Abstract
Tendon consists of highly ordered type I collagen molecules that are grouped together to form subunits of increasing diameter. At each hierarchical level, the type I collagen is interspersed with a predominantly non-collagenous matrix (NCM) (Connect. Tissue Res., 6, 1978, 11). Whilst many studies have investigated the structure, organization and function of the collagenous matrix within tendon, relatively few have studied the non-collagenous components. However, there is a growing body of research suggesting the NCM plays an important role within tendon; adaptations to this matrix may confer the specific properties required by tendons with different functions. Furthermore, age-related alterations to non-collagenous proteins have been identified, which may affect tendon resistance to injury. This review focuses on the NCM within the tensional region of developing and mature tendon, discussing the current knowledge and identifying areas that require further study to fully understand structure–function relationships within tendon. This information will aid in the development of appropriate techniques for tendon injury prevention and treatment.
Keywords: ageing, glycoprotein, interfascicular matrix, proteoglycan, structure-function
Tendon composition
Tendon matrix is predominantly composed of collagen, which makes up 60–85% of the tendon dry weight (Kjaer 2004). Approximately 95% of this is type I collagen, with small amounts of collagen types III, V, XII and XIV (Riley et al. 1994b; Birch et al. 1999; Riley 2004; Banos et al. 2008). The collagen-rich, hierarchically arranged structure results in a tissue with high tensile strength (Figure 1). Each level of the hierarchy is interspersed with a small amount of non-collagenous matrix (NCM) (Kastelic et al. 1978). The role of collagen within tendon is well characterized and has been the subject of many reviews (Kannus 2000; Silver et al. 2003; Franchi et al. 2008; Starborg et al. 2008; Kjaer et al. 2009) and as such is not the main focus of this article. By contrast, the NCM is less well defined. This matrix is primarily made up of glycoproteins (Yoon & Halper 2005), which comprise a protein core covalently linked to a carbohydrate, ranging in size from a monosaccharide to large polysaccharide chains. Glycoproteins include the proteoglycans and other molecules such as collagen oligomeric matrix protein (COMP), lubricin and tenascin-C. The NCM also includes the fibrous protein elastin that forms part of elastic fibre.
Figure 1.
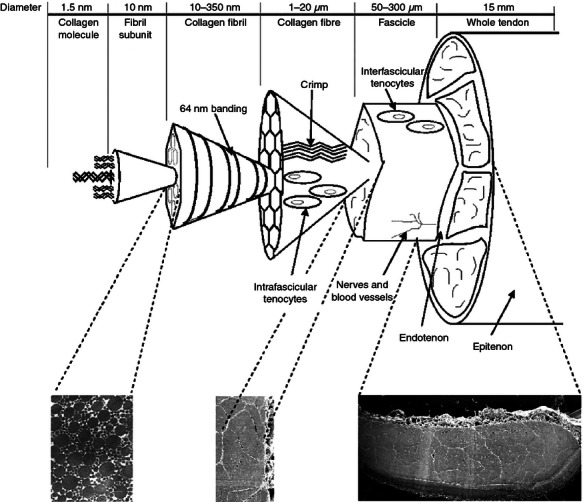
Schematic showing the hierarchical structure of tendon, in which collagen molecules assemble to form subunits of increasing diameter. At each level, the collagen is interspersed with a small amount of non-collagenous matrix. Adapted from Thorpe et al. (2010a) with permission from Wiley.
Proteoglycans and glycoproteins in tendon
Proteoglycans are the most abundant class of glycoproteins within tendon, consisting of one or several (often many) polysaccharide chains attached via 0-linkage to a core protein. Each polysaccharide chain consists of one branch of repeating disaccharide units, which can be over 100 units long, and may be sulphated in various positions and quantities. These chains are commonly referred to as glycosaminoglycan (GAG) side chains (Sharon 1986). The majority of the proteoglycans found within tendon are the small leucine-rich proteoglycans (SLRPs), with smaller amounts of the large proteoglycans aggrecan and versican (Rees et al. 2000; Yoon & Halper 2005). Decorin is the most abundant SLRP, accounting for approximately 80% of the total proteoglycan content (Samiric et al. 2004), with lower levels of biglycan, fibromodulin and lumican. Other glycoproteins identified within tendon include COMP (Smith et al. 1997), lubricin (Rees et al. 2002), tenascin-C (Riley et al. 1996) and tenomodulin (Brandau et al. 2001), whilst components of the elastic fibre are also present in varying quantities (Silver et al. 2003; Korol et al. 2007).
Whilst these non-collagenous proteins are known to be present within tendon, there is relatively little understanding of the precise location or function of specific proteins within the tendon matrix, although it has been established that the profile of this matrix differs between compressive and tensile regions within tendon. Regions that experience compressive loads, for example, where the tendon wraps round a joint, have a relative abundance of non-collagenous proteins. It is accepted that the high concentration of proteins such as aggrecan in this region increases the water content, thereby providing increased stiffness and resistance to compression (Rees et al. 2000; Jones & Riley 2005; Yoon & Halper 2005). By contrast, the tensile region of tendon has a relatively sparse NCM, with the role of specific proteins in this region yet to be established.
In addition to differences in NCM composition between compressive and tensile regions, the NCM varies between hierarchical levels. It is thought that there is little or no NCM at the lowest levels of the tendon hierarchy (i.e. between collagen molecules and subfibrils), and there is evidence to suggest that the NCM becomes more complex as the collagen subunits become larger. The proteoglycans decorin and biglycan are known to be present between fibrils, fibres and fascicles (Scott 1996; Kim et al. 2010), whereas COMP is thought to be localized only between fibrils and fibres (Sodersten et al. 2005, 2013). By contrast, components such as lubricin and elastin have only been identified between fascicles (Sun et al. 2006; Korol et al. 2007). The distribution of other NCM components within tendon remains to be fully determined.
Further, in addition to spatial variations, the NCM differs between tendon types, with greater content of non-collagenous proteins in tendons that experience high strains (Smith et al. 2002a; Batson et al. 2003; Jarvinen et al. 2003).
Finally, there are also temporal differences in non-collagenous protein content, with changes occurring to this matrix during development, maturation and ageing. Indeed, recent research suggests distinct roles for individual non-collagenous proteins in developing and mature tendons.
Distribution of non-collagenous matrix throughout tendon hierarchy
Interfibrillar matrix
The proteins comprising the interfibrillar matrix are mainly the SLRPs, which are able to bind to collagen fibrils at specific sites. These proteoglycans consist of a horseshoe-shaped core protein, which is able to bind non-covalently to a single collagen triple helix at a specific amino acid sequence in the gap region (D-band) of the fibril, such that molecules bind approximately every 68 nm along the collagen fibril (Scott 1996; Weber et al. 1996; Sweeney et al. 2008). Attached to the core protein is one or several GAG side chains, depending on the type of proteoglycan. Decorin has a single chondroitin or dermatan sulphate side chain, which binds to one edge of the core protein such that the GAG chain can align parallel or perpendicular to the axis of the collagen fibril (Weber et al. 1996). The side chain is able to interact with the side chain of another decorin molecule bound to a collagen molecule within an adjacent fibril, forming an interfibrillar bridge, shown schematically in Figure 2 (Scott 1996; Vesentini et al. 2005). Biglycan has one or two chondroitin or dermatan sulphate side chains and is thought to share a collagen binding site with decorin (Schönherr et al. 1995). Lumican and fibromodulin have 2 or 4 keratan side chains respectively (Yoon & Halper 2005) and share a binding site on type I collagen molecules, distinct from the decorin binding site (Yoon & Halper 2005; Kalamajski & Oldberg 2009).
Figure 2.
