Abstract
Epitrochlearis muscles obtained from normal male Holtzman rats used as controls (C) and rats with reduced renal mass (Nx) fed isocaloric diets of varying protein content were incubated in Krebs-Ringer buffer containing 5 mM glucose for 1 or 3 h with or without insulin.
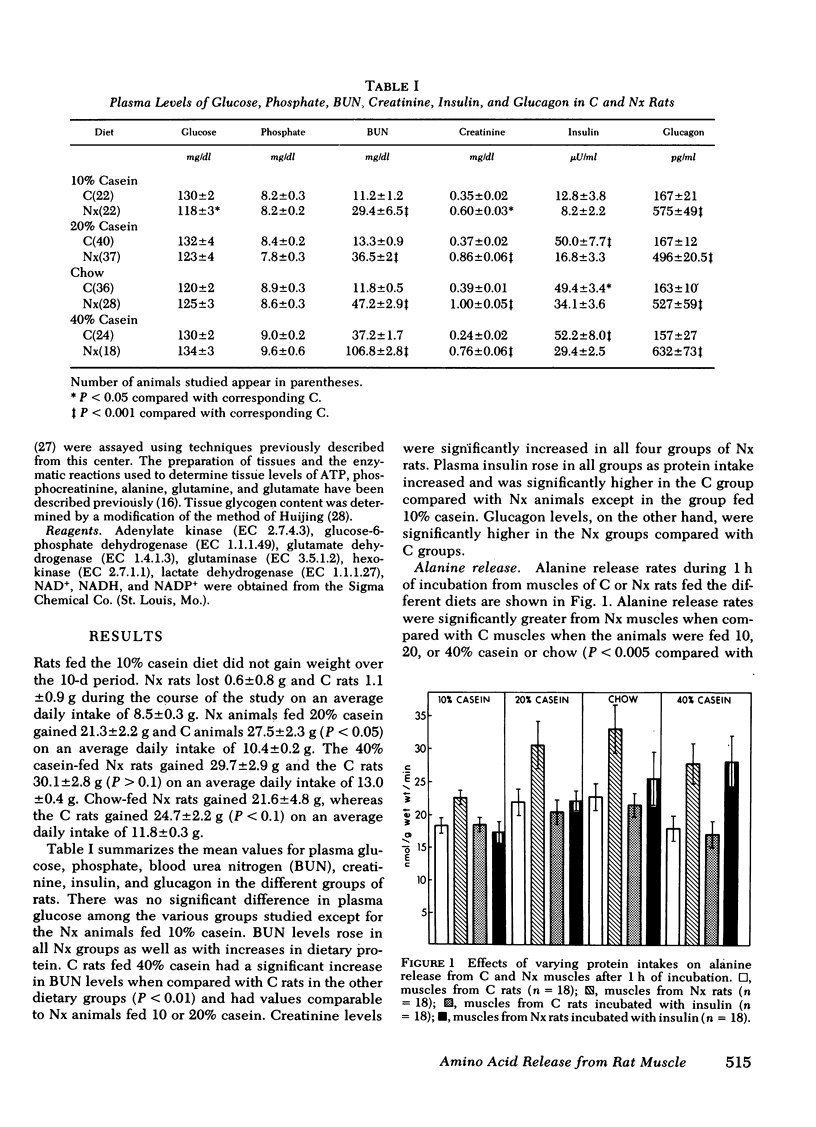
Alanine (ALA) release rates from muscles of Nx rats were increased 40% above C values after 1 h of incubation regardless of protein intake. Addition of insulin decreased the ALA release from muscles of Nx rats to C values in animals fed 10 and 20% casein and chow but did not in rats fed 40% casein. After 3 h of incubation, all ALA release rates decreased by ≅40%. The ALA release from muscles of Nx rats fed 10% casein was comparable to C values and decreased further with the addition of insulin. On the other hand, ALA release from muscles of Nx rats fed 20 and 40% casein as well as chow remained significantly elevated above C values, but responded to the addition of insulin with a reduction in release rates to C values, except from the muscles of Nx animals fed 40% casein.
