Abstract
Tendinopathies are common muskoloskeletal injuries that lead to pain and disability. Development and pathogenesis of tendinopathy is attributed to progressive pathological changes to the structure, function, and biology of tendon. The nature of this disease state, whether acquired by acute or chronic injury, is being actively investigated. Scarring, disorganized tissue, and loss of function characterize adult tendon healing. Recent work from animal models has begun to reveal the potential for adult mammalian tendon regeneration, the replacement of diseased with innate tissue. This review discusses what is known about musculoskeletal regeneration from a molecular perspective and how these findings can be applied to tendinopathy. Non-mammalian and mammalian models are discussed with emphasis on the potential of Murphy Roths Large mice to serve as a model of adult tendon regeneration. Comparison of regeneration in non-mammals, foetal mammals and adult mammals emphasizes distinctly different contributing factors to effective regeneration.
Keywords: C57BL/6J, healing, MRL, MRL/MpJ, regeneration, scarless, scar-mediated, tendon
Introduction
Over the past several decades, an increase in both sedentary lifestyle and emphasis on physical activity has vastly increased the incidence of tendon injury (Smith et al. 2002; Maffulli et al. 2003; Thomopoulos et al. 2003). Tendon injury can be broadly classified as acute or chronic, depending upon the time frame over which the injury takes place. Acute injury, a common consequence of moderate to intense physical activity, can range in severity from self-limiting injury that resolves with conservative management, such as patellar tendonitis, also known as ‘jumper's knee’ (Duri & Aichroth 1995), to complete failure, often requiring surgical intervention, such as Achilles tendon rupture (Jones et al. 2012). These well-studied acute injuries (Lin et al. 2004; Boyer 2005; Thordarson & Shean 2005; Lehfeldt et al. 2008) typically exhibit the classic healing cascade (Molloy et al. 2003) and are characterized by scar formation (Kovacevic & Rodeo 2008).
In contrast, chronic tendon injury (Kannus & Jozsa 1991) is characterized by subrupture accumulation of damage that is accompanied by an ineffective repair response. Initial studies suggested that there may have been some remodelling process (Leadbetter 1992); however, more recent studies have demonstrated a molecular response that suggests a diminishing attempt to repair as more damage is accumulated (Fung et al. 2008; Andarawis-Puri et al. 2012; Sereysky et al. 2010, 2012). Interestingly, despite the lack of overt inflammation, the molecular response to this injury includes altered regulation of inflammatory cytokines, collagen and matrix metalloproteinases (MMPs). Specifically, observations include increases in the presence and activity of MMP-13, IL-1β (Sun et al. 2008) and MMP-1 and decreases in the levels of MMP-2 and MMP-3 (Riley 2004). MMP-3 is considered a key regulator of enzymatic matrix turnover, thus its down-regulation may indicate a failure in the remodelling process.
Chronic tendon injury lacks overt inflammation and is subclinical; therefore, the disease is often attributed to overuse. As an individual is unaware of the damage accumulation taking place within the tendon, such injurious activity continues leading to the commonly observed tendinopathic clinical findings (Kannus & Jozsa 1991). Further, a logical sequela of chronic tendon injury is progression to a clinical injury, perhaps as severe as rupture (Leadbetter 1992). This is supported by epidemiological data on tendon injury and rupture, which reveals that 50% of individuals over the age of 80 have a tendon rupture, despite frequently not having an acute event to which the injury can be attributed (Tashjian 2012).
The progression of overuse tendon damage from subclinical process to clinical injury requiring surgical intervention results in severe morbidity and cost. Similarly, the scar-mediated healing typically associated with acute tendon injury also results in morbidity and cost. Yet both of these clinical scenarios have the potential for tremendous improvement. If the response of tendon to damage could be modulated to promote effective remodelling, either through repair (without overt inflammation) or through healing (with overt inflammation), improvement in postsurgical outcome or even elimination of the need for surgical intervention through conservative management could be achieved. To address this approach, we will review what is known about healing and regeneration.
Regeneration
Tissue healing is a well-studied cellular response to injury (Gurtner et al. 2008). Much of the work on tissue healing has been done using skin wounds as a model (Broughton et al. 2006) and has led to a thorough understanding of the response, which includes many cell lineages undergoing a coordinated response to yield a cascade of events that are divided into the inflammatory, proliferative and remodelling phases (Martin 1997). Importantly, healing typically concludes with the remodelling of the newly produced tissue at the wound site into scar – poorly organized extracellular matrix that does not recapitulate the native tissue's structure or function (Reinke & Sorg 2012).
In contrast, regeneration denotes a restoration of native functional tissue, with no scar formation (Larson et al. 2010). Investigations into regeneration have revealed that regeneration can be thought of as an extension of healing; both require similar cascades and phases; however, regeneration continues to remodel scar tissue, ultimately replacing it with native tissue (Baddour et al. 2012). If this process is extended to include ineffective healing, or even a lack of healing, as is the case in overuse tendinosis, then understanding the differences that exist between a healing and regeneration response may provide key insights into improving tendon's response to chronic injury.
Non-mammalian regeneration
The most common examples of regeneration exist in the setting of autotomy, or self-amputation. This phenomenon, although rarely observed in mammals, is common to starfish, who can shed an arm to evade a predator (Fleming et al. 2007), as well as vertebrates, including some species of lizards, who can shed their tails (Alibardi 2010), and subsequently regenerate the appendages. Early studies of these common adult examples of regeneration led to the identification of the blastema, the cellular structure composed of progenitor cells that are responsible for regeneration (Congdon et al. 1974; Dinsmore 1977).
Studying adult non-mammalian autotomy continues to provide insight into the function of the blastema and the molecular cascades associated with regeneration. Recent work using zebrafish fin regeneration has begun outlining the role that MMPs such as MMP-2 and tissue inhibitors of metalloproteinases (TIMPs) such as TIMP-2 play in regulating the extracellular matrix during limb regeneration (Bai et al. 2005). However, while such models provide key insights, the lack of direct applicability of findings to mammalian injury is a serious limitation that can be overcome from investigating examples of mammalian regeneration.
Mammalian regeneration
The mammalian foetal milieu has long been known to promote regeneration (Adzick et al. 1985) and has provided insights towards minimizing scar formation (Leung et al. 2012). In the setting of an acute foetal wound, there is minimal inflammatory response, as defined by the quantity of inflammatory cells (Jennings et al. 1991) and activity of inflammatory cytokines (Longaker et al. 1990). This may be a result of the fact that foetal inflammatory cells are less differentiated than those of the adult (Cowin et al. 1998) and that the duration of the foetal inflammatory phase is shorter than that of the adult (Adzick & Lorenz 1994).