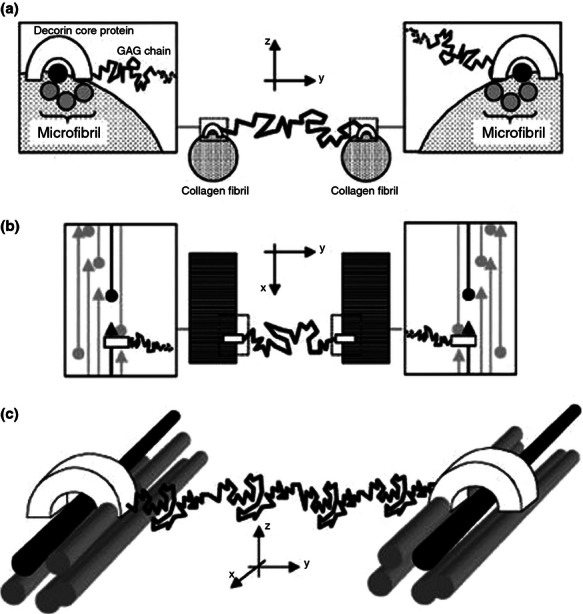
Schematic showing interaction between two collagen molecules from adjacent fibrils via decorin core proteins (white horseshoe shaped) and their glycosaminoglycan side chains (black lines). (a) Transverse view of collagen fibrils showing connections formed by decorin. (b) Longitudinal view of collagen fibrils showing decorin binding in the gap region of the collagen fibrils. (c) 3D representation of the decorin protein collagen molecule complex within a microfibril. Reprinted from Vesentini et al. (2005) with permission from Elsevier.
Of the other known interfibrillar matrix components, COMP is the most abundant glycoprotein present in tendon (Smith et al. 1997). It is a large pentameric protein, consisting of 5 subunits arranged as arms around a central cylinder (DiCesare et al. 1994). Each subunit is able to bind to type I collagen at four sites on the collagen molecule (Rosenberg et al. 1998), and therefore, one COMP molecule can provide a link between 5 adjacent collagen molecules. Other glycoproteins present within the interfibrillar matrix include tenascin-C (Riley et al. 1996), which is comprised of six arms, bound together via their N-terminal interchain cross-linking domains (Jarvinen et al. 2000), and the transmembrane protein tenomodulin, which is also found in the interfibrillar matrix, where it may provide a link between the cells and surrounding matrix (Docheva et al. 2005).
Proteins of the interfascicular matrix
Previously, little attention has been given to the interfascicular matrix (IFM), but recent research suggests it has a specialized structure, with some proteins only present at this level. Proteins identified within the IFM include elastic fibres (Korol et al. 2007), lubricin (Sun et al. 2006), GAGs (Fallon et al. 2002), decorin (Kim et al. 2010), versican (Ritty et al. 2003) and collagen types III (Sodersten et al. 2013) and VI (Ritty et al. 2003), with an absence of COMP at this structural level (Sodersten et al. 2013).
The elastic fibre complex consists of a central core of elastin, surrounded by sheath of polymers of fibrillins 1 and 2, also known as microfibrils (Kielty et al. 2002). Many other molecules have been shown to associate with the elastic fibre, including decorin and biglycan (Baccarani-Contri et al. 1990). As its name suggests, elastin is a highly elastic protein that is able to extend by more than 100% of its original length (Aaron & Gosline 1981). It is formed from tropoelastin monomers that are coiled along their axis. This coil provides high elasticity with almost no hysteresis loss (Baldock et al. 2011). Further, elastin is highly fatigue resistant (Gosline et al. 2002; Lillie & Gosline 2002) and has the capacity for energy storage (Gosline et al. 2002). However, the precise role it plays within tendon is yet to be determined.
Elastin is reported to be present in tendon at concentrations in the range of 1–10% tendon dry weight (Silver et al. 2003; Korol et al. 2007). In the canine cruciate ligament, elastin has been shown to be situated between fascicles (Smith et al. 2011). Similarly, it has been shown that rat tail tendon fascicles are surrounded by a thin sheath of elastin (Korol et al. 2007). As well as colocalizing with elastin, fibrillins are also found independent of the elastic fibre; Ritty et al. (2003) identified fibrillin-2 tubules, with a uniform distribution in the IFM of the canine flexor digitorum profundus tendon. The role of these tubules is yet to be established, but it has been suggested that they may provide a framework to maintain cell linearity and enable cell migration (Ritty et al. 2003).
Another recently identified component of tendon matrix is lubricin. Lubricin is a large mucinous glycoprotein, also known as superficial zone protein or proteoglycan 4, and was originally identified within cartilage, where it acts as a boundary lubricant (Swann et al. 1981; Flannery et al. 1999). In tendon, lubricin was first shown to be present on the tendon surface, with greater levels in the compressive than tensional regions (Rees et al. 2002). It has been demonstrated that surface lubricin modulates tendon gliding resistance, allowing tendons to glide past one another or around joints (Taguchi et al. 2008, 2009). More recent work has shown that lubricin is also localized to the IFM within canine flexor tendon (Sun et al. 2008), caprine infraspinatus tendon (Funakoshi et al. 2008) and human supraspinatus tendon (Funakoshi et al. 2009).
The large proteoglycan versican has also been shown to be localized to the IFM and is found in greatest abundance in the pericellular region (Ritty et al. 2003). Versican is able to bind hyaluronic acid and the GAG chondroitin sulphate (Wight 2002) and has also been shown to interact with fibrillin-containing microfibrils in the elastic fibre (Isogai et al. 2002). These interactions suggest versican may contribute to the structural properties of the matrix by linking microfibrils to matrix components such as proteoglycans (Isogai et al. 2002), yet the precise role of versican within tendon remains undetermined.
Although the IFM mainly consists of non-collagenous components, collagen types III and VI are also present in low concentrations within this matrix. Whilst it is known that collagen type III content is increased in injured tendon (Birch et al. 1998; Sodersten et al. 2013), the function of this fibrillar collagen within the healthy IFM is yet to be established. Type VI collagen is non-fibrillar and is often found closely associated with cells (Senga et al. 1995; Ritty et al. 2003). Type VI collagen is able to bind to macromolecules including decorin and biglycan (Wiberg et al. 2001); these interactions may provide an anchoring network for cells within the IFM (Ritty et al. 2003).
The role of the non-collagenous matrix during development and maturation
Proteoglycans in developing tendon
The majority of studies on the NCM have focused on the role of NCM proteins during tendon development. This work has established that several non-collagenous proteins play an important role in modulating collagen fibrillogenesis. As decorin is the most abundant SLRP in the tensile region of tendon, this proteoglycan has been the most investigated. Decorin is involved in the correct alignment and stabilization of fibrils during fibrillogenesis (Scott 1996); it has been shown to inhibit lateral fusion of collagen fibrils; hence, high concentrations of decorin result in the formation of thinner collagen fibrils (Birk et al. 1995). Decorin-null mice exhibit severe tendon defects, characterized by the formation of highly disorganized collagen fibrils and localized areas of calcification within the tendon (Kilts et al. 2009). Decorin may also regulate the formation of enzymatic cross-links; the decorin binding site is in the C-terminal region of the collagen fibril and is in close proximity to the predominant site for intermolecular cross-link formation (Sweeney et al. 2008). These data suggest that decorin plays a crucial role in collagen fibrillogenesis in immature tendon.
Biglycan, like decorin, is able to bind to fibrillar collagen and has also been implicated in regulation of fibrillogenesis (Yoon & Halper 2005). However, the nature of biglycan–collagen interactions has not been fully determined (Zhang et al. 2005). Biglycan knockout mice exhibit similar defects to decorin knockouts, with thin, disorganized collagen fibrils and ectopic ossification, although they are not as severe in the biglycan knockout (Corsi et al. 2002; Kilts et al. 2009). Indeed, data indicate that decorin and biglycan have some similar functions as biglycan synthesis is upregulated in decorin-null mice, suggesting the mice may be able to partially compensate for the absence of decorin (Zhang et al. 2006).
Lumican and fibromodulin also interact with fibrillar collagens. These proteoglycans are differentially expressed during tendon development (Ezura et al. 2000), and it has been proposed that they are involved in the regulation of fibril fusion; in vitro studies have shown that they are able to inhibit fibril fusion (Hedbom & Heinegard 1989). Indeed, lumican- and fibromodulin-deficient mice exhibit tendon defects characterized by larger, irregularly shaped fibrils, although these are not as severe as those seen in decorin or biglycan knockout models (Ezura et al. 2000).