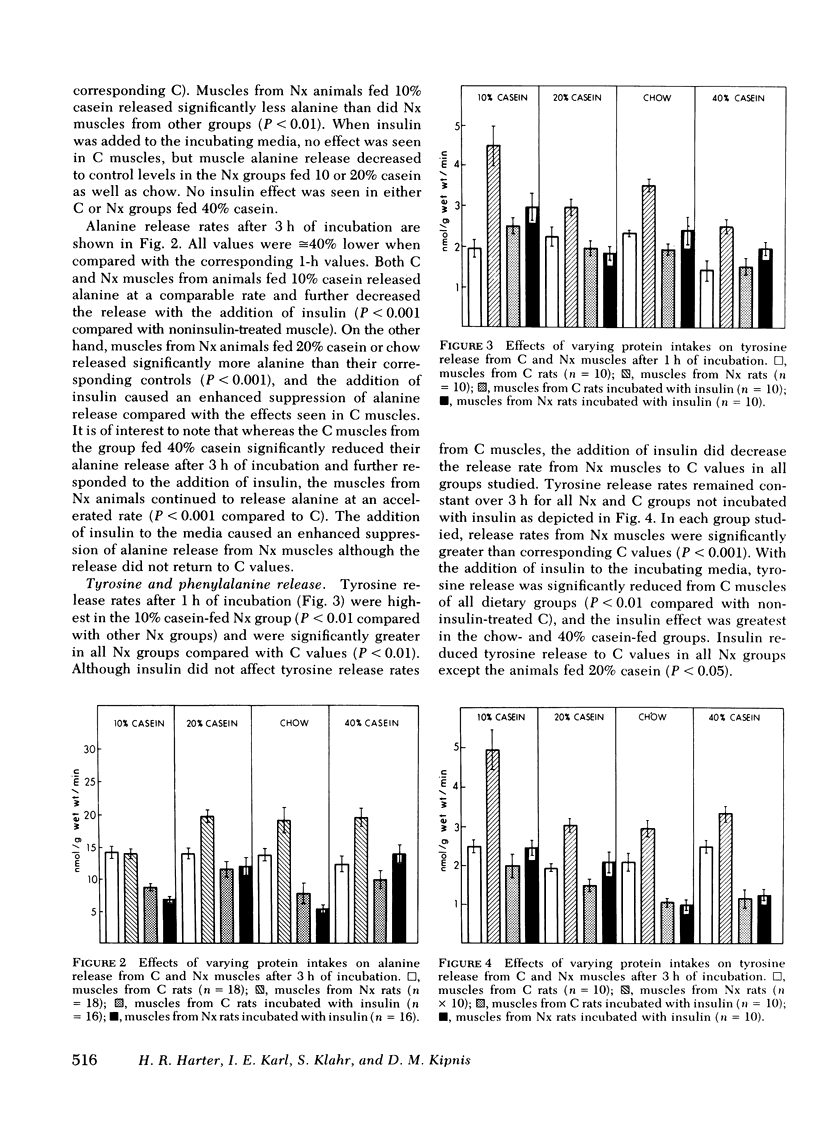
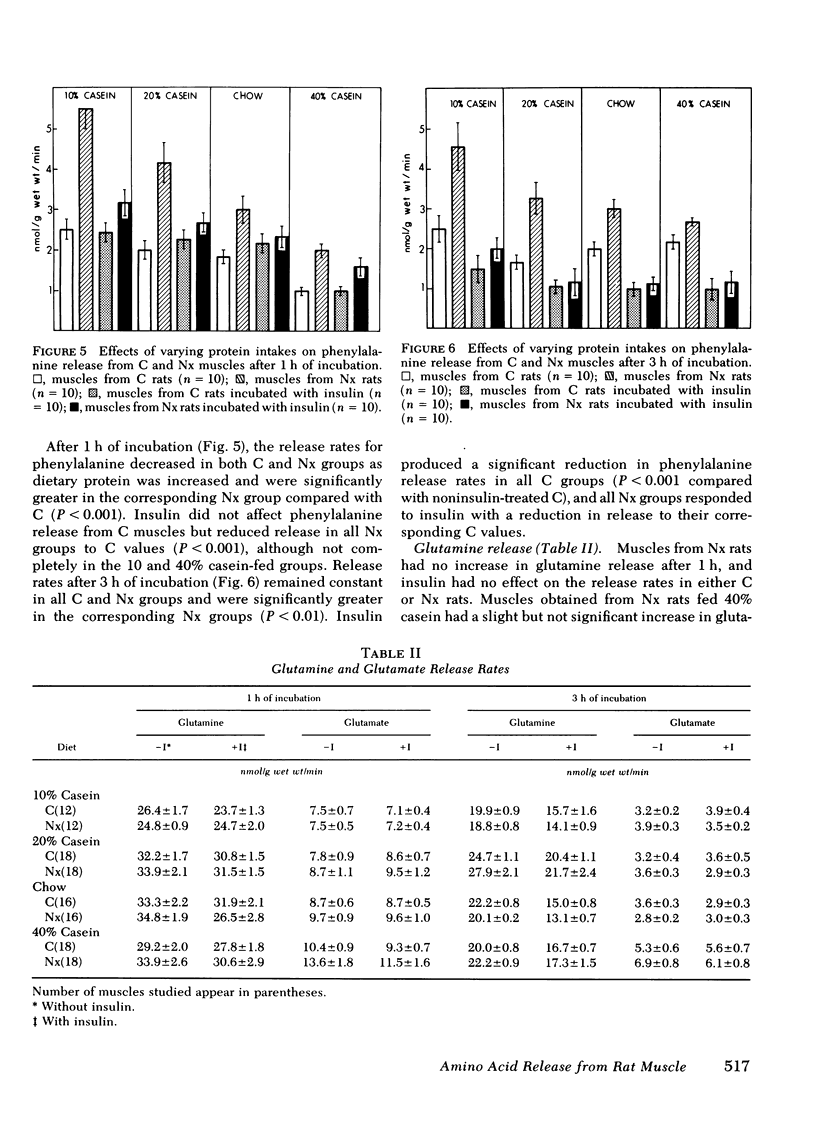
Tyrosine (TYR) and phenylalanine (PHE) release rates also were increased in muscles from Nx rats compared with C after 1 h of incubation. Release rates were highest in the Nx group fed 10% casein and decreased with increasing protein intake. Addition of insulin decreased the release rates