In contrast, an over-heightened inflammatory response correlates with scar formation in both healthy (Wynn 2008) and diseased fibroproliferative states (Wynn 2007). These findings suggest that there is an upper limit to the benefit of the inflammatory response that, when surpassed, leads to ineffective healing. This consequence of excessive inflammation is supported by studies suggesting that inflammatory cytokines are the signalling mechanism responsible for increased scar formation at wound sites subjected to mechanical forces (Wong et al. 2011) and that scar has been shown to be reduced in MCP-1 (a chemokine) knockout mice (Ferreira et al. 2006).
However, inflammation is not the only difference between the foetal and adult wound response. Studies have revealed that foetal and adult healing engages different growth factors with potentially different roles (Whitby & Ferguson 1991). For example, it has long been known that TGF-β expression (Krummel et al. 1988) and its modulators, such as fibromodulin are highly correlated with scar formation. These molecules are expressed at different levels in the foetal and adult setting (Soo et al. 2000), denoting the importance of their roles. More recent studies have begun to investigate the importance of the ratios of TGF-β1, -β2 and -β3, which also differ between the foetal and adult setting (Liu et al. 2004). These findings will be discussed in greater detail with other findings from adult mammalian regeneration models below.
A third difference between foetal and adult tissue is the presence of progenitor cells throughout the tissue, which produce the foetal milieu conducive to regeneration when tissue is injured. For example, satellite cells, the skeletal muscle progenitor cells that lie between muscle fibres, become active when muscle is injured and are responsible for repair (Charge & Rudnicki 2004). Importantly, these progenitor cells exist throughout the foetal tissue, and do not form a distinct structure. Yet structures are formed during foetal wound healing in certain tissues, such as bone (Schmitt et al. 1999), which are referred to as blastema. These structures are not typically responsible for regeneration (with the exception of MRL mice, which will be discussed below) and are therefore related to the non-mammalian blastema described above in appearance only and not function (Carlson 2005).
Several studies have suggested that it may be these precursor cells throughout the tissue that are directly responsible for regeneration. Longaker et al. (1994) found that when adult skin is transplanted onto a foetus, wounds in the adult tissue heal with scar, while adjacent identical wounds regenerate. Additionally, multiple studies have shown that foetal tissue will heal without scar when grown in an organ culture system in which the milieu is controlled, and adult levels of inflammatory cytokines and growth factors are maintained (Ihara & Motobayashi 1992; Bleacher et al. 1993). This finding suggests that if the foetal milieu is necessary for foetal regeneration (and not a consequence of it), the progenitor cells within foetal tissue are capable of establishing an effective local environment at the site of injury.
Tendon studies, utilizing a sheep model, have revealed similar findings relating to regeneration in the foetal environment. Specifically, 1 week after partial laceration, maternal (adult) tendon had healed in the presence of inflammatory cells with granulation tissue, while the foetal tendon had regenerated, with neither gross nor histological abnormalities in the tendon structure (Figure 1; Beredjiklian et al. 2003). This study also found that TGF-β1 expression remained constant in foetal tendon during repair, but was up-regulated in that of adult, further supporting its relevance to regenerative healing (Beredjiklian et al. 2003). Interestingly, assessment of the function of the repaired tendon revealed that neither cohort fully recovered naïve tendon strength (Beredjiklian et al. 2003). This finding, although surprising given the recovery of structure, is in agreement with previous, similar work done in rat (Julia et al. 1993) and may be attributable to the vastly different foetal loading environment, which represents an inherent limitation of the foetal tendon model.
Figure 1.
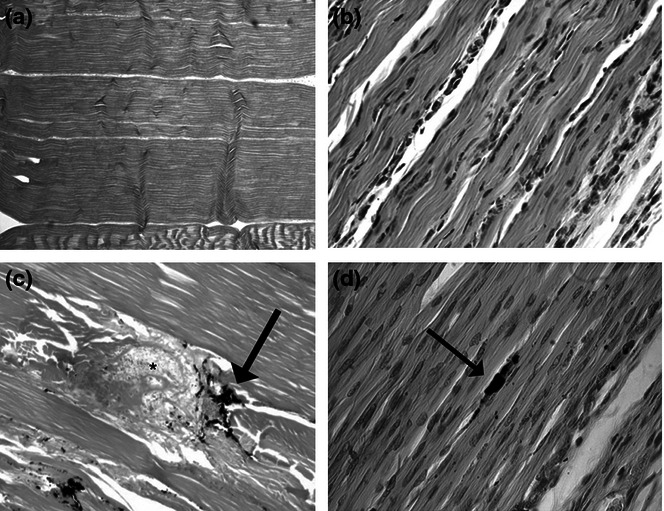
H&E stain micrographs of normal adult tendon (a, 50× magnification) and normal fetal tendon (b, 400× magnification). Injured adult tendon (c) retains disruption of the collagen fibers at the site of India ink marking (arrow) and the disruption of normal collagen organization and disorganized granulation tissue at the site of wounding (asterisk). Injured fetal tendon (d) remodels, revealing no structural abnormalities or lack of collagen fiber disorganization at the wound site (identified with charcoal, arrow). The collagen architecture appears to be completely restored (adapted from Beredjiklian et al. 2003).
The same group, building on their previous work, sought to determine whether the regenerative ability of foetal tendons is specific to the tissue or the environment. Favata et al. grafted foetal and adult tendon, attached to custom frames to maintain a basal force on the tissue, into subcutaneous pouches of adult mice. In this study, the use of a frame to ensure a mechanical force on the tendon is distinctly different from the group's previously discussed work, in which the foetal tendon experiences altered loading with likely less force as the foetus floats in amniotic fluid. Their findings were consistent with the previous study (Favata et al. 2006), confirming that, for tendon, foetal tissue alone is sufficient for regeneration. Biomechanical assessment revealed that the healed adult tendon again exhibited decreased strength; however, the loaded foetal regenerated tendon recovered naïve level strength (Favata et al. 2006). This finding denotes the capacity for foetal tendon to undergo true regeneration of both structure and function, but also highlights the limitation of the foetal model. The altered loading environment may be responsible for the disparate biomechanical function observed in the two experiments.
Yet the altered loading environment is not the only limitation of a foetal model of regeneration. The distinction between foetal regeneration and adult healing suggests a transition period. Work using foetal rats has revealed that regeneration does not last throughout foetal development, but that wounds created after approximately 75% of gestation is complete begin to result in scar (Ihara et al. 1990). Further work has continued to explore this threshold by examining the molecular response to wounds induced before and after the transition. Soo et al. (2003) found that wounds that resulted in regeneration (<75% gestation complete) yielded decreased TGF-β1 and TGF-β2 expression and increased TGF-β3 expression, while wounds that resulted in scar (more than 75% gestation complete) yielded increased TGF-β1 and TGF-β2 expression and decreased TGF-β3 expression.
This latter expression profile is similar to those observed in adult wounds (Ellis & Schor 1998; Cowin et al. 2001; Buchanan et al. 2009), confirming that the transition from regeneration to healing takes place during gestation. In addition, as noted above, the foetal milieu contains fewer inflammatory cells, and those that are present are less differentiated than in adult. However, this too suggests a transition period, and studies have revealed that the maturation of the immune system, as it relates to wound healing, takes place during gestation as well (Cowin et al. 1998).
