Abstract
Tendon injuries, often called tendinopathies, are debilitating and painful conditions, generally considered to develop as a result of tendon overuse. The aetiology of tendinopathy remains poorly understood, and whilst tendon biopsies have provided some information concerning tendon appearance in late-stage disease, there is still little information concerning the mechanical and cellular events associated with disease initiation and progression. Investigating this in situ is challenging, and numerous models have been developed to investigate how overuse may generate tendon fatigue damage and how this may relate to tendinopathy conditions. This article aims to review these models and our current understanding of tendon fatigue damage. We review the strengths and limitations of different methodologies for characterizing tendon fatigue, considering in vitro methods that adopt both viable and non-viable samples, as well as the range of different in vivo approaches. By comparing data across model systems, we review the current understanding of fatigue damage development. Additionally, we compare these findings with data from tendinopathic tissue biopsies to provide some insights into how these models may relate to the aetiology of tendinopathy. Fatigue-induced damage consistently highlights the same microstructural, biological and mechanical changes to the tendon across all model systems and also correlates well with the findings from tendinopathic biopsy tissue. The multiple testing routes support matrix damage as an important contributor to tendinopathic conditions, but cellular responses to fatigue appear complex and often contradictory.
Keywords: fatigue, mechanics, rupture, tendinopathy, tendon
Introduction
Tendons are unidirectional fibre-reinforced composites, responsible for the transmission of load from muscles to bones. Collagen constitutes the building block of the fibrous phase reinforcing a matrix of hydrated proteoglycan-rich gel at a number of hierarchical levels as demonstrated in Figure 1. The principal role of tendon is to resist tension, but it must also allow for a certain degree of compliance within musculoskeletal mechanics. These apparently conflicting demands are resolved as a direct result of the hierarchical structure of tendon and the contrasting nature of the stiff collagen fibres and the viscous, highly hydrated and proteoglycan-rich matrix surrounding them (Benjamin et al. 2008). Tendon is stronger per unit area than muscle and has a tensile strength approximately equal to that of bone, although with additional flexibility and a degree of elasticity and extensibility (James et al. 2008).
Figure 1.
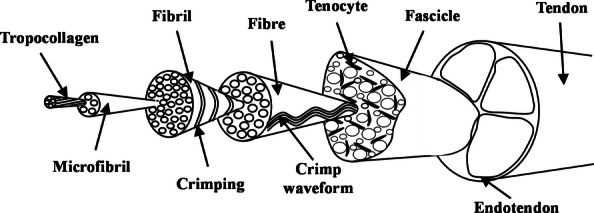
The hierarchical structure of tendon. Adapted from Screen et al. (2004a).
Like other unidirectional fibre-reinforced composites, the parallel arrangement of collagen fibres acts to resist tension, so that contractile energy is not lost during load transmission (James et al. 2008). In most everyday activities, the loads observed in tendons are comparatively low and certainly well below the tissue's ultimate tensile strength. However, in quick eccentric movements, movements where a limb must be rapidly decelerated, more significant stresses can be observed (Schechtman & Bader 1997). Stresses of between 42 and 110 MPa have been measured in vivo in the Achilles tendon, this maximum exceeding the often reported failure strength of the tendon (Komi et al. 1992; Schechtman & Bader 1997). As a result of these high in vivo loads, tendons such as the Achilles and patellar are particularly prone to overuse injuries, often referred to as tendinopathies, an effect probably worsened by material and structural inhomogeneity as well as the poor healing response of the tissue (Fung et al. 2010).
Indeed, tendon injuries such as tendinopathy and tendon rupture are prevalent, debilitating and often painful clinical problems. Each year in the United States, there are 16.4 million tendon and ligament injuries, of which at least 100,000 involve the Achilles tendon (Wang et al. 2012), and as a greater proportion of the general population become involved in physical and recreational activities, the frequency of soft tissue injury is only likely to increase. However, characterizing tendon injuries and establishing how and why damage may occur can be complex.
Tendon cannot simply be considered a fibre composite, it is a living tissue, with a structure maintained by tenocytes, the resident cell population. As such, mechanical loading can create not only structural damage, but also a cell response to remodel the matrix (Wang 2006; Franchi et al. 2007; Kjær et al. 2009). An intimate interplay exists between mechanical signalling and biochemical changes within the tendon extracellular matrix, resulting in adaptations in tendon morphology, structure and material properties with use (Kjær et al. 2009).
The interplay between mechanically induced and cell-induced matrix changes is poorly understood and has led to considerable debate concerning the aetiology of tendon injuries. Data indicate that tendinopathy often occurs in the absence of any single traumatic event, implicating a gradual accumulation of micro-injuries (Wang 2006). This has led to the hypothesis that damage to tendon probably occurs every day as a result of all manner of normal activities and that damage accumulates only when equilibrium cannot be maintained between the rate of damage and the cellular-driven rate of repair (Archambault 2003). However, there is also considerable interest in the tenocytes and how they may contribute to, or perhaps even drive, the breakdown of tissue to tendinopathic states (Riley 2005).
To understand these processes further, a range of model systems have been developed to simulate tendon overuse, characterize the development of fatigue damage and investigate how this may relate to the aetiology of tendinopathy. Monitoring fatigue in situ poses many challenges; hence, a number of in vitro and animal models have been developed to assist our understanding. This review outlines some of these methods and summarizes the current understanding of fatigue damage in tendon and the progression towards tendinopathy or rupture.
Fatigue loading
Fatigue can be considered as progressive and localized structural damage to a material as it is subjected to cyclic loading. Just as a turbine blade may suffer progressive damage during its many cycles of use, the repeated loading of tendons during normal use may well also generate microstructural damage. Fatigue testing of tendon can thus provide a means of investigating damage accumulation. There are two principal modes of fatigue testing: creep analysis, where samples are cycled to a constant peak load and increase in extension monitored (Figure 2a), and stress relaxation analysis, where loading is carried out to a constant peak displacement and the reduction in load considered (Figure 2b). With different boundary conditions, the two testing routes would be expected to elicit a different response from the loaded tendon, but are often used interchangeably. In ligaments (where the function is to connect bone to bone), it has been argued that the in-life loading is a simple creep situation, with the same loads repeatedly applied by the joint (Thornton et al. 1997). Tendons transfer the muscle force to the bone to facilitate locomotion, and as a result, load conditions may be more complex (Screen 2008).
Figure 2.
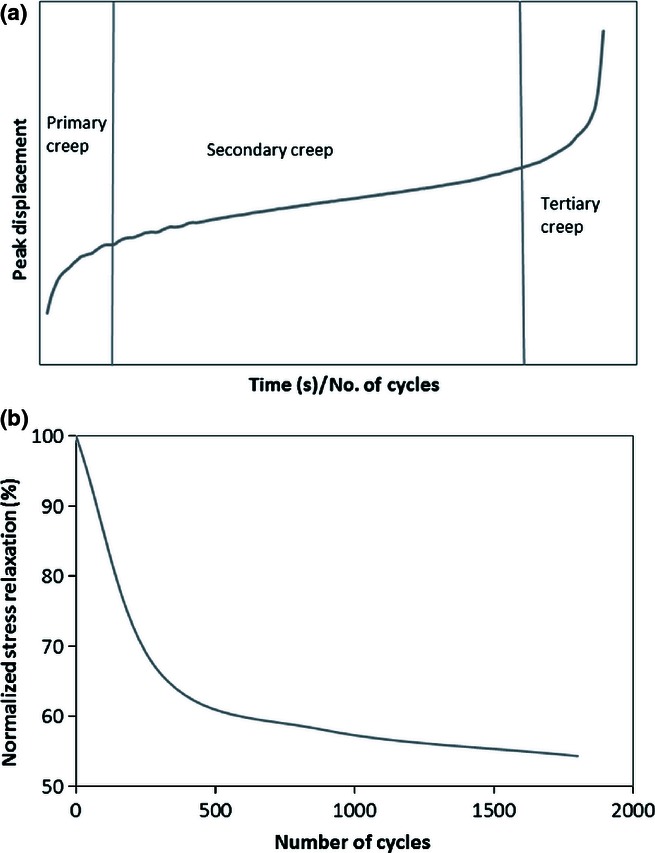
(a) Creep curve, labelled with the three stages of creep: an initial primary stage associated with rapid extension, the stable secondary stage where sample length increases steadily and tertiary creep as the sample rapidly extends to rupture; (b) typical exponential stress relaxation curve.