of Nx rats to C values in each group. After 3 h of incubation, release rates of TYR and PHE in muscles from Nx rats remained significantly above C values for all groups, but responded to the addition of insulin with a decrease to C values. Glutamine and glutamate release were not significantly affected by reduction in renal mass.
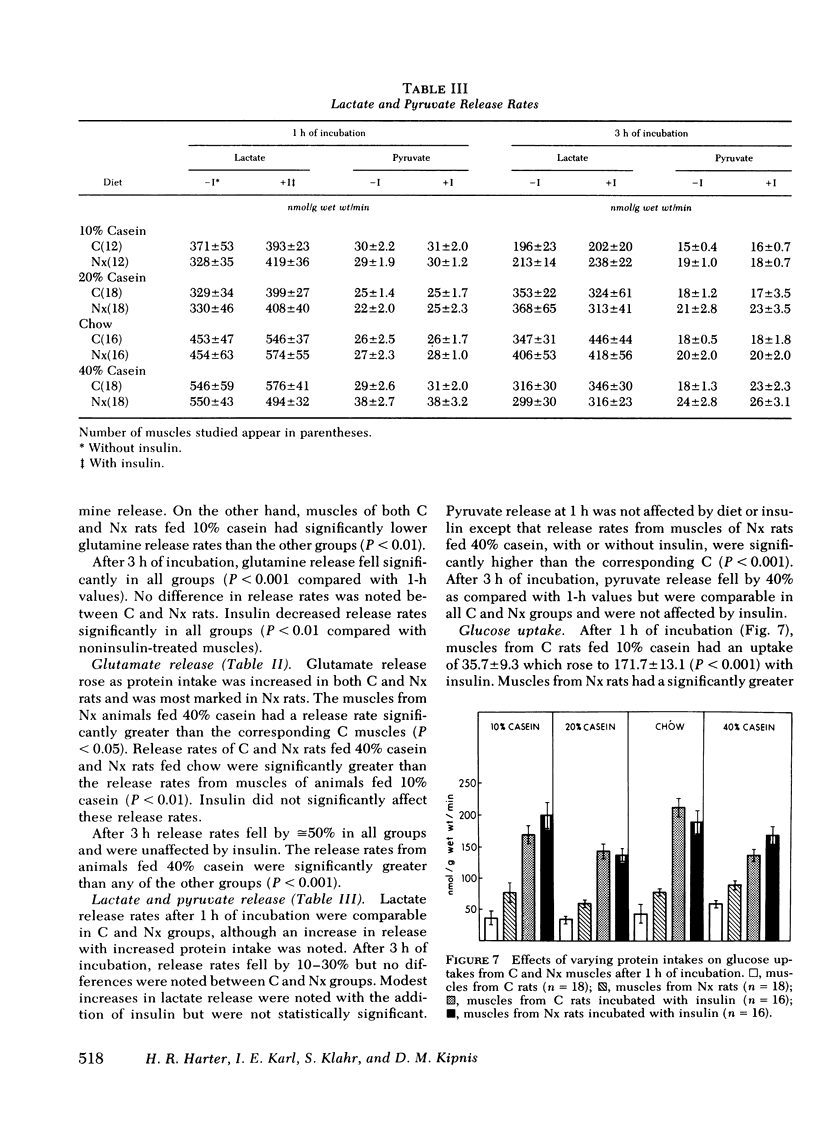