Glycoproteins in developing tendon
Collagen oligomeric matrix protein interacts with collagen, cells and other matrix proteins (Smith et al. 2002b), and it is also thought to play a role in fibrillogenesis (Sodersten et al. 2005). In skeletally immature horses, the concentration of COMP is correlated with ultimate tensile stress and elastic modulus in the superficial digital flexor tendon (SDFT), suggesting that it plays an important role during tendon development (Smith et al. 2002b). In addition, mutations in the COMP gene are associated with pseudoachondroplasia, a disease characterized by joint laxity and bone abnormalities (Maddox et al. 2000). The mechanisms by which COMP mutations induce this disease are unknown, but it has been shown that COMP mutations affect proteoglycan synthesis in cartilage (Kwak et al. 2009). Knockout studies provide conflicting data, with one study unable to identify any tendon or musculoskeletal system abnormalities in COMP-null mice (Svensson et al. 2002). However, a more recent study has reported that Achilles tendons from COMP-null mice have a smaller cross section, larger fibril diameter and contain a greater number of bifurcated or fused fibrils than wild-type controls (Pirog et al. 2010), suggesting that this protein is also involved in collagen fibrillogenesis. Indeed, the structure of COMP, enabling it to bind 5 collagen molecules simultaneously, has been shown to promote collagen–collagen interactions and the formation of microfibrils (Halasz et al. 2007). Combined, these data suggest that COMP may work in conjunction with the SLRPs during maturation to ensure proper collagen fibrillogenesis.
Tenomodulin is also found in relatively high concentrations in immature tendon and has been shown to be essential for normal tenocyte proliferation. Tenomodulin is also involved in collagen fibril alignment and organization (Docheva et al. 2005), with tenomodulin-null mice exhibiting uneven fibril surfaces within their tendons (Docheva et al. 2005). Another extracellular matrix glycoprotein, tenascin-C, shows high expression in immature tendon and is also expressed in disease states and healing tissue (Riley et al. 1996; Jarvinen et al. 2000), but the roles of tenascin-C during tendon development and healing are yet to be defined.
Overall, these data indicate that a variety of tendon NCM proteins are required for proper collagen fibrillogenesis during development. Several of these proteins are able to regulate fibril diameter, alignment, stability and organization. This allows precise control of tendon growth and will ensure that appropriate mechanical properties are achieved.
The role of non-collagenous matrix in mature tendon
Whilst tendon NCM proteins clearly have an important role during growth and development, their function in mature tendon is less well understood. With maturation, the levels of many NCM proteins tend to reduce (Jarvinen et al. 2000; Smith et al. 2002b; Shukunami et al. 2006; Zhang et al. 2006), and their roles become less well defined.
Proteoglycans in mature tendon
Several studies have investigated the role of SLRPs, particularly decorin, in mature tendon, and have suggested that this proteoglycan may contribute directly to tendon mechanical properties by aiding in the transfer of strain between discontinuous collagen fibrils via the interfibrillar bridges formed by the binding of one decorin side chain to another (Figure 2.). Individually, these bonds are weak (Vesentini et al. 2005), but combined, they may reach large enough magnitudes to transfer force between fibres. In support of this, computational simulations have shown that modelling collagen as discontinuous fibres, between which stress is transferred by decorin molecules and their GAG side chains, results in similar values for elastic modulus and ultimate stress as those recorded in mature tendon (Redaelli et al. 2003). However, there is controversy regarding the strength of the decorin protein and the interactions of this protein with collagen and other decorin molecule, whereby it has been postulated that the bonds formed would be too weak to contribute significantly to strain transfer (Provenzano & Vanderby 2006). Indeed, it has not been established if the discontinuous collagen fibril model is accurate or if fibrils span the length of the tendon, fusing to act as functionally continuous units (Provenzano & Vanderby 2006). It is clear that fibrils within immature tendon are discontinuous (Birk et al. 1995), and some tendons, including the equine SDFT and human Achilles, vary in cross-sectional area along their length (Birch et al. 2002), suggesting that not all fibrils run the entire length of the tendon. As such, these combined findings do suggest that fibrils within tendon are discontinuous, but do not confirm any role of decorin in force transfer between adjacent fascicles.
Indeed, the results of several enzymatic depletion studies have shown few alterations in tendon mechanical properties. In studies where chondroitinase has been used to remove GAG chains, in rat tail tendon fascicles (Fessel & Snedeker 2009, 2011) or human patellar tendon fascicles (Svensson et al. 2011), no changes in the failure properties were observed. With regard to gene knockout studies in mice, analysis of tendon mechanical properties in decorin-null mice did not identify differences in the maximum stress or modulus of tail tendon fascicles or flexor digitorum longus (FDL) tendons when compared with wild-type controls (Robinson et al. 2005).
Combined, these studies suggest that decorin (and other tendon proteoglycans) is unlikely to contribute directly to tendon ultimate properties. However, it has been reported that patellar tendons from decorin knockout mice have higher levels of stress relaxation when compared with controls (Robinson et al. 2005), whilst patellar tendons from decorin heterozygotes exhibit increased dynamic modulus (Dourte et al. 2012). A very recent study has shown that GAG-depleted bovine extensor fascicles exhibit more stress relaxation and a greater reduction in failure stress after the application of static load (Legerlotz et al. 2013). In addition, tail tendon fascicles from decorin-deficient mice have been shown to have reduced sensitivity to the rate of applied strain (Robinson et al. 2004). These data suggest that proteoglycans may impart viscoelasticity without dramatically altering tendon material properties. It is well established that tendon extension occurs predominantly by sliding between adjacent fibres and fibrils, rather than by fibre/fibril extension (Arnoczky et al. 2002; Screen et al. 2004; Cheng & Screen 2007; Snedeker et al. 2009a,b; Goulam Houssen et al. 2011). It has been shown that levels of fibre sliding are greater in GAG-depleted rat tail fascicles compared with controls (Screen et al. 2005), and it has additionally been demonstrated that GAG-depleted murine Achilles tendons exhibit increased fibril sliding (Rigozzi et al. 2013). Treating rat tail fascicles with a decorin binding inhibitor has been shown to result in both increased fibre sliding (Wood et al. 2003) and tendon length, but does not result in alterations in tendon ultimate properties (Esther et al. 2008). These results suggest that decorin and potentially other SLRPs play an important role in modulating fibre sliding, a mechanism that results in the characteristic viscoelastic response observed in tendons. Alterations in viscoelastic properties are more likely to impact upon tendon fatigue properties rather than failure properties, therefore supporting previous studies that have shown no alterations in failure properties of GAG-depleted tendons (Fessel & Snedeker 2009, 2011; Svensson et al. 2011) but suggesting a change that may be of greater physiological importance.
Other non-collagenous proteins in mature tendon
Other glycoproteins may also influence sliding behaviour within mature tendon but their functions remain to be fully determined. Whilst COMP is the most abundant glycoprotein found within tendon (Smith et al. 1997), COMP levels show no correlation with the failure properties of SDFTs from mature horses (Smith et al. 2002b). However, Pirog et al. (2010) have shown that Achilles tendons from COMP-null mice have a higher failure strain and exhibit greater stress relaxation during cyclic loading than tendons from wild-type controls. These data indicate that COMP may also influence fibre sliding within tendon.
At the fascicular level, the localization of lubricin to the interfascicular space has lead to the hypothesis that this glycoprotein not only promotes tendon surface gliding, but also enables sliding between adjacent fascicles (Funakoshi et al. 2008). In support of this, mouse knockout studies have shown that tail tendon fascicle gliding resistance is greater in lubricin knockout mice than in wild-type mice (Kohrs et al. 2011). Elastin is also located between fascicles, and whilst no studies have directly determined the effect of elastin on tendon mechanical properties, it has been postulated that it contributes to tendon elasticity by being stretched in the toe region of the stress strain curve, before the collagen fibrils are loaded (Gosline et al. 2002). This is supported by histochemical studies that show the greatest distribution of elastic fibres occurs in the region of canine flexor digitorum profundus tendon that experiences the greatest strains (Ritty et al. 2002). Indeed, in canine cruciate ligament, it has been suggested that elastic fibres form the complex interconnections observed between fascicles, providing a passive recoil system to enable recovery after deformation (Smith et al. 2011). Further, similar interfascicular connections and distribution of elastin have recently been observed in the equine SDFT (Figure 3.). Lubricin and elastin may therefore work in combination to enable sliding and recoil between adjacent fascicles. However, little is known about the precise distribution and organization of these proteins, and their specific role in modulating tendon mechanical properties is yet to be determined.
Figure 3.