Creep behaviour can be described by three stages of deformation, demonstrated in Figure 2(a). An initial primary stage associated with rapid extension is followed by a relatively stable secondary stage in which there is a steady increase in sample length, followed by a tertiary stage, as the sample rapidly extends to rupture. By contrast, stress relaxation curves tend to follow an exponential curve (Figure 2b), with stress steadily stabilizing after an initial rapid decrease. Whilst stress relaxation tests will not terminate in the failure of the sample, fatigue damage is generated; this has been confirmed by stopping tests and loading samples to failure, where significant decreases in quasi-static mechanical characteristics are reported (Legerlotz et al. 2011). Typically, fatigue testing of tendon samples has considered creep loading so as to allow for progressive recruitment and stretching out of the collagen fibres and ultimately investigation of tendon rupture. However, there is also a need for investigating the damage generated through stress relaxation, particularly in the light of the frequent use of this modality when investigating cell response to loading and mechanotransduction (Li et al. 2004; Wang et al. 2004; Wall et al. 2007; Qi et al. 2011).
Methods for the fatigue testing of tendon
Fatigue testing of tendon can be broadly separated into: in vitro testing of non-viable samples; ex vivo testing of viable tendon samples, and in vivo models, which in turn can be subdivided into overuse models and controlled in vivo loading. All of these models have strengths and limitations and vary in terms of their applicability for characterizing the mechanical or cellular aspects of overuse. As such, to provide a complete picture concerning the progression of fatigue damage and the possible mechanisms of tendinopathy, the evidence collected from all of these various methods of fatigue testing of tendon should be considered.
In vitro characterization of tendon fatigue properties
Whilst the quasi-static properties of tendons at various hierarchical levels have been investigated for many years (Rigby et al. 1959; Abrahams 1967; Lanir 1978), the first publications to describe fatigue analyses of tendon were not until the mid-1990s. In 1994, Wang et al. carried out cyclic creep tests on whole tendon samples and observed a series of linear relationships between the logarithm of fatigue life and the applied peak tensile stress across a wide range of loading frequencies (Wang et al. 1995). Stiffness was observed to reduce throughout the test, gradually at first, before accelerating rapidly immediately prior to failure, providing a measure of the accumulation of fatigue damage as previously observed in statically creep-loaded tendons (Wang & Ker 1995).
Other early studies of tendon fatigue considered the human extensor digitorum longus (Schechtman & Bader 1997), the tendons of the wallaby hind leg (Ker et al. 2000), the human Achilles tendon (Wren et al. 2003) and a comparison of the highly stressed plantaris and much less significantly loaded extensor digitorum lateralis of the sheep (Pike et al. 2000). An exponential decrease in cycles to failure with increasing applied peak stress was observed across all of these tendon types. Additionally, Schechtman and Bader (1997) compared the failure strain of tendon samples fatigue loaded to a variety of peak stresses, with their quasi-static failure strain, and found no significant variation, suggesting a failure mechanism determined by a limiting value of strain; in the case of the human extensor, approximately 15%. Wren et al. (2003) investigated the effects of static creep and cyclic creep loading on the mechanical properties of human Achilles tendons and in both instances found the initial strain to be the best predictor of time or cycles to failure. This again supports the hypothesis that strain is the primary mechanical parameter governing tendon damage accumulation and injury.
During cyclic creep tests to a consistent peak stress, it was observed that tendons subjected to low stresses in life failed at shorter times than those subjected to high stresses in life. However, when tests were normalized to take into account the expected stress in life, comparable fatigue lives were observed in all samples, pointing towards structural and mechanical specialization of tendons to suit their in situ load environment (Ker et al. 2000). In recent years, the specialization of tendons for specific functions has become more recognized. For example, the energy-storing bovine digital flexor tendon has been observed to exhibit significantly improved resistance to fatigue compared with the largely positional bovine digital extensor (Shepherd et al. 2012). There remains, however a need to more fully correlate response with tendon function and establish if susceptibility to fatigue damage is related to tendon injury risk.
Fatigue testing of in vitro tendon samples is complex. Because of the independent nature of the collagen fibres within tendon, effective clamping has proved problematic, as has maintaining sample hydration during these long tests (Schechtman & Bader 1997; Ker et al. 2000). Methods that grip only the outer fibres, for example, gluing, can leave the inner fibres unloaded, and as such, the material will appear less strong and less stiff than it actually is (Ker et al. 2000). The transverse stiffness of tendon is low, so direct clamping between metal plates is not ideal. It can result in serious distortion, non-uniform loading, stress concentrations and ultimately premature failure (Ker 2007). Many tests now adopt hydrated samples with air-dried ends, particularly during longer-term tests.
However, even with appropriate test methods, in vitro tests have, without fail, provided values of fatigue life vastly unrealistic for the necessary lifetime of tendons in situ. Schechtman and Bader (1997), analysing the human extensor digitorum longus, estimated that in vitro data predicted in vivo failure after about 4 months of normal walking activity. They subsequently developed a cumulative damage model to try and take into account tendon healing, suggesting a healing rate of 1% per day would be sufficient to eliminate the damage induced in the tendon during normal locomotion (Schechtman & Bader 1997). Biochemical studies, however, imply a much slower rate of turnover in tendon (Birch et al. 2008), suggesting perhaps that such a model is an oversimplification.
To try and investigate this further, more recent studies have looked to correlate changes in tendon mechanics with morphological changes during fatigue loading, aiming to determine the sequential, microstructural events associated with the build-up of fatigue damage (Fung et al. 2009). Although tendon only shows significant changes in stiffness or hysteresis with high levels of fatigue, changes in the microstructure appear to occur rapidly. Under light microscopy, non-loaded control tendons exhibit highly aligned and parallel collagen fibres with their characteristic crimp and tenocytes in a columnar fashion between the fibres. Fatigue-loaded tendons displayed isolated regions of damage within an otherwise normal collagenous architecture. Even in minimally fatigued samples, showing no mechanical changes, fibres appeared kinked (ridge-like formations were observed) and the tenocyte arrangement distorted (Fung et al. 2009). At moderate levels of fatigue, where mechanical changes first become apparent, the number of local patterns of fibre disruption increases and discontinuities and a few instances of isolated fibre tears are observed. In highly fatigued samples, dissociation is observed among the fibres, along with transversely oriented fibre discontinuities and isolated fibre ruptures (Fung et al. 2009). We have recently observed a similar damage progression during the fatigue loading of bovine extensor tendon fascicles, as shown in Figure 3 (Shepherd et al. 2012).
Figure 3.