Wound size also affects the likelihood of foetal regeneration. Cass et al., using foetal sheep, varied both foetal age and wound size, noting whether or not scar was formed. They found that the younger the foetus was, the larger the wound could be while still regenerating. Further, they found that even when using young foetuses at approximately 40% gestation (thus well below the approximately 75% threshold) a large enough wound could be induced that the chance of scarless repair is <10% (Cass et al. 1997). This work reveals that having wounded tissue edges in proximity, a well-established principle of adult wound care (Lait & Smith 1998), is also relevant in the foetal setting, supporting the theory that while it is the tissue that drives regeneration, the tissue may establish a local environment that is also necessary.
Although the mammalian foetal wound model has provided many insights into regeneration, there is much that can be learned from the few but validated adult mammalian regeneration models as well. Organwide, regeneration is known to take place commonly in liver, in which lobules are replicated in the injured liver until the organ can meet metabolic demands (Abshagen et al. 2012), and bone, in which mineral matrix is generated to meet mechanical demands (Panetta et al. 2010; Monroe et al. 2012). Yet most other adult tissue heals with scar. This finding alone – that regeneration and scar-mediated healing exist within the same adult mammalian organisms – suggests that the two responses to injury may be related and that all adult mammalian tissue may hold the potential for regeneration.
There are several other examples of adult mammalian regeneration, although not organ wide. In the 1950s, rabbits were noted to regenerate earholes (Goss & Grimes 1975). This led to a broad search for other adult mammalian species that would regenerate earholes, revealing that several other species, including rat and mouse exhibited regeneration (Williams-Boyce & Daniel 1986). Williams-Boyce & Daniel (1986) state that the regeneration is limited, noting that the phenomenon is a function of earhole location, and ‘the interpretation of “regeneration” or “wound closure”’. More recently, these qualified observations of mouse regeneration have been supported. For example, African spiny mice (Acomys) have been observed to exhibit skin autotomy; their back skin easily tears at low forces and subsequently regenerates (Seifert et al. 2012).
Work contrasting these few regeneration models with well-established and reviewed healing models (Martin 1997) has yielded many insights. Studies of healing in humans have revealed findings consistent with observations in adult mammalian models: excessively high levels of inflammation result in fibrosis and subsequently scar formation (Gurtner et al. 2008). Interestingly, Ashcroft et al. (1998) observed that while the extent of scarring increased throughout young adulthood, it decreases in the elderly. This reversion may reflect the relative decrease in levels of inflammation and chemokine expression observed in elderly wounds, a tissue response similar to that of foetal tissue (Bayat et al. 2003). In contrast, in the setting of keloid, a pathologic, fibroproliferative response to injury, inflammation is increased beyond young adult levels (Tredget et al. 1997), as is TGF-β (Tredget et al. 1998).
Investigating TGF-β isoforms in adults has revealed increased levels of TGF-β1 and -β2 and decreased levels of TGF-β3 (Ferguson & O'Kane 2004). This expression pattern is the opposite of that observed in foetal regeneration models, and taken together they strongly suggest that the ratio of these isoforms contributes to the extent of scar formation. The foetal isoform ratio was also translated to an adult model by the addition of exogenous TGF-β3 and inhibition of endogenous TGF-β1 and -β2 in the setting of adult cutaneous injury, both of which reduced scar formation (Shah et al. 1995). Further, recent work in non-regenerative mouse strains has demonstrated that a point mutation in the TGF-β1 receptor gene demonstrate a regenerative phenotype (Liu et al. 2011), supporting the role that TGF-β1 plays in tissue repair.
MRL mice
MRL/lpr is an autoimmune-prone mouse strain, initially used as a model of systemic lupus erythematosus (Theofilopoulos & Dixon 1985; Kench et al. 1999). Work using this strain, as well the LG/J strain, one of MRL's parent strains, has revealed superior adult healing capability, relative to other strains of mice (Kench et al. 1999; Li et al. 2001a,b; Blankenhorn et al. 2009). Further, through a series of bone marrow transplant experiments, this superior healing response was shown to be unrelated to the strain's altered immune system activity (Kench et al. 1999).
In 1998, a new inbred mouse strain, MRL/MpJ, (Murphy Roths large (MRL), generated by the Murphy group of The Jackson Laboratory (MpJ) was developed, isolating the superior healing response. In contrast to MRL/lpr, MRL/MpJ is not autoimmune-prone until much later in life, but retains the superior healing response of its predecessors throughout its life and in fact exhibits regeneration (Clark et al. 1998).
The regenerative healing ability of MRL/MpJ mice was initially discovered when 2-mm punches made in their ears healed with restoration of structure and no scar over the course of one month (Figure 2; Clark et al. 1998; Heber-Katz 1999). MRL/MpJ's adult regenerative capabilities have been shown to extend to myocardial tissue (Leferovich et al. 2001; Heber-Katz et al. 2004a,b), articular cartilage (Fitzgerald et al. 2008; Ward et al. 2008), digit tips (Chadwick et al. 2007), the central nervous system (Donnelly & Popovich 2008), retina (Tucker et al. 2008) and cornea (Ueno et al. 2005).
Figure 2.
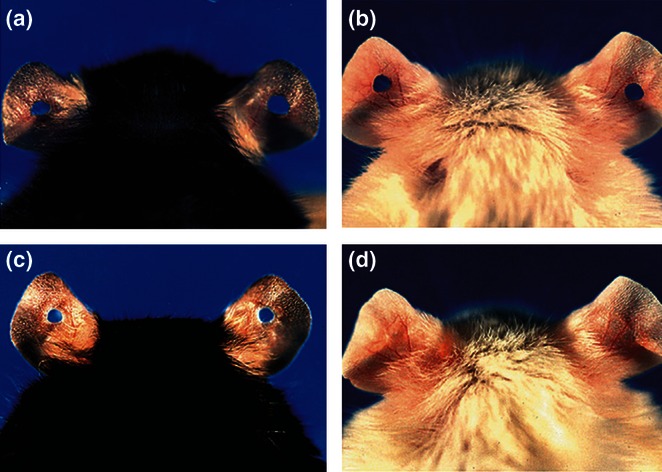
Through-and-through 2 mm holes are punched in the middle of the ear pinnae, clearly seen at day 0 in both C57BL/6J (a) and MRL/MpJ (b). By day 30, however, earholes remain clearly seen in C57BL/6J (c) but have been replaced by native tissue in MRL/MpJ (d), denoting regeneration (Adapted from Clark et al. 1998).