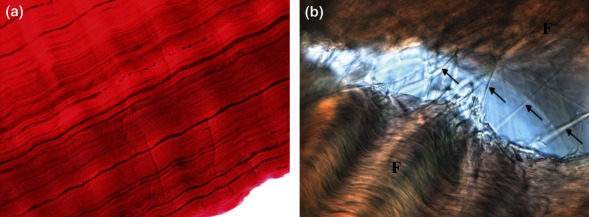
Images showing distribution of elastin and interfascicular connections in the equine superficial digital flexor tendon (SDFT). (a) Longitudinally sectioned SDFT stained with Miller's stain showing distribution of elastic fibres (black lines) between collagen bundles (E.G. Laird, unpublished data). (b) Nomarski differential interference contrast optical microscopy image of longitudinally cryosectioned SDFT showing interconnecting fibres (indicated by arrows) between adjacent fascicles (F) that have been teased apart (K.D. Smith, unpublished data).
In addition to ensuring correct tendon development, it is clear from these studies that NCM proteins also play an important role within mature tendon by modulating fibre and fascicle sliding and therefore influencing tendon viscoelastic properties. Absence or modulation of these molecules appears to result in excessive fibre sliding, which is likely to decrease the mechanical integrity of the tendon tissue, and result in increased risk of injury.
Differences in the non-collagenous matrix between tendon types
There is recent evidence showing that the structure and composition of the NCM differ between tendons with different functions, that is, those that are purely positional versus tendons with an additional function as energy stores. Examples of energy-storing tendons include the human Achilles tendon (Lichtwark & Wilson 2005) and the equine SDFT (Biewener 1998). These tendons must be able to extend and recoil efficiently and rapidly, with minimal energy loss, to maximize their energy-storing capacity (Lichtwark & Wilson 2007; Roberts & Azizi 2011). However, the high repetitive stresses and strains these tendons are exposed to make them highly prone to overstrain injury (Knobloch et al. 2008; Ely et al. 2009; Hess 2010). The equine SDFT is perhaps one of the most extreme examples of an energy-storing tendon; it experiences strains of up to 16% during gallop (Stephens et al. 1989) and may be exposed to strain rates as high as 200%/s (Herrick et al. 1978). There is little understanding of how these tendons are optimized for function; however, recent research suggests that the NCM is critical in facilitating these extremely high levels of extension and recoil in energy-storing tendons by enabling sliding between adjacent fascicles (Thorpe et al. 2012, 2013).
Several studies have compared and contrasted the composition and turnover of the NCM within the high-strain energy-storing equine SDFT and the low-strain positional common digital extensor tendon (CDET). It is well established that the SDFT has higher GAG levels than the CDET (Batson et al. 2003; Birch 2007), indicating a greater proteoglycan content. This is supported by our recent work that shows greater mRNA expression of a range of proteoglycans (aggrecan, biglycan, decorin, fibromodulin, lumican) in the SDFT than in the CDET (Thorpe 2010). It has also been shown previously that the half-life of the NCM is lower in the SDFT than in the CDET, demonstrating that turnover of this matrix occurs more rapidly in the SDFT (Thorpe et al. 2010b). In addition, gene expression data show greater expression of the stromelysins MMP-3 and MMP-10 in the SDFT when compared with the CDET (Thorpe 2010), as well as greater amounts of the pro- and active forms of MMP-3 at the protein level, as determined by casein zymography (Thorpe 2010). The stromelysin family of MMPs are able to degrade a variety of matrix molecules, including collagen type III, proteoglycans and elastin (Woessner 1991); these data therefore indicate greater metabolism of non-collagenous proteins in the energy-storing SDFT. There is further evidence showing a relatively high rate of proteoglycan turnover in flexor tendons from other species, with degradation products of biglycan and decorin identified within the bovine deep digital flexor tendon (Rees et al. 2000; Samiric et al. 2004). This relatively high rate of NCM turnover in energy-storing tendons suggests that these proteins have a particularly important role to play in extension and recoil.
In addition to greater levels of proteoglycans in the equine SDFT, it has also been shown that COMP is present in greater abundance in this energy-storing tendon than in the positional CDET (Smith et al. 2002a). A similar relationship has been observed in human tendon, with higher COMP levels in the Achilles compared with the positional anterior tibialis tendon (Smith et al. 2002a).
These results show differences in NCM content and rate of turnover between tendons with different functions. The differential expression and turnover of non-collagenous proteins may affect tendon properties via several pathways. Greater levels of SLRPs will influence regulation of collagen fibrillogenesis in developing tendon and so are likely to be responsible for the lower mass average collagen fibril diameter observed in the SDFT compared with the CDET (Birch 2007). In addition, levels of sliding throughout the hierarchy will presumably be influenced by the composition of the NCM. This is supported by several studies showing that alterations in tendon non-collagenous proteins influence mechanical properties in a tendon specific manner. Enzymatic depletion and mice knockout studies have illustrated that the effect of decorin on tendon viscoelastic properties is specific to both tendon type and region (Robinson et al. 2004, 2005; Rigozzi et al. 2009). In addition, it has been illustrated that the effect of aggrecan content on mechanical properties may also be tendon specific; accumulation of aggrecan within the tensile region of the mouse FDL tendon in an ADAMTS-5 knockout model is associated with increased cross-sectional area and decreased modulus, whilst in the Achilles tendon, the opposite effect is observed, with an increase in modulus (Wang et al. 2012). This could be attributed to differences in structure between the tendons; the murine FDL is a single fascicle tendon, whereas the Achilles has a multifascicle structure (Wang et al. 2012). These data support the hypothesis that the function of non-collagenous proteins may not only be specific to tendon type and region, but also show specificity at a particular hierarchical level.
All these data indicate that the NCM plays a more important role in energy-storing tendons than in positional tendons. Indeed, we have very recent data that directly implicate the non-collagenous IFM in determining whole tendon mechanics. We have shown that fascicles from the SDFT have a lower failure strain than the whole tendon, with fascicles failing at strains below even the working range of the SDFT (Thorpe et al. 2012). However, we have established that there is a high capacity for sliding between adjacent fascicles and that the degree of sliding shows a positive correlation with whole tendon failure strain. By contrast, we observed little interfascicular sliding in the CDET, with small differences between tendon and fascicle failure strain, and no relationship between degree of sliding and tendon strain at failure (Thorpe et al. 2012). It has also been shown that there is the capacity for interfascicular sliding in the human Achilles tendon (Haraldsson et al. 2008) and bovine deep digital flexor tendon (Purslow 2009), suggesting a similar mechanism for achieving high extension in these tendons. These data suggest that the non-collagenous IFM is specialized within energy-storing tendons to allow the greater extensions required by this tendon type whilst protecting the fascicles from damage. Whilst it has been shown that the area occupied by the IFM is greater in the equine SDFT than in the CDET (Thorpe et al. 2012), little is known about IFM structure and composition, and to the authors' knowledge, no studies have investigated IFM organization in functionally distinct tendons. Therefore, this represents an important area for future research.
Age-related alterations in the non-collagenous matrix
It is well established that the risk of injury to energy-storing tendons is increased in aged individuals (Kasashima et al. 2004; Perkins et al. 2005; Knobloch et al. 2008; Hess 2010). However, the majority of these studies have focused on the alterations to the collagenous matrix in aged tendons and relatively few studies have investigated age-related changes that occur to the tendon matrix. A few studies have documented changes that occur to the NCM with ageing, demonstrating age-related alterations in a variety of matrix proteins across several tendon types. The GAG content of rat patellar tendon has been shown to decrease with increasing age (Vailas et al. 1985), and GAG levels are also diminished in aged human supraspinatus tendon, but show no age-related decline in the common biceps tendon (Riley et al. 1994a). Collagen oligomeric matrix protein levels also show a tendon- and region-specific response to increasing age, with a decrease in COMP levels in the injury-prone tensional region of the equine SDFT, but no change in levels within the compressed region of this tendon or in the CDET (Smith et al. 1997, 2002b). In addition, it has been demonstrated that aged rat tail tendon has an increased elastin content, which is accompanied by decreased elastic modulus and failure stress (Vogel 1980). Whilst few studies have investigated changes in the levels of specific proteoglycans within aged tendon, it has been shown that a truncated form of decorin is present within aged skin (Carrino et al. 2000). If a similar accumulation occurs in aged tendon, it could result in altered collagen–proteoglycan interactions. This small body of research indicates that ageing results in alterations to tendon non-collagenous protein content and structure, which appears to be specific to both tendon type and region.