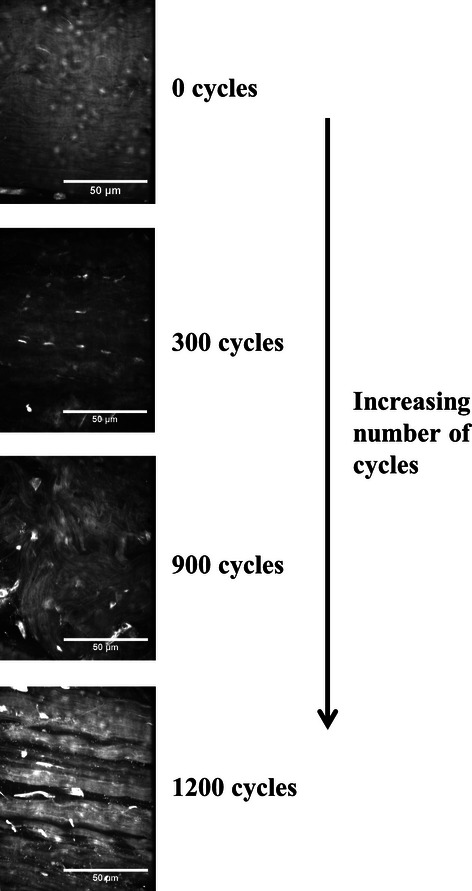
Progression of fatigue damage in bovine extensor tendon fascicles with increasing number of cycles of creep loading (25% of the ultimate tensile stress). Confocal images of fascicles stained with 5 mM acridine orange solution. Kinked fibres were observed after just 300 cycles with much more severe damage including widening of the interfibre space and fibre breakages at 900 and 1200 cycles.
Ex vivo testing – maintaining viable tendon samples
Whilst some of the studies described in the previous section utilized tendon samples very soon after sacrifice of the animal, attempts were not made to maintain sample viability during testing; thus, no conclusions involving the cellular contributions to overuse changes could be drawn. By contrast, ex vivo studies, defined for the purpose of this review as studies carried out on viable tendon tissue, have intentionally maintained sample viability, with the aim of correlating mechanical fatigue with its biological effects. The literature concerning the effect of loading or displacement on viable tendon samples is significant, but much of it considers the application of static load; only tests directly considering fatigue loading will be mentioned here. In addition, the considerable literature concerning isolated cells and the mechanotransduction response to cyclic loading is also not discussed.
Structural comparisons between fatigue-loaded viable and non-viable samples indicate the same progression of microstructural fatigue damage (Parent et al. 2011). Perhaps this is not surprising, as the time frame of experiments and lack of systemic in vivo conditions mean that repair responses are unlikely to be representative. Nevertheless, the effect of loading conditions on immediate cellular metabolism and early changes to matrix turnover can be investigated. Indeed, a study into the effect of stress deprivation and cyclic tensile loading on the mechanical and histological properties of the canine flexor digitorum profundus confirmed that cells can mediate tissue integrity and subsequently tissue mechanics in ex vivo settings. Differences between stress-deprived and stress-loaded samples were only observed in the presence of viable cells (Hannafin et al. 1995).
Banes et al. (1999a,b) carried out some of the early ex vivo work on viable tendon samples, concentrating particularly on cellular mechanotransduction responses. The cyclic creep loading of whole avian tendon samples was found to stimulate DNA and collagen synthesis, in a response highly dependent upon gap junctions and the interconnectivity and signalling of the cells.
Considerable controversy exists as to the possible role of systemic inflammatory reactions in overuse tendinopathy, and as such, the initiation of inflammatory pathways has been investigated in ex vivo fatigue studies. In one cyclic creep study, aggressively loaded samples were found to exhibit significantly reduced mechanical strengths as well as higher concentrations of the inflammatory mediator prostaglandin E2 (PGE2). It was suggested that the release of PGE2 could contribute to the pathology of overuse tendinopathies by stimulating matrix metalloproteinase expression (Flick et al. 2006). Devkota et al. (2007) also hypothesized that PGE2 was a key contributor to the induction of fatigue damage, showing that tendons undergoing loading over long periods of time became significantly weaker than control groups and that cellular turnover and collagenase production also increased. However, in contrast to these findings, a cyclic stress relaxation study found MMP-1 production to be inhibited for low-strain, low-frequency cyclic loading and eliminated entirely at high strains and high frequencies (Lavagnino et al. 2003).
Further controversy exists concerning the behaviour of the cells themselves in response to fatigue. In a light microscopy study of high-strain cyclic stress relaxation loading, loaded tendon samples were found to be associated with the presence of numerous apoptotic cells, whilst control tendons (unloaded) showed very few apoptotic cells (Scott et al. 2005). Another study, however, found no change in cell viability between unloaded and aggressively loaded avian tendon explants (Flick et al. 2006). These examples highlight the variability in cellular response and matrix metabolism during fatigue loading of viable tendon and the considerable challenges that exist in drawing firm conclusions. There is undoubtedly a need to correlate cellular response with tendon function and to consider more fully the significance, if any, of the choice of cyclic creep or stress relaxation testing.
Whilst the majority of the testing of viable tendon has considered the bulk tendon level, fascicular behaviour has also been investigated. The considerable issues associated with the gripping of whole tendon samples can be overcome by fascicle testing, and cross sections are generally much more consistent along the length than observed in the bulk material. Furthermore, considering fatigue properties at smaller hierarchical levels than the bulk tendon can provide additional information about a structure, although careful validation is required and care must be taken not to assume that bulk tendon properties are defined at the fascicular level. Indeed, when considering quasi-static properties, Thorpe et al. (2011) presented work that showed the interfascicular matrix to have a significant effect on bulk tendon mechanics.
Mechanical stimulation of tendon fascicles has been shown to upregulate collagen production and enhance its retention within the matrix, as well as upregulating IL-6 production in a possible adaptive response to exercise (Screen et al. 2005; Maeda et al. 2007; Legerlotz et al. 2011). In an investigation into the mechanics of cyclically stress-relaxed fascicles, it was found that 5 h of cyclic loading at 30% failure strain was required to see a statistically significant decrease in the quasi-static ultimate tensile strength, yet when the strain was increased to 60% of the strain to failure, a statistically significant decrease was observed in just 15 min (Legerlotz et al. 2011). In the same study, it was observed that changes in cell metabolism in response to loading were observed well before any changes in the fascicle mechanics (Legerlotz et al. 2011).
The use of fascicles in the characterization of fatigue behaviour may also allow for more straightforward imaging of the effect of fatigue on cellular morphology and processes in combination with an understanding of matrix structure changes (Figure 4). Furthermore, postfatigue microstraining experiments, using the methods developed by Screen et al. (2004b), could be considered to investigate the effect of fatigue damage on mechanics at the fibre level.
Figure 4.
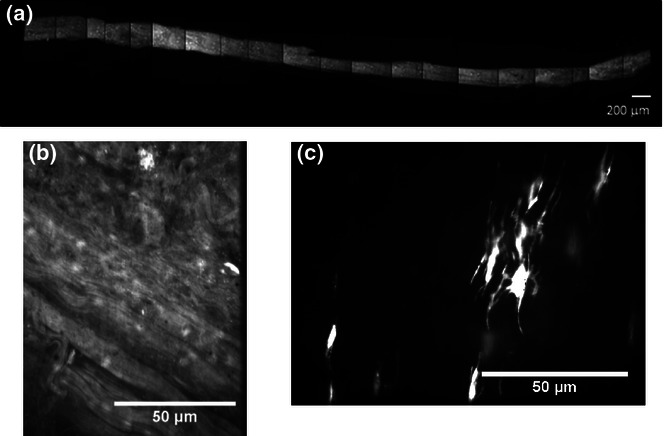
Characterization of fatigue damage in the tendon fascicle (bovine extensor tendon fascicle after 300 cycles of creep loading) using a combination of high-concentration acridine orange staining for collagen structure and calcein AM for cellular morphology: (a) low-magnification composite image of the fascicle length, (b) matrix structure after 300 cycles of creep loading and (c) calcein AM staining of tenocytes within the tendon fascicle after fatigue loading.
In vivo studies
Overuse animal models
The in vitro testing methods discussed thus far can provide very controlled loading conditions, but cannot mirror the complexity of the native tissue environment. In vivo, so-called animal overuse models, overcome this by enabling consideration of cellular responses within the native tissue environment. However, the degree of reproducibility of loading can be harder to control.