However, there do appear to be limits to MRL/MpJ's regeneration. Colwell et al. (2006) performed studies that simultaneously confirmed MRL's capability to regenerate earholes, but also found that their healing of incisional and excisional dorsal cutaneous wounds were no different than non-regenerating mouse strains. Additionally, several groups were not able to reproduce prior myocardial regeneration, demonstrated via cryogenic injury, when using an ischaemic injury model (Oh et al. 2004; Abdullah et al. 2005; Robey & Murry 2008), and one has reported data conflicting with the regenerative response to cryogenic injury (Grisel et al. 2008). Nonetheless, MRL/MpJ represents a promising adult mammalian model of tendon regeneration.
Molecular mechanisms of MRL/MpJ healing and regeneration
Investigations into the mechanism of MRL/MpJ regeneration began broadly. Li et al. used microarray hybridization to measure the expression of more than 8700 genes at the wound site at various timepoints postinjury in both MRL/MpJ and C57BL/6J, a non-regenerating mouse strain. This approach revealed that, similar to previous examples of regeneration, MRL/MpJ inflammation was down-regulated relative to C57BL/6J, and wound repair genes were up-regulated (Li et al. 2001a,b). Several studies have tried to use this or a similar approach to identify a quantitative trait locus responsible for these broad differences (McBrearty et al. 1998; Li et al. 2000; Masinde et al. 2005), but as of yet, none has been successful, as multiple loci have been identified.
Further complicating the issue, MRL/MpJ exhibits sexual dimorphism (female mice regenerate more quickly) with overlapping, but not identical sets of quantitative trait loci being responsible for regeneration in each sex (Blankenhorn et al. 2003). Interestingly, oestrogen is known to alter healing by modulating TGF-β1 levels (Ashcroft et al. 1997), but does not appear to alter MRL/MpJ regeneration (Blankenhorn et al. 2003), suggesting that the two processes are regulated separately.
Investigating downstream mechanisms of MRL/MpJ's regeneration revealed that MRL/MpJ has increased expression of MMP-2 and -9 (Tucker et al. 2008), and decreased expression of TIMP-2 and -3 (Heber-Katz et al. 2004a,b). MRL/MpJ has also been noted to have lower levels of inflammation as measured by TNF-α, IL-1β and macrophage inflammatory proteins after injury, relative to non-regenerative mouse strains (Lewis et al. 2012). TGF-β levels are also altered, and will be discussed in the context of tendon below.
MRL/MpJ mice were also noted to have functional blastema at wound sites, which were not present in non-regenerating controls (Gourevitch et al. 2003). These structures, only observed in mammalian regeneration during the foetal period, as discussed above, strongly suggest that MRL/MpJ's regenerative capability is a remnant of the foetal period which is not lost as the mouse matures (Edwards 2008). This is further supported by analysis of the blastema cells, which revealed that they express progenitor cell markers, including Msx-1 and Pref-1 (Vorotnikova et al. 2010). Further studies using bone marrow transplants have revealed that the blastema arises from dedifferentiated adult tissue, and not a hematopoietic stem cell source (Kench et al. 1999).These blastema cells have been noted to be missing p21 (Arthur & Heber-Katz 2011), a kinase inhibitor that prevents dedifferentiation (Hong et al. 2009). The importance of this finding was emphasized when a p21 knockout strain was developed, inducing a regenerative phenotype in a previously non-regenerative mouse strain (Bedelbaeva et al. 2010).
The value of progenitor cells in wound healing has also been demonstrated by a recent study in which the addition of mesenchymal stem cells from either MRL or a non-regenerative mouse strain prevents post-traumatic arthritis when introduced to an intra-articular fracture in a non-regenerating mouse strain (Diekman et al. 2012). These findings suggest that MRL/MpJ's superior healing response is a function of ability of the mature cells within its tissue to dedifferentiate and form a functional blastema at the site of injury.
Specific to tendon healing, MRL/MpJ mice present a very promising venue of research. Tendon's response to injury, when present, has been noted to be similar to the foetal state of tendon development (Ingraham et al. 2003). However, similar to other examples discussed above, the process is altered at some point, resulting in the formation of scar and not true tendon. This may be due to tendon's relative lack of vascularity and cellularity, making cellular recruitment difficult and resulting in a typically poor healing response.
Thus, MRL/MpJ is ideally suited to this task, given its development of a blastema which allows wounded tissue to be more self-sufficient during healing or regeneration. Further, MRL/MpJ's demonstrated ability to mount a foetal-like regeneration response in multiple tissue types, including connective tissue, suggests that MRL/MpJ may provide an adult, mammalian, regeneration model in the setting of tendon injury.
Given the important role that inflammation plays in healing and MRL/MpJ regeneration, we chose to investigate MRL/MpJ's response to tendon injury using two distinct models, an acute injury model with the expected overt inflammation, and a non-inflammatory subrupture fatigue injury model. All procedures were approved by the Institutional Animal Care and Use Committee. To investigate MRL/MpJ's response to acute tendon injury, MRL/MpJ and C57BL/6J mice underwent partial laceration of one patellar tendon, by modifying a previously published protocol (Lin et al. 2006; Ricchetti et al. 2008; Andarawis-Puri et al. 2009; Ansorge et al. 2009; Beason et al. 2011, 2012). Tendons were then harvested at 4 and 8 weeks postinjury. Each tendon was assessed histologically using toluidine blue to assess cellularity and gross morphology, picrosirius red to assess collagen structure and immunohistochemistry to assess MMP-2 and TGF-β1 protein levels. In addition to its previously discussed role in repair and regeneration, MMP-2 was examined because it is active in integration of undifferentiated blastema cells in limb regeneration (Vinarsky et al. 2005) and is suspected to play a similar role in MRL tendon regeneration. In addition, MMP-2 activates TGF-β and may further contribute to the differences in the role of TGF-β1 in healing and regeneration.
As expected, MMP-2 and TGF-β1 protein levels significantly differed in lacerated MRL/MpJ than C57BL/6J tendons. In general, TGF-β1 protein levels were higher at all timepoints in MRL/MpJ than B6, with the greatest increase in MRL/MpJ TGF-β1 protein level being observed 4 weeks after injury (Figure 3). Interestingly, while MMP-2 protein levels were generally higher in MRL/MpJ than C57BL/6J tendons, MRL/MpJ tendons exhibited the lowest MMP-2 protein level 4 weeks after injury, the timepoint at which MRL/MpJ TGF-β1 protein level was highest (Figure 4). Toluidine blue staining revealed that C57BL/6J demonstrated an increase in proteoglycan content for both strains 4 weeks after injury and remained high for B6 but was modulated to baseline levels for MRL by week 8. MRL/MpJ tendons exhibited increased vascularization at 4 and 8 weeks postinjury, which was not observed in C57BL/6J. The result of these responses was improved structural recovery of MRL/MpJ relative to C57BL/6J at 8 weeks postinjury, as determined by picrosirius red staining (Figure 5; Sereysky et al. 2013).
Figure 3.