If the relationship between these compositional changes and mechanical properties is to be understood, we need to characterize how these changes influence tendon extension mechanisms. Indeed, our recent work demonstrates that the non-collagenous IFM shows age-related alterations in mechanical properties that are specific to tendon type. The stiffness of the IFM is increased in SDFTs from old horses; however, the IFM in the CDET does not show any age-related changes in mechanical properties (Thorpe et al. 2013). These findings suggest that the IFM is compromised in aged energy-storing SDFTs, resulting in reduced capacity for interfascicular sliding. A reduction in sliding between adjacent fascicles may result in a decrease in the ability of the tendon to respond to repetitive loading, potentially contributing to the increased risk of fatigue-induced injury with ageing. Whilst our data show that the area occupied by the IFM decreases with increasing age in the SDFT (Thorpe et al. 2013), the specific changes that occur to particular IFM components in aged tendons have not been investigated and so represent a further area for future study.
Conclusions
It is apparent that several significant advances have been made recently to further understand the role that non-collagenous proteins have in modulating tendon properties. However, a substantial amount of research is still required to develop a complete understanding of the role of each specific component at each hierarchical level and to determine the specializations that occur in tendons with differing mechanical demands. In addition, work needs to be undertaken to elucidate the changes that occur to this matrix with ageing and injury. This information is critical to fully understand structure–function relationships within tendon, which will aid in the development of novel, effective techniques for tendon injury prevention and treatment.
Acknowledgments
The authors would like to thank Dr Elizabeth Laird and Dr Kinley Smith for the provision of histological images.
Funding source
This work is funded by a project grant (VETS/PRJ/752) from the Horserace Betting Levy Board, UK.
Conflict of interest
The authors have no conflicts of interest.
References
- Aaron BB, Gosline JM. Elastin as a random-network elastomer: a mechanical and optical analysis of single elastin fibers. Biopolymers. 1981;20:1247–1260. [Google Scholar]
- Arnoczky SP, Lavagnino M, Whallon JH, Hoonjan A. In situ cell nucleus deformation in tendons under tensile load; a morphological analysis using confocal laser microscopy. J. Orthop. Res. 2002;20:29–35. doi: 10.1016/S0736-0266(01)00080-8. [DOI] [PubMed] [Google Scholar]
- Baccarani-Contri M, Vincenzi D, Cicchetti F, Mori G, Pasquali-Ronchetti I. Immunocytochemical localization of proteoglycans within normal elastin fibers. Eur. J. Cell Biol. 1990;53:305–312. [PubMed] [Google Scholar]
- Baldock C, Oberhauser AF, Ma L, et al. Shape of tropoelastin, the highly extensible protein that controls human tissue elasticity. Proc. Natl Acad. Sci. USA. 2011;108:4322–4327. doi: 10.1073/pnas.1014280108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banos CC, Thomas AH, Kuo CK. Collagen fibrillogenesis in tendon development: current models and regulation of fibril assembly. Birth Defects Res. C Embryo Today. 2008;84:228–244. doi: 10.1002/bdrc.20130. [DOI] [PubMed] [Google Scholar]
- Batson EL, Paramour RJ, Smith TJ, Birch HL, Patterson-Kane JC, Goodship AE. Are the material properties and matrix composition of equine flexor and extensor tendons determined by their functions? Equine Vet. J. 2003;35:314–318. doi: 10.2746/042516403776148327. [DOI] [PubMed] [Google Scholar]
- Biewener AA. Muscle-tendon stresses and elastic energy storage during locomotion in the horse. Comp. Biochem. Physiol. B, Biochem. Mol. Biol. 1998;120:73–87. doi: 10.1016/s0305-0491(98)00024-8. [DOI] [PubMed] [Google Scholar]
- Birch HL. Tendon matrix composition and turnover in relation to functional requirements. Int. J. Exp. Pathol. 2007;88:241–248. doi: 10.1111/j.1365-2613.2007.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch HL, Bailey AJ, Goodship AE. Macroscopic ‘degeneration’ of equine superficial digital flexor tendon is accompanied by a change in extracellular matrix composition. Equine Vet. J. 1998;30:534–539. doi: 10.1111/j.2042-3306.1998.tb04530.x. [DOI] [PubMed] [Google Scholar]
- Birch HL, Bailey JV, Bailey AJ, Goodship AE. Age-related changes to the molecular and cellular components of equine flexor tendons. Equine Vet. J. 1999;31:391–396. doi: 10.1111/j.2042-3306.1999.tb03838.x. [DOI] [PubMed] [Google Scholar]
- Birch HL, Smith TJ, Poulton C, Peiffer D, Goodship AE. Do regional variations in flexor tendons predispose to site-specific injuries? Equine Vet. J. Suppl. 2002;34:288–292. doi: 10.1111/j.2042-3306.2002.tb05435.x. [DOI] [PubMed] [Google Scholar]
- Birk DE, Nurminskaya MV, Zycband EI. Collagen fibrillogenesis in situ: fibril segments undergo post-depositional modifications resulting in linear and lateral growth during matrix development. Dev. Dyn. 1995;202:229–243. doi: 10.1002/aja.1002020303. [DOI] [PubMed] [Google Scholar]
- Brandau O, Meindl A, Fassler R, Aszodi A. A novel gene, tendin, is strongly expressed in tendons and ligaments and shows high homology with chondromodulin-I. Dev. Dyn. 2001;221:72–80. doi: 10.1002/dvdy.1126. [DOI] [PubMed] [Google Scholar]
- Carrino DA, Sorrell JM, Caplan AI. Age-related Changes in the Proteoglycans of Human Skin. Arch. Biochem. Biophys. 2000;373:91–101. doi: 10.1006/abbi.1999.1545. [DOI] [PubMed] [Google Scholar]
- Cheng VWT, Screen HRC. The micro-structural strain response of tendon. J. Mater. Sci. 2007;42:8957–8965. [Google Scholar]
- Corsi A, Xu T, Chen XD, et al. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J. Bone Miner. Res. 2002;17:1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- DiCesare PE, MÖRgelin M, Mann K, Paulsson M. Cartilage oligomeric matrix protein and thrombospondin 1. Eur. J. Biochem. 1994;223:927–937. doi: 10.1111/j.1432-1033.1994.tb19070.x. [DOI] [PubMed] [Google Scholar]
- Docheva D, Hunziker EB, Fassler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol. Cell. Biol. 2005;25:699–705. doi: 10.1128/MCB.25.2.699-705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourte LM, Pathmanathan L, Jawad AF, et al. Influence of decorin on the mechanical, compositional, and structural properties of the mouse patellar tendon. J. Biomech. Eng. 2012;134:031005. doi: 10.1115/1.4006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely ER, Avella CS, Price JS, Smith RK, Wood JL, Verheyen KL. Descriptive epidemiology of fracture, tendon and suspensory ligament injuries in National Hunt racehorses in training. Equine Vet. J. 2009;41:372–378. doi: 10.2746/042516409x371224. [DOI] [PubMed] [Google Scholar]
- Esther RJ, Creighton RA, Draeger RW, Weinhold PS, Dahners LE. Effect of NKISK on tendon lengthening: an in vivo model for various clinically applicable dosing regimens. J. Orthop. Res. 2008;26:971–976. doi: 10.1002/jor.20594. [DOI] [PubMed] [Google Scholar]
- Ezura Y, Chakravarti S, Oldberg A, Chervoneva I, Birk DE. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J. Cell Biol. 2000;151:779–788. doi: 10.1083/jcb.151.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon J, Blevins FT, Vogel K, Trotter J. Functional morphology of the supraspinatus tendon. J. Orthop. Res. 2002;20:920–926. doi: 10.1016/S0736-0266(02)00023-2. [DOI] [PubMed] [Google Scholar]
- Fessel G, Snedeker JG. Evidence against proteoglycan mediated collagen fibril load transmission and dynamic viscoelasticity in tendon. Matrix Biol. 2009;28:503–510. doi: 10.1016/j.matbio.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Fessel G, Snedeker JG. Equivalent stiffness after glycosaminoglycan depletion in tendon–an ultra-structural finite element model and corresponding experiments. J. Theor. Biol. 2011;268:77–83. doi: 10.1016/j.jtbi.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Flannery CR, Hughes CE, Schumacher BL, et al. Articular Cartilage Superficial Zone Protein (SZP) Is Homologous to Megakaryocyte Stimulating Factor Precursor and Is a Multifunctional Proteoglycan with Potential Growth-Promoting, Cytoprotective, and Lubricating Properties in Cartilage Metabolism. Biochem. Biophys. Res. Commun. 1999;254:535–541. doi: 10.1006/bbrc.1998.0104. [DOI] [PubMed] [Google Scholar]