Animal models of tendinopathy can be divided into two – chemically induced models and mechanically induced ones. Chemically induced models include the injection of collagenase to induce collagen breakdown (Foland et al. 1992; Soslowsky et al. 1996), cytokines to induce an inflammatory response (Stone et al. 1999) or the injection of prostaglandins (Sullo et al. 2001; Khan et al. 2005) or fluoroquinolone (Kato et al. 1995; Simonin et al. 2000) both believed to have an involvement in tendinopathy. These models allow a study of the interplay between inflammatory cells, mechanical loading and tissue healing (Lake et al. 2008) and are typically less labour intensive than mechanical means and generally provide more consistent tendon damage (Dirks & Warden 2011); however, they cannot be considered a route for fatigue testing of tendon and thus will not be considered further.
Mechanically induced models should be beneficial because they are designed to induce injury through repetitive loading, similar to how tendinopathy is believed to develop in the human condition. However, the process is typically sped up in the in vivo model, with a more rigorous loading protocol, and it should not be assumed that damage accumulation will be entirely consistent with chronic tendinopathy. Mechanical means of generating fatigue damage include electrical muscle stimulation, where the muscle is stimulated so as to produce contraction and loading of the tendon, repetitive stretching and grabbing activities, and treadmill running, which is typically either downhill or uphill, to ensure eccentric muscle contraction and the exertion of greater forces (Dirks & Warden 2011). Small animals are generally used for these mechanical models and with no clear evidence of tendinopathy in these animals during life, and it is difficult to establish how the aetiology of overuse may relate between species or how the pathophysiology may vary.
In 1999, Soslowsky et al. (2000) used a treadmill running model to investigate the histological and biomechanical effects of overuse activity on the supraspinatus of the rat. Tendons in the exercised animals exhibited larger diameters at all time points and significantly decreased modulus and ultimate tensile stress (UTS), in addition to increased cellularity and a reduction in the organization of the collagen structure. In a similar, more recent model, hypervascularity, glycosaminoglycan accumulation and collagen fragmentation have also been observed in response to fatigue (Scott et al. 2007). Scott et al. (2007) observed that in a downhill running rat model, early tendinosis was associated with local stimulation of tenocytes and not extrinsic inflammation or apoptosis. Conversely, Millar et al. (2009) reported fatigue-induced expression of cytokines – IL-18, IL-15 and IL-64, increased expression of mediators of apoptosis and increased levels of heat shock proteins. They also reported increased expression of cartilage-associated genes, but reduced levels of typical tendon genes, with overuse (Millar et al. 2009).
When a similar downhill treadmill running model was used in order to induce injury in the rat Achilles, no significant variations were observed between control and exercise groups in either biomechanics or geometrics (Huang et al. 2004). The absence of damage was hypothesized to result from either the larger size of the Achilles compared with the supraspinatus or because Achilles damage only results from acute injury in rats (Huang et al. 2004). Another study that considered uphill running in rats found running and non-running animals to exhibit grossly similar Achilles tendon with cross-sectional areas that did not exhibit significant variation (Glazebrook et al. 2008). However, histology showed the tendons of the running rats to be significantly more disorganized with a higher cellular density (Glazebrook et al. 2008).
Voluntary forelimb repetitive reaching and grasping tasks have also been used to create overuse in the rat (Barbe et al. 2003). After 5–6 weeks, the repeated task was observed to result in significantly decreased motor performance and widespread tissue responses, with rats unable to maintain their baseline reach rate. Furthermore, at week 6, fraying of tendon fibrils was observed in the midforelimb. Electrical stimulation studies also report degenerative changes in the tendon at the 5–6-week time frame, including the presence of inflammatory cells, increased numbers of capillaries, oedema and fibrosis in the paratenon (Backman et al. 1990). Whilst not all overuse in vivo models report increased vascularity or inflammatory cells, microtears do appear to be consistently evident (Nakama et al. 2005).
An additional finding of electrical stimulation models highlights the complex interplay between mechanical and cellular response to overuse. It is commonplace in these models to use the contralateral limb as the control. However, Asundi et al. (2008) investigated the mRNA levels of many tendinopathy-associated proteins in both loaded and contralateral limbs and found no differences. In gene expression analysis, changes in contralateral limbs have also been observed during electrical stimulation, possibly as a result of central neuronal mechanisms (Andersson et al. 2011). Not only do these studies highlight that the contralateral limb should perhaps not be considered a suitable control but they also point to a clear cellular contribution to degenerative matrix changes with tendinopathy.
Controlled in vivo loading
Whilst overuse models overcome some of the limitations of ex vivo testing, they may not necessarily ensure measurable and standardized loading across the tendons due to natural variations in gait, anatomical size, strength or temperament (Fung et al. 2010; Andarawis-Puri & Flatow 2011). To address this limitation, novel, controlled, in vivo models have also been developed, for applying specified loading conditions (albeit acutely) to tendons in small animal models, initially in the rat (Fung et al. 2010). The limb of the anaesthetized animal to be fatigued is secured to an adjustable testing frame (Figure 5), and the required tendon fatigue loading protocol applied directly to the in situ tendon in order to explore the mechanisms of tendon degeneration. Following completion of the loading protocol, animals are allowed to resume normal cage activity, prior to euthanasia and tendon characterization at a later time point.
Figure 5.
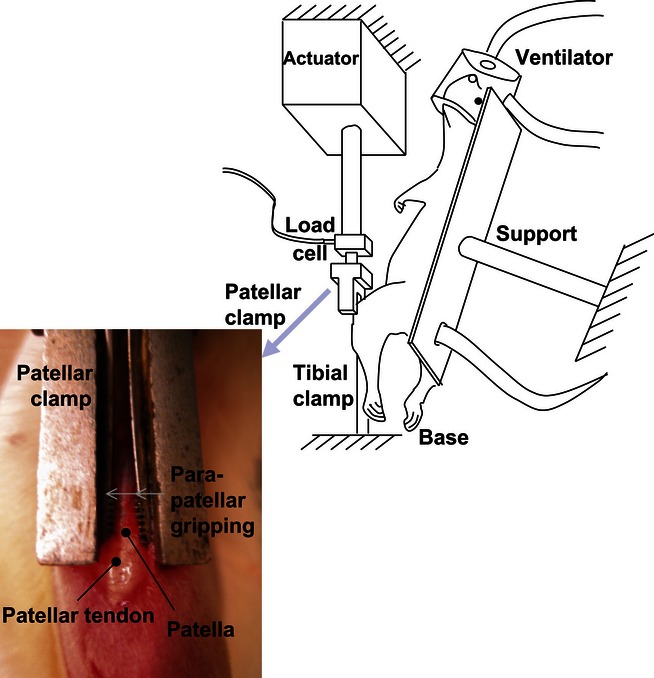
Experimental set-up of controlled in vivo loading in a rat model. (Reprinted from Fung et al. 2010, with permission from Elsevier).
The biomechanics and microstructural progression of fatigue damage can be characterized using mechanical testing and second harmonic generation imaging (SHG) (Fung et al. 2010; Andarawis-Puri & Flatow 2011; Andarawis-Puri et al. 2012; Neviaser et al. 2012). However, gene expression changes (Fung et al. 2010) and the presence of MMPs and inflammatory cytokines can also be more appropriately assessed (Sun et al. 2008). Whilst there is concern that small animal models do not develop tendinopathy in the same manner as humans and large mammals, the breadth of these investigations has nevertheless facilitated a wider understanding of how fatigue damage may progress under controlled conditions in vivo.
The advent of SHG imaging has additionally provided a unique insight into collagen fibril architecture in minimally processed tendon allowing a clear description of the progression of fatigue damage (Figure 6) as summarized in the paper by Neviaser et al. (2012). By correlating this with mechanical data (Fung et al. 2010), a more complete picture of fatigue accumulation can be developed (Table 1). It is notable that the progression of structural and mechanical changes with fatigue damage appears to be broadly consistent across all of the reviewed methods of fatigue damage generation.
Figure 6.