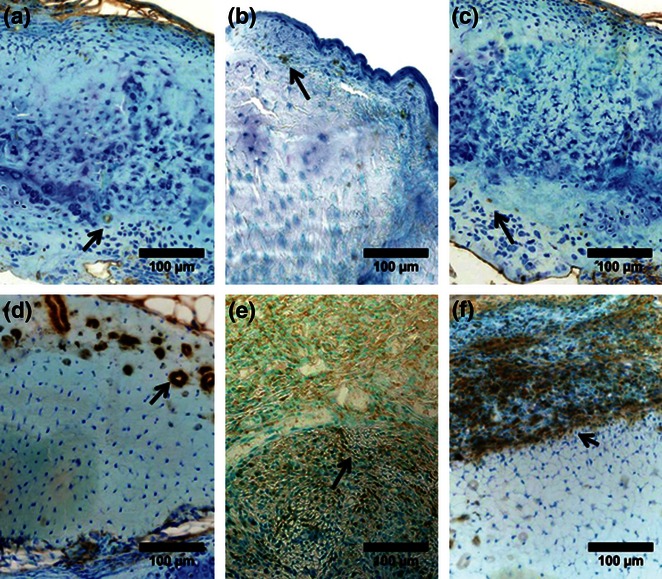
TGF-β presence (brown, positive staining, arrows) post-laceration in C57BL/6J control (a), week 4 (b), and week 8 (c), and in MRL/MpJ control (d), week 4 (e), and week 8 (f), revealing higher levels in MRL/MpJ at all timepoints (adapted from Sereysky et al. 2013).
Figure 4.
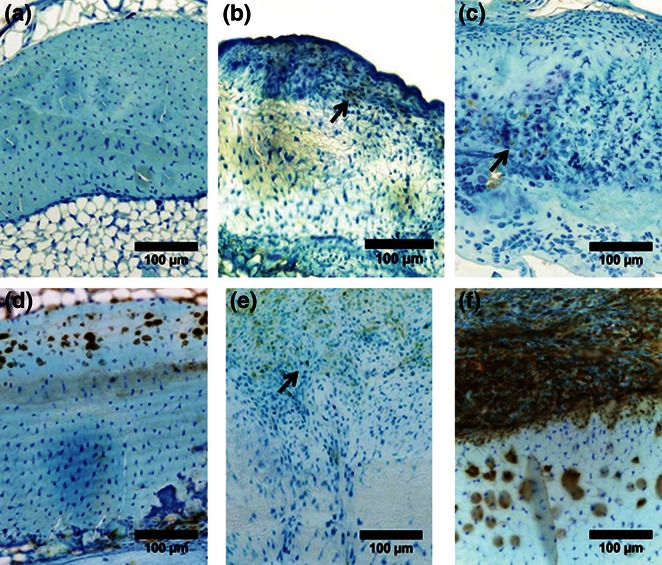
MMP-2 presence (brown, positive staining, arrows) post-laceration in C57BL/6J control (a), week 4 (b), and week 8 (c), and in MRL/MpJ control (d), week 4 (e), and week 8 (f), revealing higher levels in MRL/MpJ at all timepoints (adapted from Sereysky et al. 2013).
Figure 5.
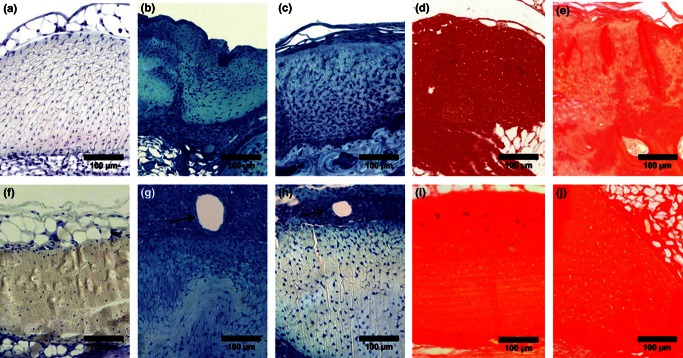
Toluidine Blue stain for proteoglycan content (degree of blue color) post-laceration in C57BL/6J control (a), week 4 (b), and week 8 (c), and in MRL/MpJ control (f), week 4 (g), and week 8 (h), revealing high levels in C57BL/6J through week 8, while MRL/MpJ returned to naïve between weeks 4 and 8. Picrosirius red stain for collagen maturity and organization (degree of red color) post-laceration in C57Bl/6J control (d), and week 8 (e), and in MRL/MpJ control (i) and week 8 (j), revealing restoration of naïve collagen structure in MRL/MpJ at week 8 (adapted from Sereysky et al. 2013).
The role of TGF-β1 is highly complex and contextual. It is essential to wound healing (Ellis & Schor 1998) but is associated with scar formation and fibrosis (Shah et al. 1995). TGF-β1 is integral in tendon foetal development (Krummel et al. 1988), leads to collagen synthesis (Varga et al. 1987) and can induce apoptosis (Perlman et al. 2001). For instance, exogenous addition of TGF-β1 has been shown to improve mechanical properties of injured tendons (Majewski et al. 2009), while TGF-β1 injections into healthy tendons result in a change of phenotype that is consistent with tendinopathy (Bell et al. 2013). Investigating the role of TGF-β in MRL/MpJ's tendon repair will provide insight into the duration, levels of activity and TGF-β-activated pathways that are associated with regeneration. These data strongly suggest that MRL/MpJ mounts a superior repair response and should be further investigated as a possible model of tendon regeneration in the setting of acute tendon injury.
To investigate MRL/MpJ's response to subrupture fatigue injury, MRL/MpJ and C57BL/6J mice underwent a surgical procedure in which fatigue loading is applied directly to the patellar tendon to induce subrupture damage (Sereysky et al.2010, 2012). Tendons were then harvested at various timepoints postinjury, and assessed for recovery of structure via second harmonic generation imaging (Sereysky et al. 2010), function via mechanical testing, biological response via change in expression of a panel of collagens, MMPs and inflammatory cytokines. C57BL/6J demonstrated an immediate and maintained change in structure (disorganization of collagen fibres) and function (decreased failure load). In contrast, MRL demonstrated similar immediate changes (suggestive of similar injury), but recovered naïve structure by 4 weeks postinjury and function by 8 weeks postinjury (Figure 6; Sereysky 2011; Sereysky et al. 2013). These findings suggest that C57BL/6J has no effective response to overuse tendinosis (similar to humans), while MRL/MpJ exhibits an improved repair response and possibly regeneration. Expression profiles show that in comparison with C57BL/6J, MRL/MpJ exhibited increased (or maintained) expression of collagens, greater increases in MMP-2 and -3 and maintained expression of inflammatory cytokines and TGF-β1. These findings suggest that MRL/MpJ's regenerative capability in the setting of overuse tendinosis is due to increased anabolic and catabolic activity as a function of maintenance of naïve inflammatory levels. In the context of prior work discussed above, these findings suggest that while excessive inflammation results in scar, some inflammation (at least a naïve level) is required for regeneration, while a decrease in inflammation from naïve levels, as observed in C57BL/6J, results in no effective repair response.