- Franchi M, Raspanti M, Dell'Orbo C, et al. Different crimp patterns in collagen fibrils relate to the subfibrillar arrangement. Connect. Tissue Res. 2008;49:85–91. doi: 10.1080/03008200801913635. [DOI] [PubMed] [Google Scholar]
- Funakoshi T, Schmid T, Hsu H, Spector M. Lubricin distribution in the goat infraspinatus tendon: a basis for interfascicular lubrication. J. Bone Joint Surg. Am. 2008;90:803–814. doi: 10.2106/JBJS.G.00627. [DOI] [PubMed] [Google Scholar]
- Funakoshi T, Martin SD, Schmid TM, Spector M. Distribution of lubricin in the ruptured human rotator cuff and biceps tendon: a pilot study. Clin. Orthop. Relat. Res. 2009;468:1588–1599. doi: 10.1007/s11999-009-1108-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosline J, Lillie M, Carrington E, Guerette P, Ortlepp C, Savage K. Elastic proteins: biological roles and mechanical properties. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002;357:121–132. doi: 10.1098/rstb.2001.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulam Houssen Y, Gusachenko I, Schanne-Klein MC, Allain JM. Monitoring micrometer-scale collagen organization in rat-tail tendon upon mechanical strain using second harmonic microscopy. J. Biomech. 2011;44:2047–2052. doi: 10.1016/j.jbiomech.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Halasz K, Kassner A, Morgelin M, Heinegard D. COMP acts as a catalyst in collagen fibrillogenesis. J. Biol. Chem. 2007;282:31166–31173. doi: 10.1074/jbc.M705735200. [DOI] [PubMed] [Google Scholar]
- Haraldsson BT, Aagaard P, Qvortrup K, et al. Lateral force transmission between human tendon fascicles. Matrix Biol. 2008;27:86–95. doi: 10.1016/j.matbio.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Hedbom E, Heinegard D. Interaction of a 59-kDa connective tissue matrix protein with collagen I and collagen II. J. Biol. Chem. 1989;264:6898–6905. [PubMed] [Google Scholar]
- Herrick WC, Kingsbury HB, Lou DY. A study of the normal range of strain, strain rate, and stiffness of tendon. J. Biomed. Mater. Res. 1978;12:877–894. doi: 10.1002/jbm.820120610. [DOI] [PubMed] [Google Scholar]
- Hess GW. Achilles tendon rupture: a review of etiology, population, anatomy, risk factors, and injury prevention. Foot Ankle Spec. 2010;3:29–32. doi: 10.1177/1938640009355191. [DOI] [PubMed] [Google Scholar]
- Isogai Z, Aspberg A, Keene DR, Ono RN, Reinhardt DP, Sakai LY. Versican Interacts with Fibrillin-1 and Links Extracellular Microfibrils to Other Connective Tissue Networks. J. Biol. Chem. 2002;277:4565–4572. doi: 10.1074/jbc.M110583200. [DOI] [PubMed] [Google Scholar]
- Jarvinen TA, Kannus P, Jarvinen TL, Jozsa L, Kalimo H, Jarvinen M. Tenascin-C in the pathobiology and healing process of musculoskeletal tissue injury. Scand. J. Med. Sci. Sports. 2000;10:376–382. doi: 10.1034/j.1600-0838.2000.010006376.x. [DOI] [PubMed] [Google Scholar]
- Jarvinen TA, Jozsa L, Kannus P, et al. Mechanical loading regulates the expression of tenascin-C in the myotendinous junction and tendon but does not induce de novo synthesis in the skeletal muscle. J. Cell Sci. 2003;116:857–866. doi: 10.1242/jcs.00303. [DOI] [PubMed] [Google Scholar]
- Jones GC, Riley GP. ADAMTS proteinases: a multi-domain, multi-functional family with roles in extracellular matrix turnover and arthritis. Arthritis Res. Ther. 2005;7:160–169. doi: 10.1186/ar1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalamajski S, Oldberg A. Homologous sequence in lumican and fibromodulin leucine-rich repeat 5–7 competes for collagen binding. J. Biol. Chem. 2009;284:534–539. doi: 10.1074/jbc.M805721200. [DOI] [PubMed] [Google Scholar]
- Kannus P. Structure of the tendon connective tissue. Scand. J. Med. Sci. Sports. 2000;10:312–320. doi: 10.1034/j.1600-0838.2000.010006312.x. [DOI] [PubMed] [Google Scholar]
- Kasashima Y, Takahashi T, Smith RK, et al. Prevalence of superficial digital flexor tendonitis and suspensory desmitis in Japanese Thoroughbred flat racehorses in 1999. Equine Vet. J. 2004;36:346–350. doi: 10.2746/0425164044890580. [DOI] [PubMed] [Google Scholar]
- Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connect. Tissue Res. 1978;6:11–23. doi: 10.3109/03008207809152283. [DOI] [PubMed] [Google Scholar]
- Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J. Cell Sci. 2002;115:2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- Kilts T, Ameye L, Syed-Picard F, et al. Potential roles for the small leucine-rich proteoglycans biglycan and fibromodulin in ectopic ossification of tendon induced by exercise and in modulating rotarod performance. Scand. J. Med. Sci. Sports. 2009;19:536–546. doi: 10.1111/j.1600-0838.2009.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Yoon JH, Zhang J, Eric Mueller PO, Halper J. Glycan profiling of a defect in decorin glycosylation in equine systemic proteoglycan accumulation, a potential model of progeroid form of Ehlers-Danlos syndrome. Arch. Biochem. Biophys. 2010;501:221–231. doi: 10.1016/j.abb.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Kjaer M. Role of Extracellular Matrix in Adaptation of Tendon and Skeletal Muscle to Mechanical Loading. Physiol. Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Kjaer M, Langberg H, Heinemeier K, et al. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand. J. Med. Sci. Sports. 2009;19:500–510. doi: 10.1111/j.1600-0838.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- Knobloch K, Yoon U, Vogt PM. Acute and overuse injuries correlated to hours of training in master running athletes. Foot Ankle Int. 2008;29:671–676. doi: 10.3113/FAI.2008.0671. [DOI] [PubMed] [Google Scholar]
- Kohrs RT, Zhao C, Sun Y-L, et al. Tendon fascicle gliding in wild type, heterozygous, and lubricin knockout mice. J. Orthop. Res. 2011;29:384–389. doi: 10.1002/jor.21247. [DOI] [PubMed] [Google Scholar]
- Korol RM, Finlay HM, Josseau MJ, Lucas AR, Canham PB. Fluorescence spectroscopy and birefringence of molecular changes in maturing rat tail tendon. J. Biomed. Opt. 2007;12:024011–024011. doi: 10.1117/1.2714055. [DOI] [PubMed] [Google Scholar]
- Kwak YH, Roh JY, Lee KS, Park HW, Kim HW. Altered synthesis of cartilage-specific proteoglycans by mutant human cartilage oligomeric matrix protein. Clin. Orthop. Surg. 2009;1:181–187. doi: 10.4055/cios.2009.1.4.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerlotz K, Riley GP, Screen HRC. GAG depletion increases the stress relaxation response of tendon fascicles, but does not influence recovery. Acta Biomater. 2013 doi: 10.1016/j.actbio.2013.02.028. doi: 10.1016/j.actbio.2013.02.028. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtwark GA, Wilson AM. In vivo mechanical properties of the human Achilles tendon during one-legged hopping. J. Exp. Biol. 2005;208:4715–4725. doi: 10.1242/jeb.01950. [DOI] [PubMed] [Google Scholar]
- Lichtwark GA, Wilson AM. Is Achilles tendon compliance optimised for maximum muscle efficiency during locomotion? J. Biomech. 2007;40:1768–1775. doi: 10.1016/j.jbiomech.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Lillie MA, Gosline JM. The viscoelastic basis for the tensile strength of elastin. Int. J. Biol. Macromol. 2002;30:119–127. doi: 10.1016/s0141-8130(02)00008-9. [DOI] [PubMed] [Google Scholar]
- Maddox BK, Mokashi A, Keene DR, Bachinger HP. A cartilage oligomeric matrix protein mutation associated with pseudoachondroplasia changes the structural and functional properties of the type 3 domain. J. Biol. Chem. 2000;275:11412–11417. doi: 10.1074/jbc.275.15.11412. [DOI] [PubMed] [Google Scholar]
- Perkins NR, Reid SWJ, Morris RS. Risk factors for injury to the superficial digital flexor tendon and suspensory apparatus in Thoroughbred racehorses in New Zealand. NZ Vet. J. 2005;53:184–192. doi: 10.1080/00480169.2005.36503. [DOI] [PubMed] [Google Scholar]
- Pirog KA, Jaka O, Katakura Y, et al. A mouse model offers novel insights into the myopathy and tendinopathy often associated with pseudoachondroplasia and multiple epiphyseal dysplasia. Hum. Mol. Genet. 2010;19:52–64. doi: 10.1093/hmg/ddp466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Vanderby R. Collagen fibril morphology and organization: implications for force transmission in ligament and tendon. Matrix Biol. 2006;25:71–84. doi: 10.1016/j.matbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Purslow PP. The shear modulus of connections between tendon fascicles. 2009. pp. 134–136. Proceedings of the Science and Technology for Humanity (TIC-STH), IEEE Toronto International Conference.