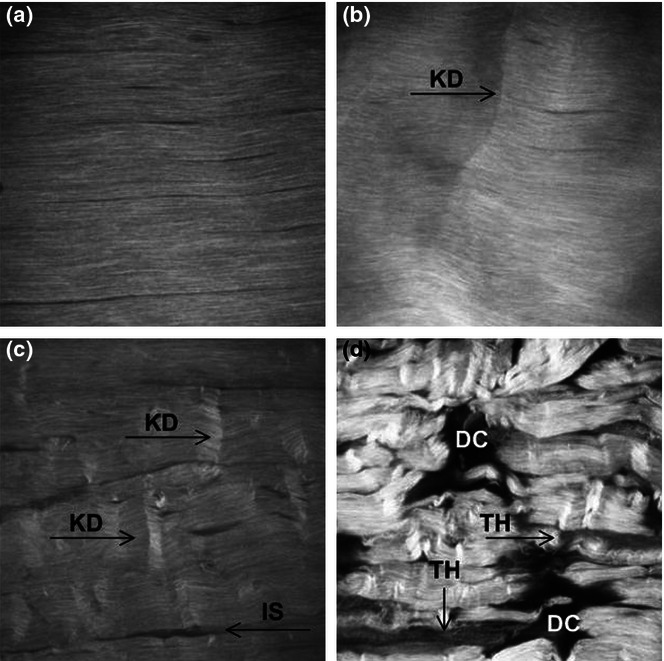
Second harmonic generation imaging of the progression of fatigue damage during controlled in vivo loading. (A) Control tendons without fatigue, (B) Low fatigue demonstrating isolated kinked fibre deformations (KD), (C) Moderate fatigue showing increased kinking and widening of the interfibre space (IS), (D) High fatigue showing severe matrix disruption, poor fibre alignment, and greater widening of interface space. Regions of low signal intensity suggest fibre thinning (TH) and more severely, matrix discontinuities (DC). Field of view = 400 microns (Reprinted from Fung et al (2010), with permission from Elsevier).
Table 1.
The progression of fatigue damage during controlled in vivo loading; the effect on collagen fibril architecture and mechanics. Data collated from Neviaser et al. (2012) to Fung et al. (2010)
| Level of fatigue | Collagen fibril architecture | Mechanics |
|---|---|---|
| No fatigue | Aligned collagen fibrils, exhibiting typical collagen crimp | |
| Low-level fatigue | Kinked fibre deformations | Increased stiffness and decreased hysteresis |
| Moderate fatigue | Kinked fibre deformation with widening of the interfibre space | Mechanics did not differ from low-level fatigue samples |
| High-level fatigue | Severe matrix disruption, fibre thinning, angulations and fibre discontinuities | Reduction in stiffness and increase in energy dissipation alongside steep increase in tendon strain |
The particular advantage of controlled in vivo testing lies in the assessment of systemically controlled molecular changes at all stages of fatigue damage generation (Andarawis-Puri & Flatow 2011). In one study, the temporal profiling of gene expression with loading indicated that the molecular profile after 100 cycles (representative of exercise) is anabolic, whilst after 7200 cycles (moderate-level fatigue loading), this had become catabolic (Sun et al. 2010). Studies have also shown that significant variation in the expression of collagen 1 and 3 as well as interleukin-1β and MMP-13 is only observed in moderate and high levels of fatigue (Andarawis-Puri & Flatow 2011). This suggests a threshold of loading that once overcome changes the tendon response from beneficial to degenerative. It may be that a certain amount of accumulated damage is necessary to induce molecular inflammation, and once induced, this may play a role in altering the response of the tendon to further loading (Andarawis-Puri & Flatow 2011).
Controlled in vivo loading rigs have more recently been developed to investigate fatigue damage generation in mice, mainly to take advantage of the abundance of well-characterized in-bred genetic strains of mice and the established technologies for knocking out their specific genes (Sereysky et al. 2012). Collagen architecture and damage accumulation have been shown to be similar to that of the rat, with damage initially leading to changes in structure (increase in damage area fraction), which subsequently result in change in function. Indeed, one outcome of these studies is to concur with the earlier in vitro studies, suggesting that damage area fraction should be considered an early indicator of fatigue damage (Sereysky et al. 2012).
However, mouse and rat models may not be ideal for characterizing tendinopathy, and there remains an incomplete understanding of the behaviour and physiology of these animals. As previously outlined, there is no evidence of the development of tendinopathy in small rodents during normal life and it is suggested that they may also use different mechanisms for balancing, locomotion and pain perception compared with humans, and as such, fatigue response could also differ (Lui et al. 2011). Furthermore, with ethical limitations to the amount of time for which animals can be anaesthetized, damage can only be generated acutely. Tendinopathy is not believed to be an acute condition, but instead occurs over months or possibly years. Nevertheless, in vivo models such as this can provide valuable insights into the cumulative damage process as the amount of induced fatigue damage can be carefully controlled.
Tendinopathy – correlating tissue biopsies with fatigue-damaged samples
Although tissue biopsies can provide information on molecular changes, microstructural variation and mechanics in late-stage tendinopathy, they do not tell us about the progression of the disease. However, comparison of biopsy data with that achieved from the fatigue loading of tendon is critical in order to ascertain the value of various fatigue loading protocols.
Generally, biopsies from tendinopathic tissue have demonstrated an abnormal fibril and fibre morphology compared with normal controls (Józsa & Kannus 1997; Movin et al. 1997; Tallon et al. 2001; Kongsgaard et al. 2010). For example, Movin et al. (1997) considered biopsy specimens from patients with long-standing achillodynia and found that slight and moderate cases exhibited a separation of the fibres with increased waviness, whilst extreme cases showed a total loss of fine fibre structure and hyalinization. However, whilst Järvinen et al. found spontaneously ruptured tendons to display focal regions with decreased collagen fibre thickness, decreased crimp angle and disrupted crimp continuity (Järvinen et al. 2004), Magnusson et al. (2002) did not observe any change in crimp morphology although a decrease in the number of larger diameter fibrils in the core of ruptured tendons was reported. In agreement with observations of damage within fatigue-loaded samples, Józsa and Kannus (1997) reported only patches where fragmentation and fraying of collagen fibres were seen, along with loss of the normal wavy alignment.
Not only are the microstructural changes in tissue biopsies generally consistent with those in fatigue models, but there is also evidence they may precede cellular, molecular and mechanical changes. Kongsgaard et al. (2010) observed that whilst fibril morphology was abnormal in tendinopathic biopsy specimens, in vivo tendon mechanical properties did not appear altered. In another study, only once, cases of tendinopathy had developed to a moderate stage were decreases in tenocyte number observed, and as pathologic changes progressed (largely in spontaneously ruptured specimens), the nuclei became progressively more rounded (Tallon et al. 2001). An increase in the glycosaminoglycan and non-collagenous extracellular matrix has also been observed in late-stage tendinopathy (Movin et al. 1997).
Another key area of interest is the role of inflammation in tendinopathy and rupture. Just as in many of the fatigue models discussed, an absence of inflammatory cells is most commonly reported in tissue biopsies from both tendinopathic and ruptured tendon (Jozsa et al. 1990; Kannus & Jozsa 1991; Fredberg & Stengaard-Pedersen 2008). However, in a study from 2003, immunohistochemical staining identified acute inflammation in all of 60 ruptured tendons (Cetti et al. 2003). Biochemically, the presence of inflammatory mediators such as COX-2 and PGE-2 is reported in some studies (Fu et al. 2002; Fredberg & Stengaard-Pedersen 2008), but others found no upregulation of mRNA for the investigated cytokines and cytokine receptors in chronic Achilles tendinopathy, suggesting no chemical inflammation (Alfredson et al. 2003). It is important to note that tendinopathy and spontaneous tendon rupture may have different aetiologies, but there is little clear evidence of how they may differ or if inflammation may be related to just a subset of tendon conditions.