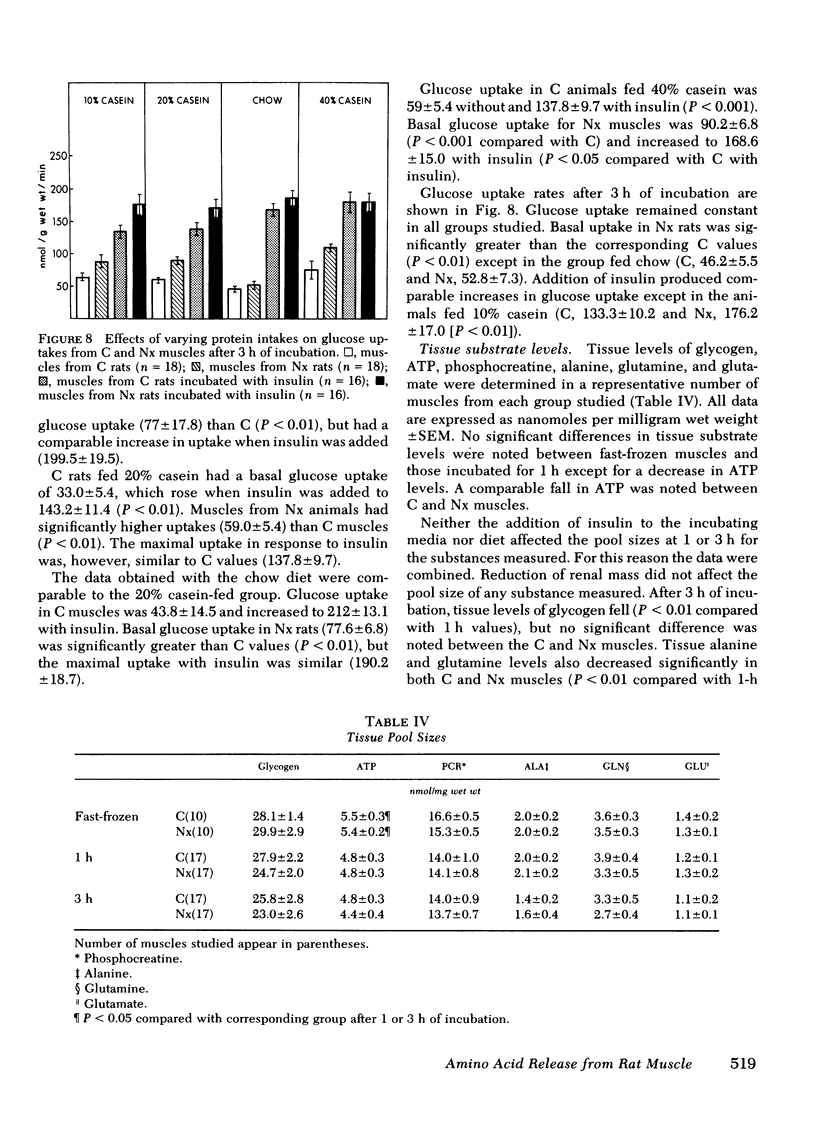
Base-line glucose uptake by all groups of muscles from Nx rats was significantly greater than corresponding C values, but maximal insulin-stimulated glucose uptake was comparable in all groups. Tissue pool sizes for glycogen, ATP, phosphocreatine, ALA, glutamate, and glutamine were unaffected by reduction in renal mass.
