprenal.1977.233.6.F600. [DOI] [PubMed] [Google Scholar]
- McGiff J. C., Crowshaw K., Terragno N. A., Lonigro A. J. Release of a prostaglandin-like substance into renal venous blood in response to angiotensin II. Circ Res. 1970 Jul;27(1 Suppl 1):121–130. [PubMed] [Google Scholar]
- Myers B. D., Deen W. M., Brenner B. M. Effects of norepinephrine and angiotensin II on the determinants of glomerular ultrafiltration and proximal tubule fluid reabsorption in the rat. Circ Res. 1975 Jul;37(1):101–110. doi: 10.1161/01.res.37.1.101. [DOI] [PubMed] [Google Scholar]
- Oster P., Hackenthal E., Hepp R. Radioimmunoassay of angiotensin II in rat plasma. Experientia. 1973 Mar 15;29(3):353–354. doi: 10.1007/BF01926526. [DOI] [PubMed] [Google Scholar]
- Robertson C. R., Deen W. M., Troy J. L., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. 3. Hemodynamics and autoregulation. Am J Physiol. 1972 Nov;223(5):1191–1200. doi: 10.1152/ajplegacy.1972.223.5.1191. [DOI] [PubMed] [Google Scholar]
- Stein J. H., Osgood R. W., Boonjarern S., Cox J. W., Ferris T. F. Segmental sodium reabsorption in rats with mild and severe volume depletion. Am J Physiol. 1974 Aug;227(2):351–359. doi: 10.1152/ajplegacy.1974.227.2.351. [DOI] [PubMed] [Google Scholar]
- Steven K. Effect of peritubular infusion of angiotensin II on rat proximal nephron function. Kidney Int. 1974 Aug;6(2):73–80. doi: 10.1038/ki.1974.82. [DOI] [PubMed] [Google Scholar]
- Tucker B. J., Blantz R. C. An analysis of the determinants of nephron filtration rate. Am J Physiol. 1977 Jun;232(6):F477–F483. doi: 10.1152/ajprenal.1977.232.6.F477. [DOI] [PubMed] [Google Scholar]
- Tucker B. J., Blantz R. C. Factors determining superficial nephron filtration in the mature, growing rat. Am J Physiol. 1977 Feb;232(2):F97–104. doi: 10.1152/ajprenal.1977.232.2.F97. [DOI] [PubMed] [Google Scholar]
- Weiner M. W., Weinman E. J., Kashgarian M., Hayslett J. P. Accelerated reabsorption in the proximal tubule produced by volume depletion. J Clin Invest. 1971 Jul;50(7):1379–1385. doi: 10.1172/JCI106620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusman R. M., Keiser H. R. Prostaglandin biosynthesis by rabbit renomedullary interstitial cells in tissue culture. Stimulation by angiotensin II, bradykinin, and arginine vasopressin. J Clin Invest. 1977 Jul;60(1):215–223. doi: 10.1172/JCI108758. [DOI] [PMC free article] [PubMed] [Google Scholar]