Conclusion
Whilst tissue biopsies may provide some information concerning late-stage tendinopathy in situ, there is still little information about the early-stage disease. This means that despite the prevalence of tendinopathy, its clinical management is limited. The correlation between the data from fatigue models and the microstructural, biological and mechanical changes observed in tendinopathic tissue biopsies does suggest that tendinopathy is associated with the fatigue damage of tendon. Nevertheless, data from the contralateral limbs in in vivo models highlight the importance of the cells, and whilst interactions between cell response and structurally induced damage remain unclear, they are undoubtedly critical.
There is unfortunately no one method that can provide a complete understanding of the development of tendinopathy. Testing of non-viable bulk tendon samples is comparatively straightforward and provides the clearest information concerning the mechanics of fatigue damage, for example, the importance of strain as the primary mechanical parameter governing tendon damage accumulation and injury. It also provides evidence concerning the nature of failure, but it cannot take into account in vivo processes of healing and remodelling or provide any information concerning cellular response to fatigue damage. Ex vivo testing of viable tendon samples provides information concerning the initial cellular and matrix response to loading but still cannot take into account in vivo healing, and considerable complications exist in maintaining tissue viability. The ex vivo testing of fascicles may provide the opportunity of understanding the effect of fatigue on tendon's various hierarchical levels but care should be taken not to assume bulk tendon properties are defined at the fascicular level. Overuse in vivo models enable the consideration of cellular responses within the native tissue environment and can allow consideration of mechanics and tissue degradation after a considerable fatigue period, but the degree of reproducibility of loading and thus its effects can be harder to control. In vivo fatigue testing allows the application of very controlled levels of fatigue to natural tissue, but with ethical consideration regarding animals under anaesthesia, one can only ever use small animals and generate acute damage.
Multiple testing routes support the hypothesis that matrix damage is the primary indicator of fatigue damage and that this is observed before any decrease in mechanical properties. The nature and progression of fatigue damage show real similarity between the various fatigue testing routes, both with and without the presence of viable cells, and also correlate strongly with biopsy samples. Observed microstructural changes supported the changes in mechanical properties, whilst cellular responses to fatigue, including the expression of inflammatory mediators, were more complex and contradictions existed between the various studies. It appears likely, however, that there is a threshold of loading that once overcome, changes the response of loading of the tendon from beneficial to degenerative. The role of inflammation in tendinopathy still remains controversial but it appears likely that inflammatory factors will only ever occur with quite a significant degree of matrix damage. Whilst close similarities exist between matrix damage in fatigue loading and in tendinopathic specimens, the tendinopathic situation should not be oversimplified. The exact in vivo loading conditions of human tendon remain unknown and the healing of some cases of tendinopathy poorly investigated. Care must also be taken with the assumption that tendinopathy and spontaneous tendon rupture have the same aetiology and follow the same progression of microdamage accumulation. Further research is essential to better understand the loading environment in situ so as to improve further the models of fatigue damage generation.
Conflict of interest
The authors have no conflict of interest.
Funding source
This work was supported by the Wellcome Trust, Grant No. WT087112.
References
- Abrahams M. Mechanical behaviour of tendon in vitro. Med. Biol. Eng. Comput. 1967;5:433–443. doi: 10.1007/BF02479137. [DOI] [PubMed] [Google Scholar]
- Alfredson H, Lorentzon M, Backman S, Backman A, Lerner UH. cDNA-arrays and real-time quantitative PCR techniques in the investigation of chronic Achilles tendinosis. J. Orthop. Res. 2003;21:970–975. doi: 10.1016/S0736-0266(03)00107-4. [DOI] [PubMed] [Google Scholar]
- Andarawis-Puri N, Flatow EL. Tendon fatigue in response to mechanical loading. J. Musculoskelet. Neuronal Interact. 2011;11:106–114. [PMC free article] [PubMed] [Google Scholar]
- Andarawis-Puri N, Sereysky JB, Jepsen KJ, Flatow EL. The relationships between cyclic fatigue loading, changes in initial mechanical properties, and the in vivo temporal mechanical response of the rat patellar tendon. J. Biomech. 2012;45:59–65. doi: 10.1016/j.jbiomech.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson G, Forsgren S, Scott A, et al. Tenocyte hypercellularity and vascular proliferation in a rabbit model of tendinopathy: contralateral effects suggest the involvement of central neuronal mechanisms. Br. J. Sports Med. 2011;45:399–406. doi: 10.1136/bjsm.2009.068122. [DOI] [PubMed] [Google Scholar]
- Archambault J. Tendon micromechanics and research methods in tendinopathy. J. Musculoskelet. Neuronal Interact. 2003;3:326–328. [PubMed] [Google Scholar]
- Asundi K, King K, Rempel D. Evaluation of gene expression through qRT-PCR in cyclically loaded tendons: an in vivo model. Eur. J. Appl. Physiol. 2008;102:265–270. doi: 10.1007/s00421-007-0582-9. [DOI] [PubMed] [Google Scholar]
- Backman C, Boquist L, Fridén J, Lorentzon R, Toolanen G. Chronic achilles paratenonitis with tendinosis: An experimental model in the rabbit. J. Orthop. Res. 1990;8:541–547. doi: 10.1002/jor.1100080410. [DOI] [PubMed] [Google Scholar]
- Banes AJ, Horesovsky G, Larson C, et al. Mechanical load stimulates expression of novel genes in vivo and in vitro in avian flexor tendon cells. Osteoarthr. Cartil. 1999a;7:141–153. doi: 10.1053/joca.1998.0169. [DOI] [PubMed] [Google Scholar]
- Banes AJ, Weinhold P, Yang X, et al. Gap junctions regulate responses of tendon cells ex vivo to mechanical loading. Clin. Orthop. Relat. Res. 1999b;367:S356–S370. doi: 10.1097/00003086-199910001-00034. [DOI] [PubMed] [Google Scholar]
- Barbe MF, Barr AE, Gorzelany I, Amin M, Gaughan JP, Safadi FF. Chronic repetitive reaching and grasping results in decreased motor performance and widespread tissue responses in a rat model of MSD. J. Orthop. Res. 2003;21:167–176. doi: 10.1016/S0736-0266(02)00086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Kaiser E, Milz S. Structure–function relationships in tendons: a review. J. Anat. 2008;212:211–228. doi: 10.1111/j.1469-7580.2008.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch HL, Worboys S, Eissa S, Jackson B, Strassburg S, Clegg PD. Matrix metabolism rate differs in functionally distinct tendons. Matrix Biol. 2008;27:182–189. doi: 10.1016/j.matbio.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Cetti R, Junge J, Vyberg M. Spontaneous rupture of the Achilles tendon is preceded by widespread and bilateral tendon damage and ipsilateral inflammation – a clinical and histopathologic study of 60 patients. Acta Orthop. Scand. 2003;74:78–84. doi: 10.1080/00016470310013707. [DOI] [PubMed] [Google Scholar]