- Redaelli A, Vesentini S, Soncini M, Vena P, Mantero S, Montevecchi FM. Possible role of decorin glycosaminoglycans in fibril to fibril force transfer in relative mature tendons–a computational study from molecular to microstructural level. J. Biomech. 2003;36:1555–1569. doi: 10.1016/s0021-9290(03)00133-7. [DOI] [PubMed] [Google Scholar]
- Rees SG, Flannery CR, Little CB, Hughes CE, Caterson B, Dent CM. Catabolism of aggrecan, decorin and biglycan in tendon. Biochem. J. 2000;350(Pt 1):181–188. [PMC free article] [PubMed] [Google Scholar]
- Rees SG, Davies JR, Tudor D, et al. Immunolocalisation and expression of proteoglycan 4 (cartilage superficial zone proteoglycan) in tendon. Matrix Biol. 2002;21:593–602. doi: 10.1016/s0945-053x(02)00056-2. [DOI] [PubMed] [Google Scholar]
- Rigozzi S, Muller R, Snedeker JG. Local strain measurement reveals a varied regional dependence of tensile tendon mechanics on glycosaminoglycan content. J. Biomech. 2009;42:1547–1552. doi: 10.1016/j.jbiomech.2009.03.031. [DOI] [PubMed] [Google Scholar]
- Rigozzi S, Müller R, Stemmer A, Snedeker JG. Tendon glycosaminoglycan proteoglycan sidechains promote collagen fibril sliding-AFM observations at the nanoscale. J. Biomech. 2013;46:813–818. doi: 10.1016/j.jbiomech.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology (Oxford) 2004;43:131–142. doi: 10.1093/rheumatology/keg448. [DOI] [PubMed] [Google Scholar]
- Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL. Glycosaminoglycans of human rotator cuff tendons: changes with age and in chronic rotator cuff tendinitis. Ann. Rheum. Dis. 1994a;53:367–376. doi: 10.1136/ard.53.6.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL. Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann. Rheum. Dis. 1994b;53:359–366. doi: 10.1136/ard.53.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley GP, Harrall RL, Cawston TE, Hazleman BL, Mackie EJ. Tenascin-C and human tendon degeneration. Am. J. Pathol. 1996;149:933–943. [PMC free article] [PubMed] [Google Scholar]
- Ritty TM, Ditsios K, Starcher BC. Distribution of the elastic fiber and associated proteins in flexor tendon reflects function. Anat. Rec. 2002;268:430–440. doi: 10.1002/ar.10175. [DOI] [PubMed] [Google Scholar]
- Ritty TM, Roth R, Heuser JE. Tendon cell array isolation reveals a previously unknown fibrillin-2-containing macromolecular assembly. Structure. 2003;11:1179–1188. doi: 10.1016/s0969-2126(03)00181-3. [DOI] [PubMed] [Google Scholar]
- Roberts TJ, Azizi E. Flexible mechanisms: the diverse roles of biological springs in vertebrate movement. J. Exp. Biol. 2011;214:353–361. doi: 10.1242/jeb.038588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PS, Lin TW, Reynolds PR, Derwin KA, Iozzo RV, Soslowsky LJ. Strain-rate sensitive mechanical properties of tendon fascicles from mice with genetically engineered alterations in collagen and decorin. J. Biomech. Eng. 2004;126:252–257. doi: 10.1115/1.1695570. [DOI] [PubMed] [Google Scholar]
- Robinson PS, Huang TF, Kazam E, Iozzo RV, Birk DE, Soslowsky LJ. Influence of decorin and biglycan on mechanical properties of multiple tendons in knockout mice. J. Biomech. Eng. 2005;127:181–185. doi: 10.1115/1.1835363. [DOI] [PubMed] [Google Scholar]
- Rosenberg K, Olsson H, Morgelin M, Heinegard D. Cartilage oligomeric matrix protein shows high affinity zinc-dependent interaction with triple helical collagen. J. Biol. Chem. 1998;273:20397–20403. doi: 10.1074/jbc.273.32.20397. [DOI] [PubMed] [Google Scholar]
- Samiric T, Ilic MZ, Handley CJ. Characterisation of proteoglycans and their catabolic products in tendon and explant cultures of tendon. Matrix Biol. 2004;23:127–140. doi: 10.1016/j.matbio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Schönherr E, Witsch-Prehm P, Harrach B, Robenek H, Rauterberg J, Kresse H. Interaction of Biglycan with Type I Collagen. J. Biol. Chem. 1995;270:2776–2783. doi: 10.1074/jbc.270.6.2776. [DOI] [PubMed] [Google Scholar]
- Scott JE. Proteodermatan and proteokeratan sulfate (decorin, lumican/fibromodulin) proteins are horseshoe shaped. Implications for their interactions with collagen. Biochemistry. 1996;35:8795–8799. doi: 10.1021/bi960773t. [DOI] [PubMed] [Google Scholar]
- Screen HRC, Bader DL, Lee DA, Shelton JC. Local strain measurement within tendon. Strain. 2004;40:157–163. [Google Scholar]
- Screen HRC, Shelton JC, Bader DL, Lee DA. Cyclic tensile strain upregulates collagen synthesis in isolated tendon fascicles. Biochem. Biophys. Res. Commun. 2005;336:424–429. doi: 10.1016/j.bbrc.2005.08.102. [DOI] [PubMed] [Google Scholar]
- Senga K, Kobayashi M, Hattori H, et al. Type VI collagen in mouse masseter tendon, from osseous attachment to myotendinous junction. Anat. Rec. 1995;243:294–302. doi: 10.1002/ar.1092430303. [DOI] [PubMed] [Google Scholar]
- Sharon N. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Nomenclature of glycoproteins, glycopeptides and peptidoglycans. Recommendations 1985. Eur. J. Biochem. 1986;159:1–6. doi: 10.1111/j.1432-1033.1986.tb09825.x. [DOI] [PubMed] [Google Scholar]
- Shukunami C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev. Biol. 2006;298:234–247. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Silver FH, Freeman JW, Seehra GP. Collagen self-assembly and the development of tendon mechanical properties. J. Biomech. 2003;36:1529–1553. doi: 10.1016/s0021-9290(03)00135-0. [DOI] [PubMed] [Google Scholar]
- Smith RK, Zunino L, Webbon PM, Heinegard D. The distribution of cartilage oligomeric matrix protein (COMP) in tendon and its variation with tendon site, age and load. Matrix Biol. 1997;16:255–271. doi: 10.1016/s0945-053x(97)90014-7. [DOI] [PubMed] [Google Scholar]
- Smith RK, Birch HL, Goodman S, Heinegard D, Goodship AE. The influence of ageing and exercise on tendon growth and degeneration–hypotheses for the initiation and prevention of strain-induced tendinopathies. Comp. Biochem. Physiol., Part A Mol. Integr. Physiol. 2002a;133:1039–1050. doi: 10.1016/s1095-6433(02)00148-4. [DOI] [PubMed] [Google Scholar]
- Smith RK, Gerard M, Dowling B, Dart AJ, Birch HL, Goodship AE. Correlation of cartilage oligomeric matrix protein (COMP) levels in equine tendon with mechanical properties: a proposed role for COMP in determining function-specific mechanical characteristics of locomotor tendons. Equine Vet. J. Suppl. 2002b:241–244. doi: 10.1111/j.2042-3306.2002.tb05426.x. [DOI] [PubMed] [Google Scholar]
- Smith KD, Vaughan-Thomas A, Spiller DG, Innes JF, Clegg PD, Comerford EJ. The organisation of elastin and fibrillins 1 and 2 in the cruciate ligament complex. J. Anat. 2011;218:600–607. doi: 10.1111/j.1469-7580.2011.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedeker JG, Ben Arav A, Zilberman Y, Pelled G, Gazit D. Functional fibered confocal microscopy: a promising tool for assessing tendon regeneration. Tissue Eng. Part C Methods. 2009a;15:485–491. doi: 10.1089/ten.tec.2008.0612. [DOI] [PubMed] [Google Scholar]