Figure 6.
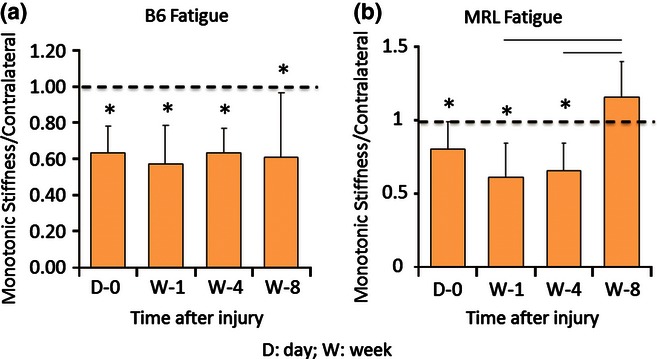
Stiffness of sub-rupture fatigue injured tendon, normalized by contralateral tendon. C57BL/6J does not recover naïve function by week 8 post-injury (a). MRL/MpJ recovers naïve function between weeks 4 and 8 post-injury (b) (adapted from Sereysky et al. 2013).
Conclusions and future directions
By investigating vastly different models of regeneration, insights can be gained into different necessary components of the regenerative mechanism. For example, our comparison of regeneration in non-mammals, foetal mammals and adult mammals emphasizes distinctly different contributing factors to effective regeneration. Non-mammals provide insight into the functional blastema inherent to MRL remodelling. Foetal mammals reveal the relevance of inflammation to the quality of the remodelling process, guiding our close examination of inflammation in adults. Adult mammals are the most complex models and yet most similar to the complexity of adult human tendon healing. They are the ideal platform for investigating the interacting mechanisms that lead to regeneration and identifying therapeutic interventions.
Examining these models of regeneration also raises several uncertainties that must be addressed with future work. The role of inflammation in remodelling, while large, has yet to be thoroughly investigated. The majority of published data suggest that more inflammation leads to more scar, tempting one to conclude that complete inhibition of inflammation may be a viable therapeutic strategy. Yet our work with MRL suggests that some basal level of inflammation may be necessary for MRL to undergo its superior remodelling/regeneration in tendon, while a lack of inflammation may result in a lack of response, in the case of chronic injury. Taken together, these findings suggest that the role of inflammation is necessary but complex, and deserving of further investigation.
A second uncertainty that must be addressed is the role of wounded tissue and its environment in the setting of regeneration. Early studies identified many differences between the regenerative and non-regenerative environments, yet transplant studies found that regeneration took place using regenerative tissue in a non-regenerative environment. These latter findings suggest that tissue alone defines regenerative capacity. However, if this were the case, the role of inflammatory cytokines, which interact with the environment, would be minimal. As this is not the case, future studies must be performed to elucidate the contributing roles of tissue and environment, as well as their interactions.
The final model reviewed, MRL/MpJ, is one of the most promising models of adult, mammalian tendon remodelling presently known. The improved repair and healing in MRL/MpJ mice suggest that they may serve as an adult model of tendon regeneration. Further work must be done to determine whether true regeneration takes place. However, regardless of whether MRL/MpJ's response is true regeneration or simply a superior, scar-mediated repair/healing response, there is much to be learned from this model. Whether using MRL/MpJ or other adult mammalian regeneration models, characterizing the molecular environment associated with adult tendon regeneration may identify therapeutic targets for clinical interventions, leading to reduced morbidity and cost of tendon injury.
Acknowledgments
The authors gratefully acknowledge Damien Laudier and Nisha George for their contributions.
Conflict of interest
The authors have no conflicts of interest to disclose.
Funding source
This study was supported by National Institutes of Health grants GM007280 (JBS) and AR052743 (ELF).
References
- Abdullah I, Lepore JJ, Epstein JA, Parmacek MS, Gruber PJ. MRL mice fail to heal the heart in response to ischemia-reperfusion injury. Wound Repair Regen. 2005;13:205–208. doi: 10.1111/j.1067-1927.2005.130212.x. [DOI] [PubMed] [Google Scholar]
- Abshagen K, Eipel C, Vollmar B. A critical appraisal of the hemodynamic signal driving liver regeneration. Langenbecks Arch. Surg. 2012;397:579–590. doi: 10.1007/s00423-012-0913-0. [DOI] [PubMed] [Google Scholar]
- Adzick NS, Lorenz HP. Cells, matrix, growth factors, and the surgeon. The biology of scarless fetal wound repair. Ann. Surg. 1994;220:10–18. doi: 10.1097/00000658-199407000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adzick NS, Harrison MR, Glick PL, et al. Comparison of fetal, newborn, and adult wound healing by histologic, enzyme-histochemical, and hydroxyproline determinations. J. Pediatr. Surg. 1985;20:315–319. doi: 10.1016/s0022-3468(85)80210-4. [DOI] [PubMed] [Google Scholar]
- Alibardi L. Morphological and cellular aspects of tail and limb regeneration in lizards. A model system with implications for tissue regeneration in mammals. Adv. Anat. Embryol. Cell Biol. 2010;207:iii. v–x, 1–109. [PubMed] [Google Scholar]
- Andarawis-Puri N, Ricchetti ET, Soslowsky LJ. Rotator cuff tendon strain correlates with tear propagation. J. Biomech. 2009;42:158–163. doi: 10.1016/j.jbiomech.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andarawis-Puri N, Sereysky JB, Sun HB, Jepsen KJ, Flatow EL. Molecular response of the patellar tendon to fatigue loading explained in the context of the initial induced damage and number of fatigue loading cycles. J. Orthop. Res. 2012;30:1327–1334. doi: 10.1002/jor.22059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge HL, Beredjiklian PK, Soslowsky LJ. CD44 deficiency improves healing tendon mechanics and increases matrix and cytokine expression in a mouse patellar tendon injury model. J. Orthop. Res. 2009;27:1386–1391. doi: 10.1002/jor.20891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur LM, Heber-Katz E. The role of p21 in regulating mammalian regeneration. Stem Cell Res. Ther. 2011;2:30. doi: 10.1186/scrt71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft GS, Dodsworth J, Van Boxtel E, et al. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-beta1 levels. Nat. Med. 1997;3:1209–1215. doi: 10.1038/nm1197-1209. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Horan MA, Ferguson MW. Aging alters the inflammatory and endothelial cell adhesion molecule profiles during human cutaneous wound healing. Lab. Invest. 1998;78:47–58. [PubMed] [Google Scholar]