The results indicate that Nx is associated with accelerated ALA, TYR, and PHE release from muscle. ALA release rose with increasing protein intake and decreased to values observed from C muscles after addition of insulin except in Nx animals fed 40% casein. TYR and PHE release decreased with increasing protein intake and also decreased to C values with the addition of insulin. The data also suggest that ALA release is not dependent upon glucose uptake in muscles from either C or Nx rats.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagdade J. D., Shafrir E., Wilson D. E. Mechanism(s) of hyperlipidemia in chronic uremia. Trans Am Soc Artif Intern Organs. 1976;22:42–45. [PubMed] [Google Scholar]
- Bianchi R., Mariani G., Pilo A., Toni M. G. Effects of long-term low protein diet on albumin metabolism in chronic uremia. Nephron. 1975;15(6):409–423. doi: 10.1159/000180524. [DOI] [PubMed] [Google Scholar]
- Bihler I. The action of cardiotonic steroids on sugar-transport in muscle, in vitro. Biochim Biophys Acta. 1968 Nov 5;163(3):401–410. doi: 10.1016/0005-2736(68)90125-9. [DOI] [PubMed] [Google Scholar]
- Bilbrey G. L., Faloona G. R., White M. G., Knochel J. P. Hyperglucagonemia of renal failure. J Clin Invest. 1974 Mar;53(3):841–847. doi: 10.1172/JCI107624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. L., Houghton B. J., Souhami R. L., Richards P. The effects of low-protein diet and uraemia upon urea-cycle enzymes and transaminases in rats. Clin Sci. 1972 Sep;43(3):371–376. doi: 10.1042/cs0430371. [DOI] [PubMed] [Google Scholar]
- Coles G. A., Peters D. K., Jones J. H. Albumin metabolism in chronic renal failure. Clin Sci. 1970 Sep;39(3):423–435. doi: 10.1042/cs0390423. [DOI] [PubMed] [Google Scholar]
- Felig P. The glucose-alanine cycle. Metabolism. 1973 Feb;22(2):179–207. doi: 10.1016/0026-0495(73)90269-2. [DOI] [PubMed] [Google Scholar]
- Garber A. J., Karl I. E., Kipnis D. M. Alanine and glutamine synthesis and release from skeletal muscle. I. Glycolysis and amino acid release. J Biol Chem. 1976 Feb 10;251(3):826–835. [PubMed] [Google Scholar]
- Garber A. J., Karl I. E., Kipnis D. M. Alanine and glutamine synthesis and release from skeletal muscle. II. The precursor role of amino acids in alanine and glutamine synthesis. J Biol Chem. 1976 Feb 10;251(3):836–843. [PubMed] [Google Scholar]
- Garber A. J. Skeletal muscle protein and amino acid metabolism in experimental chronic uremia in the rat: accelerated alanine and glutamine formation and release. J Clin Invest. 1978 Sep;62(3):623–632. doi: 10.1172/JCI109169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber A. J. The regulation of skeletal muscle alanine and glutamine formation and release in experimental chronic uremia in the rat: subsensitivity of adenylate cyclase and amino acid release to epinephrine and serotonin. J Clin Invest. 1978 Sep;62(3):633–641. doi: 10.1172/JCI109170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Chang T. W. Regulation and significance of amino acid metabolism in skeletal muscle. Fed Proc. 1978 Jul;37(9):2301–2307. [PubMed] [Google Scholar]
- HARTROFT P. M., EISENSTEIN A. B. Alterations in the adrenal cortex of the rat induced by sodium deficiency: correlation of histologic changes with steroid hormone secretion. Endocrinology. 1957 May;60(5):641–651. doi: 10.1210/endo-60-5-641. [DOI] [PubMed] [Google Scholar]
- Hampers C. L., Soeldner J. S., Doak P. B., Merrill J. P. Effect of chronic renal failure and hemodialysis on carbohydrate metabolism. J Clin Invest. 1966 Nov;45(11):1719–1731. doi: 10.1172/JCI105478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter H. R., Mercado A., Rutherford W. E., Rodriguez H., Slatopolsky E., Klahr S. Effects of phosphate depletion and parathyroid hormone on renal glucose reabsorption. Am J Physiol. 1974 Dec;227(6):1422–1427. doi: 10.1152/ajplegacy.1974.227.6.1422. [DOI] [PubMed] [Google Scholar]