- Snedeker JG, Pelled G, Zilberman Y, et al. An analytical model for elucidating tendon tissue structure and biomechanical function from in vivo cellular confocal microscopy images. Cells Tissues Organs. 2009b;190:111–119. doi: 10.1159/000189211. [DOI] [PubMed] [Google Scholar]
- Sodersten F, Ekman S, Eloranta ML, Heinegard D, Dudhia J, Hultenby K. Ultrastructural immunolocalization of cartilage oligomeric matrix protein (COMP) in relation to collagen fibrils in the equine tendon. Matrix Biol. 2005;24:376–385. doi: 10.1016/j.matbio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Sodersten F, Hultenby K, Heinegard D, Johnston C, Ekman S. Immunolocalization of collagens (I and III) and cartilage oligomeric matrix protein (COMP) in the normal and injured equine superficial digital flexor tendon. Connect. Tissue Res. 2013;54:62–69. doi: 10.3109/03008207.2012.734879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starborg T, Lu Y, Kadler KE, Holmes DF. Electron microscopy of collagen fibril structure in vitro and in vivo including three-dimensional reconstruction. Methods Cell Biol. 2008;88:319–345. doi: 10.1016/S0091-679X(08)00417-2. [DOI] [PubMed] [Google Scholar]
- Stephens PR, Nunamaker DM, Butterweck DM. Application of a Hall-effect transducer for measurement of tendon strains in horses. Am. J. Vet. Res. 1989;50:1089–1095. [PubMed] [Google Scholar]
- Sun Y, Berger EJ, Zhao C, Jay GD, An K-N, Amadio PC. Expression and mapping of lubricin in canine flexor tendon. J. Orthop. Res. 2006;24:1861–1868. doi: 10.1002/jor.20239. [DOI] [PubMed] [Google Scholar]
- Sun HB, Li YH, Fung DT, Majeska RJ, Schaffler MB, Flatow EL. Coordinate regulation of IL-1 beta and MMP-13 in rat tendons following subrupture fatigue damage. Clin. Orthop. Relat. Res. 2008;466:1555–1561. doi: 10.1007/s11999-008-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson L, Aszodi A, Heinegard D, et al. Cartilage oligomeric matrix protein-deficient mice have normal skeletal development. Mol. Cell. Biol. 2002;22:4366–4371. doi: 10.1128/MCB.22.12.4366-4371.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson RB, Hassenkam T, Hansen P, Kjaer M, Magnusson SP. Tensile force transmission in human patellar tendon fascicles is not mediated by glycosaminoglycans. Connect. Tissue Res. 2011;52:415–421. doi: 10.3109/03008207.2010.551569. [DOI] [PubMed] [Google Scholar]
- Swann DA, Slayter HS, Silver FH. The molecular structure of lubricating glycoprotein-I, the boundary lubricant for articular cartilage. J. Biol. Chem. 1981;256:5921–5925. [PubMed] [Google Scholar]
- Sweeney SM, Orgel JP, Fertala A, et al. Candidate cell and matrix interaction domains on the collagen fibril, the predominant protein of vertebrates. J. Biol. Chem. 2008;283:21187–21197. doi: 10.1074/jbc.M709319200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi M, Sun Y-L, Zhao C, et al. Lubricin surface modification improves extrasynovial tendon gliding in a Canine model in vitro. J. Bone Joint Surg. Am. 2008;90:129–135. doi: 10.2106/JBJS.G.00045. [DOI] [PubMed] [Google Scholar]
- Taguchi M, Sun Y-L, Zhao C, et al. Lubricin surface modification improves tendon gliding after tendon repair in a canine model in vitro. J. Orthop. Res. 2009;27:257–263. doi: 10.1002/jor.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe CT. Extracellular Matrix Synthesis and Degradation in Functionally Distinct Tendons. London: Institute of Orthopaedics and Musculoskeletal Science, University College London; 2010. p. 267. [Google Scholar]
- Thorpe CT, Clegg PD, Birch HL. A review of tendon injury: why is the equine superficial digital flexor tendon most at risk? Equine Vet. J. 2010a;42:174–180. doi: 10.2746/042516409X480395. [DOI] [PubMed] [Google Scholar]
- Thorpe CT, Streeter I, Pinchbeck GL, Goodship AE, Clegg PD, Birch HL. Aspartic acid racemization and collagen degradation markers reveal an accumulation of damage in tendon collagen that is enhanced with aging. J. Biol. Chem. 2010b;285:15674–15681. doi: 10.1074/jbc.M109.077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe CT, Udeze CP, Birch HL, Clegg PD, Screen HRC. Specialization of tendon mechanical properties results from interfascicular differences. J. R. Soc. Interface. 2012;9:3108–3117. doi: 10.1098/rsif.2012.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe CT, Udeze CP, Birch HL, Clegg PD, Screen HRC. Capacity for sliding between tendon fascicles decreases with ageing in injury prone equine tendons: a possible mechanism for age-related tendinopathy? Eur. Cell. Mater. 2013;25:48–60. doi: 10.22203/ecm.v025a04. [DOI] [PubMed] [Google Scholar]
- Vailas AC, Pedrini VA, Pedrini-Mille A, Holloszy JO. Patellar tendon matrix changes associated with aging and voluntary exercise. J. Appl. Physiol. 1985;58:1572–1576. doi: 10.1152/jappl.1985.58.5.1572. [DOI] [PubMed] [Google Scholar]
- Vesentini S, Redaelli A, Montevecchi FM. Estimation of the binding force of the collagen molecule-decorin core protein complex in collagen fibril. J. Biomech. 2005;38:433–443. doi: 10.1016/j.jbiomech.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Vogel HG. Influence of maturation and aging on mechanical and biochemical properties of connective tissue in rats. Mech. Ageing Dev. 1980;14:283–292. doi: 10.1016/0047-6374(80)90002-0. [DOI] [PubMed] [Google Scholar]
- Wang VM, Bell RM, Thakore R, et al. Murine tendon function is adversely affected by aggrecan accumulation due to the knockout of ADAMTS5. J. Orthop. Res. 2012;30:620–626. doi: 10.1002/jor.21558. [DOI] [PubMed] [Google Scholar]
- Weber IT, Harrison RW, Iozzo RV. Model structure of decorin and implications for collagen fibrillogenesis. J. Biol. Chem. 1996;271:31767–31770. doi: 10.1074/jbc.271.50.31767. [DOI] [PubMed] [Google Scholar]
- Wiberg C, Hedbom E, Khairullina A, et al. Biglycan and decorin bind close to the N-terminal region of the collagen VI triple helix. J. Biol. Chem. 2001;276:18947–18952. doi: 10.1074/jbc.M100625200. [DOI] [PubMed] [Google Scholar]
- Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr. Opin. Cell Biol. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- Woessner JF. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–2154. [PubMed] [Google Scholar]
- Wood ML, Luthin WN, Lester GE, Dahners LE. Tendon creep is potentiated by NKISK and relaxin which produce collagen fiber sliding. Iowa Orthop. J. 2003;23:75–79. [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Halper J. Tendon proteoglycans: biochemistry and function. J. Musculoskelet. Neuronal Interact. 2005;5:22–34. [PubMed] [Google Scholar]
- Zhang G, Young BB, Ezura Y, et al. Development of tendon structure and function: regulation of collagen fibrillogenesis. J. Musculoskelet. Neuronal Interact. 2005;5:5–21. [PubMed] [Google Scholar]
- Zhang G, Ezura Y, Chervoneva I, et al. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J. Cell. Biochem. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]