- Baddour JA, Sousounis K, Tsonis PA. Organ repair and regeneration: an overview. Birth Defects Res. C Embryo Today. 2012;96:1–29. doi: 10.1002/bdrc.21006. [DOI] [PubMed] [Google Scholar]
- Bai S, Thummel R, Godwin AR, et al. Matrix metalloproteinase expression and function during fin regeneration in zebrafish: analysis of MT1-MMP, MMP2 and TIMP2. Matrix Biol. 2005;24:247–260. doi: 10.1016/j.matbio.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Bayat A, McGrouther DA, Ferguson MW. Skin scarring. BMJ. 2003;326:88–92. doi: 10.1136/bmj.326.7380.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beason DP, Abboud JA, Kuntz AF, Bassora R, Soslowsky LJ. Cumulative effects of hypercholesterolemia on tendon biomechanics in a mouse model. J. Orthop. Res. 2011;29:380–383. doi: 10.1002/jor.21255. [DOI] [PubMed] [Google Scholar]
- Beason DP, Kuntz AF, Hsu JE, Miller KS, Soslowsky LJ. Development and evaluation of multiple tendon injury models in the mouse. J. Biomech. 2012;45:1550–1553. doi: 10.1016/j.jbiomech.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedelbaeva K, Snyder A, Gourevitch D, et al. Lack of p21 expression links cell cycle control and appendage regeneration in mice. Proc. Natl Acad. Sci. USA. 2010;107:5845–5850. doi: 10.1073/pnas.1000830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R, Li J, Gorski DJ, et al. Controlled treadmill exercise eliminates chondroid deposits and restores tensile properties in a new murine tendinopathy model. J. Biomech. 2013;46:498–505. doi: 10.1016/j.jbiomech.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Beredjiklian PK, Favata M, Cartmell JS, Flanagan CL, Crombleholme TM, Soslowsky LJ. Regenerative versus reparative healing in tendon: a study of biomechanical and histological properties in fetal sheep. Ann. Biomed. Eng. 2003;31:1143–1152. doi: 10.1114/1.1616931. [DOI] [PubMed] [Google Scholar]
- Blankenhorn EP, Troutman S, Clark LD, Zhang XM, Chen P, Heber-Katz E. Sexually dimorphic genes regulate healing and regeneration in MRL mice. Mamm. Genome. 2003;14:250–260. doi: 10.1007/s00335-002-2222-3. [DOI] [PubMed] [Google Scholar]
- Blankenhorn EP, Bryan G, Kossenkov AV, et al. Genetic loci that regulate healing and regeneration in LG/J and SM/J mice. Mamm. Genome. 2009;20:720–733. doi: 10.1007/s00335-009-9216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleacher JC, Adolph VR, Dillon PW, Krummel TM. Isolated fetal mouse limbs: gestational effects on tissue repair in an unperfused system. J. Pediatr. Surg. 1993;28:1312–1314. doi: 10.1016/s0022-3468(05)80319-7. discussion 1314–5. [DOI] [PubMed] [Google Scholar]
- Boyer MI. Flexor tendon biology. Hand Clin. 2005;21:159–166. doi: 10.1016/j.hcl.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Broughton G, 2nd, Janis JE, Attinger CE. The basic science of wound healing. Plast. Reconstr. Surg. 2006;117(7 Suppl):12S–34S. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- Buchanan EP, Longaker MT, Lorenz HP. Fetal skin wound healing. Adv. Clin. Chem. 2009;48:137–161. doi: 10.1016/s0065-2423(09)48006-5. [DOI] [PubMed] [Google Scholar]
- Carlson BM. Some principles of regeneration in mammalian systems. Anat. Rec. B. New Anat. 2005;287:4–13. doi: 10.1002/ar.b.20079. [DOI] [PubMed] [Google Scholar]
- Cass DL, Bullard KM, Sylvester KG, Yang EY, Longaker MT, Adzick NS. Wound size and gestational age modulate scar formation in fetal wound repair. J. Pediatr. Surg. 1997;32:411–415. doi: 10.1016/s0022-3468(97)90593-5. [DOI] [PubMed] [Google Scholar]
- Chadwick RB, Bu L, Yu H, et al. Digit tip regrowth and differential gene expression in MRL/Mpj, DBA/2, and C57BL/6 mice. Wound Repair Regen. 2007;15:275–284. doi: 10.1111/j.1524-475X.2007.00216.x. [DOI] [PubMed] [Google Scholar]
- Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Clark LD, Clark RK, Heber-Katz E. A new murine model for mammalian wound repair and regeneration. Clin. Immunol. Immunopathol. 1998;88:35–45. doi: 10.1006/clin.1998.4519. [DOI] [PubMed] [Google Scholar]
- Colwell AS, Krummel TM, Kong W, Longaker MT, Lorenz HP. Skin wounds in the MRL/MPJ mouse heal with scar. Wound Repair Regen. 2006;14:81–90. doi: 10.1111/j.1524-475X.2005.00092.x. [DOI] [PubMed] [Google Scholar]
- Congdon JD, Vitt LJ, King WW. Geckos: adaptive significance and energetics of tail autotomy. Science. 1974;184:1379–1380. doi: 10.1126/science.184.4144.1379. [DOI] [PubMed] [Google Scholar]
- Cowin AJ, Brosnan MP, Holmes TM, Ferguson MW. Endogenous inflammatory response to dermal wound healing in the fetal and adult mouse. Dev. Dyn. 1998;212:385–393. doi: 10.1002/(SICI)1097-0177(199807)212:3<385::AID-AJA6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Cowin AJ, Brosnan MP, Holmes TM, Ferguson MW. Expression of TGF-beta and its receptors in murine fetal and adult dermal wounds. Eur. J. Dermatol. 2001;11:424–431. [PubMed] [Google Scholar]
- Diekman BO, Wu CL, Louer CR, et al. Intra-articular delivery of purified mesenchymal stem cells from C57BL/6 or MRL/MpJ superhealer mice prevents post-traumatic arthritis. Cell Transplant. 2012 doi: 10.3727/096368912X653264. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinsmore CE. Tail regeneration in the plethodontid salamander, Plethodon cinereus: induced autotomy versus surgical amputation. J. Exp. Zool. 1977;199:163–175. doi: 10.1002/jez.1401990202. [DOI] [PubMed] [Google Scholar]
- Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duri ZA, Aichroth PM. Patellar tendonitis: clinical and literature review. Knee Surg. Sports Traumatol. Arthrosc. 1995;3:95–100. doi: 10.1007/BF01552382. [DOI] [PubMed] [Google Scholar]
- Edwards RG. From embryonic stem cells to blastema and MRL mice. Reprod. Biomed. Online. 2008;16:425–461. doi: 10.1016/s1472-6483(10)60605-0. [DOI] [PubMed] [Google Scholar]