- Huijing F. A rapid enzymic method for glycogen estimation in very small tissue samples. Clin Chim Acta. 1970 Dec;30(3):567–572. doi: 10.1016/0009-8981(70)90246-9. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S., Li J. B., Rannels S. R. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem. 1977 Feb 25;252(4):1476–1483. [PubMed] [Google Scholar]
- Karl I. E., Garber A. J., Kipnis D. M. Alanine and glutamine synthesis and release from skeletal muscle. III. Dietary and hormonal regulation. J Biol Chem. 1976 Feb 10;251(3):844–850. [PubMed] [Google Scholar]
- Karl I. E., Pagliara A. S., Kipnis D. M. A microfluorometric enzymatic assay for the determination of alanine and pyruvate in plasma and tissues. J Lab Clin Med. 1972 Sep;80(3):434–441. [PubMed] [Google Scholar]
- Kramer H. J., Bäcker A., Krück F. Inhibition of intestinal (Na+--K+)-ATPase in experimental uremia. Clin Chim Acta. 1974 Jan 19;50(1):13–18. doi: 10.1016/0009-8981(74)90072-2. [DOI] [PubMed] [Google Scholar]
- Leichter S. B., Pagliara A. S., Grieder M. H., Pohl S., Rosai J., Kipnis D. M. Uncontrolled diabetes mellitus and hyperglucagonemia associated with an islet cell carcinoma. Am J Med. 1975 Feb;58(2):285–293. doi: 10.1016/0002-9343(75)90579-3. [DOI] [PubMed] [Google Scholar]
- Mannan A., Wiebe L. I., Noujaim A. A., Secord D. C. Carbohydrate metabolism in the chronically uremic rat. Clin Biochem. 1975 Jun;8(3):199–205. doi: 10.1016/s0009-9120(75)92010-x. [DOI] [PubMed] [Google Scholar]
- Orskov H., Christensen N. J. Growth hormone in uremia. I. Plasma growth hormone, insulin and glucagon after oral and intravenous glucose in uremic subjects. Scand J Clin Lab Invest. 1971 Feb;27(1):51–60. doi: 10.3109/00365517109080189. [DOI] [PubMed] [Google Scholar]
- Richards P. Protein metabolism in uraemia. Nephron. 1975;14(2):134–152. doi: 10.1159/000180444. [DOI] [PubMed] [Google Scholar]
- Rubenfeld S., Garber A. J. Abnormal carbohydrate metabolism in chronic renal failure. The potential role of accelerated glucose production, increased gluconeogenesis, and impaired glucose disposal. J Clin Invest. 1978 Jul;62(1):20–28. doi: 10.1172/JCI109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin I. B., Kelmers A. D., Goldstein G. The determination of transfer ribonucleic acid by aminoacylation. I. Leucine and phenylalanine transfer ribonucleic acid from E. coli B. Anal Biochem. 1967 Sep;20(3):533–544. doi: 10.1016/0003-2697(67)90298-9. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Schmahl F. W., Goodman M. N. Regulation of alanine formation and release in rat muscle in vivo: effect of starvation and diabetes. Am J Physiol. 1977 Aug;233(2):E109–E114. doi: 10.1152/ajpendo.1977.233.2.E109. [DOI] [PubMed] [Google Scholar]
- Shankel S. W., Robson A. M., Bricker N. S. On the mechanism of the splay in the glucose titration curve in advanced experimental renal disease in the rat. J Clin Invest. 1967 Feb;46(2):164–172. doi: 10.1172/JCI105519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear L. Internal redistribution of tissue protein synthesis in uremia. J Clin Invest. 1969 Jul;48(7):1252–1257. doi: 10.1172/JCI106090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Vyhmeister I., Kopple J. D., Swendseid M. E. Effect of protein intake on weight gain and plasma amino acid levels in uremic rats. Am J Physiol. 1976 May;230(5):1455–1459. doi: 10.1152/ajplegacy.1976.230.5.1455. [DOI] [PubMed] [Google Scholar]
- Young G. A., Parsons F. M. Impairment of phenylalanine hydroxylation in chronic renal insufficiency. Clin Sci. 1973 Jul;45(1):89–97. doi: 10.1042/cs0450089. [DOI] [PubMed] [Google Scholar]