- Devkota AC, Tsuzaki M, Almekinders LC, Banes AJ, Weinhold PS. Distributing a fixed amount of cyclic loading to tendon explants over longer periods induces greater cellular and mechanical responses. J. Orthop. Res. 2007;25:1078–1086. doi: 10.1002/jor.20389. [DOI] [PubMed] [Google Scholar]
- Dirks RC, Warden SJ. Models for the study of tendinopathy. J. Musculoskelet. Neuronal Interact. 2011;11:141–149. [PubMed] [Google Scholar]
- Flick J, Devkota A, Tsuzaki M, Almekinders L, Weinhold P. Cyclic loading alters biomechanical properties and secretion of PGE2 and NO from tendon explants. Clin. Biomech. 2006;21:99–106. doi: 10.1016/j.clinbiomech.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Foland JW, Trotter GW, Powers BE, Wrigley RH, Smith FW. Effect of sodium hyaluronate in collagenase-induced superficial digital flexor tendinitis in horses. Am. J. Vet. Res. 1992;53:2371–2376. [PubMed] [Google Scholar]
- Franchi M, Trire A, Quaranta M, Orsini E, Ottani V. Collagen structure of tendon relates to function. ScientificWorldJournal. 2007;7:404–420. doi: 10.1100/tsw.2007.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredberg U, Stengaard-Pedersen K. Chronic tendinopathy tissue pathology, pain mechanisms, and etiology with a special focus on inflammation. Scand. J. Med. Sci. Sports. 2008;18:3–15. doi: 10.1111/j.1600-0838.2007.00746.x. [DOI] [PubMed] [Google Scholar]
- Fu SC, Wang W, Pau HM, Wong YP, Chan KM, Rolf CG. Increased expression of transforming growth factor-beta1 in patellar tendinosis. Clin. Orthop. Relat. Res. 2002;17:4–183. doi: 10.1097/00003086-200207000-00022. [DOI] [PubMed] [Google Scholar]
- Fung DT, Wang VM, Laudier DM, et al. Subrupture tendon fatigue damage. J. Orthop. Res. 2009;27:264–273. doi: 10.1002/jor.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung DT, Wang VM, Andarawis-Puri N, et al. Early response to tendon fatigue damage accumulation in a novel in vivo model. J. Biomech. 2010;43:274–279. doi: 10.1016/j.jbiomech.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook MA, Wright JR, Langman M, Stanish WD, Lee JM. Histological analysis of Achilles tendons in an overuse rat model. J. Orthop. Res. 2008;26:840–846. doi: 10.1002/jor.20546. [DOI] [PubMed] [Google Scholar]
- Hannafin JA, Arnoczky SP, Hoonjan A, Torzilli PA. Effect of stress deprivation and cyclic tensile loading on the material and morphologic properties of canine flexor digitorum profundus tendon: An in vitro study. J. Orthop. Res. 1995;13:907–914. doi: 10.1002/jor.1100130615. [DOI] [PubMed] [Google Scholar]
- Huang T-F, Perry S, Soslowsky L. The effect of overuse activity on Achilles tendon in an animal model: a biomechanical study. Ann. Biomed. Eng. 2004;32:336–341. doi: 10.1023/b:abme.0000017537.26426.76. [DOI] [PubMed] [Google Scholar]
- James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J. Hand Surg. Am. 2008;33:102–112. doi: 10.1016/j.jhsa.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Järvinen TAH, Teppo LN, Järvinen TLN, Kannus K, Józsa L, Järvinen M. Collagen fibres of the spontaneously ruptured human tendons display decreased thickness and crimp angle. J. Orthop. Res. 2004;22:1303–1309. doi: 10.1016/j.orthres.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Józsa L, Kannus P. Histopathological findings in spontaneous tendon ruptures. Scand. J. Med. Sci. Sports. 1997;7:113–118. doi: 10.1111/j.1600-0838.1997.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Jozsa L, Reffy A, Kannus P, Demel S, Elek E. Pathological alterations in human tendons. Arch. Orthop. Trauma Surg. 1990;110:15–21. doi: 10.1007/BF00431359. [DOI] [PubMed] [Google Scholar]
- Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon – a controlled study of 891 patients. J. Bone Joint Surg. Am. 1991;73A:1507–1525. [PubMed] [Google Scholar]
- Kato M, Takada S, Kashida Y, Nomura M. Histological examination on achilles tendon lesions induced by quinolone antibacterial agents in juvenile rats. Toxicol. Pathol. 1995;23:385–392. doi: 10.1177/019262339502300315. [DOI] [PubMed] [Google Scholar]
- Ker RF. Mechanics of tendon, from an engineering perspective. Int. J. Fatigue. 2007;29:1001–1009. [Google Scholar]
- Ker RF, Wang XT, Pike AVL. Fatigue quality of mammalian tendons. J. Exp. Biol. 2000;203:1317–1327. doi: 10.1242/jeb.203.8.1317. [DOI] [PubMed] [Google Scholar]
- Khan MH, Li Z, Wang JH-C. Repeated exposure of tendon to prostaglandin-E2 leads to localized tendon degeneration. Clin. J. Sport Med. 2005;15:27–33. doi: 10.1097/00042752-200501000-00006. [DOI] [PubMed] [Google Scholar]
- Kjær M, Langberg H, Heinemeier K, et al. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand. J. Med. Sci. Sports. 2009;19:500–510. doi: 10.1111/j.1600-0838.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- Komi PV, Fukashiro S, Järvinen M. Biomechanical loading of Achilles tendon during normal locomotion. Clin. Sports Med. 1992;11:521–531. [PubMed] [Google Scholar]
- Kongsgaard M, Qvortrup K, Larsen J, et al. Fibril morphology and tendon mechanical properties in patellar tendinopathy: effects of heavy slow resistance training. Am. J. Sports Med. 2010;38:749–756. doi: 10.1177/0363546509350915. [DOI] [PubMed] [Google Scholar]
- Lake SP, Ansorge HL, Soslowsky LJ. Animal models of tendinopathy. Disabil. Rehabil. 2008;30:1530–1541. doi: 10.1080/09638280701785460. [DOI] [PubMed] [Google Scholar]
- Lanir Y. Structure–strength relations in mammalian tendon. Biophys. J. 1978;24:541–554. doi: 10.1016/S0006-3495(78)85400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavagnino M, Arnoczky SP, Tian T, Vaupel Z. Effect of amplitude and frequency of cyclic tensile strain on the inhibition of MMP-1 mRNA expression in tendon cells: an in vitro study. Connect. Tissue Res. 2003;44:181–187. doi: 10.1080/03008200390215881. [DOI] [PubMed] [Google Scholar]
- Legerlotz K, Jones GC, Screen HRC, Riley GP. Cyclic loading of tendon fascicles using a novel fatigue loading system increases interleukin-6 expression by tenocytes. Scand. J. Med. Sci. Sports. 2011;23:31–37. doi: 10.1111/j.1600-0838.2011.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Yang G, Khan M, Stone D, Woo SL-Y, Wang JH-C. Inflammatory response of human tendon fibroblasts to cyclic mechanical stretching. Am. J. Sports Med. 2004;32:435–440. doi: 10.1177/0095399703258680. [DOI] [PubMed] [Google Scholar]
- Lui PPY, Maffulli N, Rolf C, Smith RKW. What are the validated animal models for tendinopathy? Scand. J. Med. Sci. Sports. 2011;21:3–17. doi: 10.1111/j.1600-0838.2010.01164.x. [DOI] [PubMed] [Google Scholar]
- Maeda E, Shelton JC, Bader DL, Lee DA. Time dependence of cyclic tensile strain on collagen production in tendon fascicles. Biochem. Bioph. Res. Co. 2007;362:399–404. doi: 10.1016/j.bbrc.2007.08.029. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Qvortrup K, Larsen JO, et al. Collagen fibril size and crimp morphology in ruptured and intact Achilles tendons. Matrix Biol. 2002;21:369–377. doi: 10.1016/s0945-053x(02)00011-2. [DOI] [PubMed] [Google Scholar]
- Millar NL, Wei AQ, Molloy TJ, Bonar F, Murrell GAC. Cytokines and apoptosis in supraspinatus tendinopathy. J. Bone Joint Surg. Br. 2009;91-B:417–424. doi: 10.1302/0301-620X.91B3.21652. [DOI] [PubMed] [Google Scholar]
- Movin T, Gad A, Reinholt FP, Rolf C. Tendon pathology in long-standing achillodynia: Biopsy findings in 40 patients. Acta Orthop. 1997;68:170–175. doi: 10.3109/17453679709004002. [DOI] [PubMed] [Google Scholar]
- Nakama LH, King KB, Abrahamsson S, Rempel DM. Evidence of tendon microtears due to cyclical loading in an in vivo tendinopathy model. J. Orthop. Res. 2005;23:1199–1205. doi: 10.1016/j.orthres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Neviaser A, Andarawis-Puri N, Flatow E. Basic mechanisms of tendon fatigue damage. J. Shoulder Elbow Surg. 2012;21:158–163. doi: 10.1016/j.jse.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent G, Huppé N, Langelier E. Low stress tendon fatigue is a relatively rapid process in the context of overuse injuries. Ann. Biomed. Eng. 2011;39:1535–1545. doi: 10.1007/s10439-011-0254-0. [DOI] [PubMed] [Google Scholar]
- Pike AV, Ker RF, Alexander RM. The development of fatigue quality in high- and low-stressed tendons of sheep (Ovis aries. J. Exp. Biol. 2000;203:2187–2193. doi: 10.1242/jeb.203.14.2187. [DOI] [PubMed] [Google Scholar]
- Qi J, Chi L, Bynum D, Banes AJ. Gap junctions in IL-1β-mediated cell survival response to strain. J. Appl. Physiol. 2011;110:1425–1431. doi: 10.1152/japplphysiol.00477.2010. [DOI] [PubMed] [Google Scholar]
- Rigby BJ, Hirai N, Spikes JD, Eyring H. The mechanical properties of rat tail tendon. J. Gen. Physiol. 1959;43:265–283. doi: 10.1085/jgp.43.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley G. Chronic tendon pathology: molecular basis and therapeutic implications. Expert Rev. Mol. Med. 2005;7:1–25. doi: 10.1017/S1462399405008963. [DOI] [PubMed] [Google Scholar]
- Schechtman H, Bader DL. In vitro fatigue of human tendons. J. Biomech. 1997;30:829–835. doi: 10.1016/s0021-9290(97)00033-x. [DOI] [PubMed] [Google Scholar]
- Scott A, Khan KM, Heer J, Cook JL, Lian O, Duronio V. High strain mechanical loading rapidly induces tendon apoptosis: an ex vivo rat tibialis anterior model. Br. J. Sports Med. 2005;39:e25. doi: 10.1136/bjsm.2004.015164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A, Cook JL, Hart DA, Walker DC, Duronio V, Khan KM. Tenocyte responses to mechanical loading in vivo: A role for local insulin-like growth factor 1 signaling in early tendinosis in rats. Arthritis Rheum. 2007;56:871–881. doi: 10.1002/art.22426. [DOI] [PubMed] [Google Scholar]
- Screen HRC. Investigating load relaxation mechanics in tendon. J. Mech. Behav. Biomed. Mater. 2008;1:51–58. doi: 10.1016/j.jmbbm.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Screen HRC, Lee DA, Bader DL, Shelton JC. An investigation into the effects of the hierarchical structure of tendon fascicles on micromechanical properties. Proc. Inst. Mech. Eng. H. 2004a;218:109–119. doi: 10.1243/095441104322984004. [DOI] [PubMed] [Google Scholar]
- Screen HRC, Bader DL, Lee DA, Shelton JC. Local strain measurement within tendon. Strain. 2004b;40:157–163. [Google Scholar]
- Screen HRC, Shelton JC, Bader DL, Lee DA. Cyclic tensile strain upregulates collagen synthesis in isolated tendon fascicles. Biochem. Biophys. Res. Commun. 2005;336:424–429. doi: 10.1016/j.bbrc.2005.08.102. [DOI] [PubMed] [Google Scholar]
- Sereysky JB, Andarawis-Puri N, Jepsen KJ, Flatow EL. Structural and mechanical effects of in vivo fatigue damage induction on murine tendon. J. Orthop. Res. 2012;30:965–972. doi: 10.1002/jor.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JH, Legerlotz K, Demirci T, Riley GP, Screen HRC. Norwich, UK: 2012. The Fatigue Behaviour of Functionally Distinct Bovine Tendons. BSMB Special Meeting – Tendinopathy – from basic science to treatment. [Google Scholar]
- Simonin M-A, Gegout-Pottie P, Minn A, Gillet P, Netter P, Terlain B. Pefloxacin-induced Achilles tendon toxicity in rodents: biochemical changes in proteoglycan synthesis and oxidative damage to collagen. Antimicrob. Agents Chemother. 2000;44:867–872. doi: 10.1128/aac.44.4.867-872.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J. Shoulder Elbow Surg. 1996;5:383–392. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- Soslowsky LJ, Thomopoulos S, Tun S, et al. Neer award 1999 overuse activity injures the supraspinatus tendon in an animal model: A histologic and biomechanical study. J. Shoulder Elbow Surg. 2000;9:79–84. [PubMed] [Google Scholar]
- Stone D, Green C, Rao U, et al. Cytokine-induced tendinitis: A preliminary study in rabbits. J. Orthop. Res. 1999;17:168–177. doi: 10.1002/jor.1100170204. [DOI] [PubMed] [Google Scholar]
- Sullo A, Maffulli N, Capasso G, Testa V. The effects of prolonged peritendinous administration of PGE1 to the rat Achilles tendon: a possible animal model of chronic Achilles tendinopathy. J. Orthop. Sci. 2001;6:349–357. doi: 10.1007/s007760100031. [DOI] [PubMed] [Google Scholar]
- Sun HB, Li Y, Fung DT, Majeska RJ, Schaffler MB, Flatow EL. Coordinate regulation of IL-1beta and MMP-13 in rat tendons following subrupture fatigue damage. Clin. Orthop. Relat. Res. 2008;466:1555–1561. doi: 10.1007/s11999-008-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HB, Andarawis-Puri N, Li Y, et al. Cycle-dependent matrix remodeling gene expression response in fatigue-loaded rat patellar tendons. J. Orthop. Res. 2010;28:1380–1386. doi: 10.1002/jor.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon C, Maffulli N, Ewen SW. Ruptured Achilles tendons are significantly more degenerated than tendinopathic tendons. Med. Sci. Sports Exerc. 2001;33:1983–1990. doi: 10.1097/00005768-200112000-00002. [DOI] [PubMed] [Google Scholar]
- Thornton GM, Oliynyk A, Frank CB, Shrive NG. Ligament creep cannot be predicted from stress relaxation at low stress: A biomechanical study of the rabbit medial collateral ligament. J. Orthop. Res. 1997;15:652–656. doi: 10.1002/jor.1100150504. [DOI] [PubMed] [Google Scholar]
- Thorpe CT, Udeze CP, Birch HL, Clegg PD, Screen HRC. Tendon material properties cannot be predicted by the material properties of the tendon fascicles. Int. J. Exp. Pathol. 2011;92:A10. [Google Scholar]
- Wall ME, Weinhold PS, Siu T, Brown TD, Banes AJ. Comparison of cellular strain with applied substrate strain in vitro. J. Biomech. 2007;40:173–181. doi: 10.1016/j.jbiomech.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Wang JHC. Mechanobiology of tendon. J. Biomech. 2006;39:1563–1582. doi: 10.1016/j.jbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Wang XT, Ker RF. Creep rupture of wallaby tail tendons. J. Exp. Biol. 1995;198:831–845. doi: 10.1242/jeb.198.3.831. [DOI] [PubMed] [Google Scholar]
- Wang XT, Ker RF, Alexander RM. Fatigue rupture of wallaby tail tendons. J. Exp. Biol. 1995;198:847–852. doi: 10.1242/jeb.198.3.847. [DOI] [PubMed] [Google Scholar]
- Wang JHC, Yang G, Li Z, Shen W. Fibroblast responses to cyclic mechanical stretching depend on cell orientation to the stretching direction. J. Biomech. 2004;37:573–576. doi: 10.1016/j.jbiomech.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Wang JHC, Guo Q, Li B. Tendon biomechanics and mechanobiology – a minireview of basic concepts and recent advancements. J. Hand Ther. 2012;25:133–141. doi: 10.1016/j.jht.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren TAL, Lindsey DP, Beaupré GS, Carter DR. Effects of creep and cyclic loading on the mechanical properties and failure of human Achilles tendons. Ann. Biomed. Eng. 2003;31:710–717. doi: 10.1114/1.1569267. [DOI] [PubMed] [Google Scholar]