- Ellis IR, Schor SL. Differential motogenic and biosynthetic response of fetal and adult skin fibroblasts to TGF-beta isoforms. Cytokine. 1998;10:281–289. doi: 10.1006/cyto.1997.0294. [DOI] [PubMed] [Google Scholar]
- Favata M, Beredjiklian PK, Zgonis MH, et al. Regenerative properties of fetal sheep tendon are not adversely affected by transplantation into an adult environment. J. Orthop. Res. 2006;24:2124–2132. doi: 10.1002/jor.20271. [DOI] [PubMed] [Google Scholar]
- Ferguson MW, O'Kane S. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2004;359:839–850. doi: 10.1098/rstb.2004.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira AM, Takagawa S, Fresco R, Zhu X, Varga J, DiPietro LA. Diminished induction of skin fibrosis in mice with MCP-1 deficiency. J. Invest. Dermatol. 2006;126:1900–1908. doi: 10.1038/sj.jid.5700302. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J, Rich C, Burkhardt D, Allen J, Herzka AS, Little CB. Evidence for articular cartilage regeneration in MRL/MpJ mice. Osteoarthr. Cartil. 2008;16:1319–1326. doi: 10.1016/j.joca.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Fleming PA, Muller D, Bateman PW. Leave it all behind: a taxonomic perspective of autotomy in invertebrates. Biol. Rev. Camb. Philos. Soc. 2007;82:481–510. doi: 10.1111/j.1469-185X.2007.00020.x. [DOI] [PubMed] [Google Scholar]
- Fung DT, Basta-Pljakic J, Laudier DM, Jepsen KJ, Schaffler MB, Flatow EL. Early In vivo Response in Tendons to Low-level Fatigue Damage. San Francisco, CA: Transactions of the 54th Meeting of the Orthopaedic Research Society; 2008. [Google Scholar]
- Goss RJ, Grimes LN. Epidermal downgrowths in regenerating rabbit ear holes. J. Morphol. 1975;146:533–542. doi: 10.1002/jmor.1051460408. [DOI] [PubMed] [Google Scholar]
- Gourevitch D, Clark L, Chen P, Seitz A, Samulewicz SJ, Heber-Katz E. Matrix metalloproteinase activity correlates with blastema formation in the regenerating MRL mouse ear hole model. Dev. Dyn. 2003;226:377–387. doi: 10.1002/dvdy.10243. [DOI] [PubMed] [Google Scholar]
- Grisel P, Meinhardt A, Lehr HA, Kappenberger L, Barrandon Y, Vassalli G. The MRL mouse repairs both cryogenic and ischemic myocardial infarcts with scar. Cardiovasc. Pathol. 2008;17:14–22. doi: 10.1016/j.carpath.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- Heber-Katz E. The regenerating mouse ear. Semin. Cell Dev. Biol. 1999;10:415–419. doi: 10.1006/scdb.1999.0328. [DOI] [PubMed] [Google Scholar]
- Heber-Katz E, Leferovich J, Bedelbaeva K, Gourevitch D, Clark L. The scarless heart and the MRL mouse. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004a;359:785–793. doi: 10.1098/rstb.2004.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber-Katz E, Leferovich JM, Bedelbaeva K, Gourevitch D. Spallanzani's mouse: a model of restoration and regeneration. Curr. Top. Microbiol. Immunol. 2004b;280:165–189. doi: 10.1007/978-3-642-18846-6_5. [DOI] [PubMed] [Google Scholar]
- Hong H, Takahashi K, Ichisaka T, et al. Suppression of induced pluripotent stem cell generation by the p53–p21 pathway. Nature. 2009;460:1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara S, Motobayashi Y. Wound closure in foetal rat skin. Development. 1992;114:573–582. doi: 10.1242/dev.114.3.573. [DOI] [PubMed] [Google Scholar]
- Ihara S, Motobayashi Y, Nagao E, Kistler A. Ontogenetic transition of wound healing pattern in rat skin occurring at the fetal stage. Development. 1990;110:671–680. doi: 10.1242/dev.110.3.671. [DOI] [PubMed] [Google Scholar]
- Ingraham JM, Hauck RM, Ehrlich HP. Is the tendon embryogenesis process resurrected during tendon healing? Plast. Reconstr. Surg. 2003;112:844–854. doi: 10.1097/01.PRS.0000070180.62037.FC. [DOI] [PubMed] [Google Scholar]
- Jennings RW, Adzick NS, Longaker MT, Duncan BW, Scheuenstuhl H, Hunt TK. Ontogeny of fetal sheep polymorphonuclear leukocyte phagocytosis. J. Pediatr. Surg. 1991;26:853–855. doi: 10.1016/0022-3468(91)90155-m. [DOI] [PubMed] [Google Scholar]
- Jones MP, Khan RJ, Carey Smith RL. Surgical interventions for treating acute achilles tendon rupture: key findings from a recent cochrane review. J. Bone Joint Surg. Am. 2012;94:e88. doi: 10.2106/JBJS.J.01829. [DOI] [PubMed] [Google Scholar]
- Julia MV, Albert A, Morales L, Miro D, Sancho MA, Garcia X. Wound healing in the fetal period: the resistance of the scar to rupture. J. Pediatr. Surg. 1993;28:1458–1462. doi: 10.1016/0022-3468(93)90430-s. [DOI] [PubMed] [Google Scholar]
- Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J. Bone Joint Surg. Am. 1991;73:1507–1525. [PubMed] [Google Scholar]
- Kench JA, Russell DM, Fadok VA, et al. Aberrant wound healing and TGF-beta production in the autoimmune-prone MRL/+ mouse. Clin. Immunol. 1999;92:300–310. doi: 10.1006/clim.1999.4754. [DOI] [PubMed] [Google Scholar]
- Kovacevic D, Rodeo SA. Biological augmentation of rotator cuff tendon repair. Clin. Orthop. Relat. Res. 2008;466:622–633. doi: 10.1007/s11999-007-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel TM, Michna BA, Thomas BL, et al. Transforming growth factor beta (TGF-beta) induces fibrosis in a fetal wound model. J. Pediatr. Surg. 1988;23:647–652. doi: 10.1016/s0022-3468(88)80638-9. [DOI] [PubMed] [Google Scholar]
- Lait ME, Smith LN. Wound management: a literature review. J. Clin. Nurs. 1998;7:11–17. doi: 10.1046/j.1365-2702.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plast. Reconstr. Surg. 2010;126:1172–1180. doi: 10.1097/PRS.0b013e3181eae781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadbetter WB. Cell-matrix response in tendon injury. Clin. Sports Med. 1992;11:533–578. [PubMed] [Google Scholar]
- Leferovich JM, Bedelbaeva K, Samulewicz S, et al. Heart regeneration in adult MRL mice. Proc. Natl Acad. Sci. USA. 2001;98:9830–9835. doi: 10.1073/pnas.181329398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehfeldt M, Ray E, Sherman R. MOC-PS(SM) CME article: treatment of flexor tendon laceration. Plast. Reconstr. Surg. 2008;121(4 Suppl):1–12. doi: 10.1097/01.prs.0000305927.51554.fb. [DOI] [PubMed] [Google Scholar]
- Leung A, Crombleholme TM, Keswani SG. Fetal wound healing: implications for minimal scar formation. Curr. Opin. Pediatr. 2012;24:371–378. doi: 10.1097/MOP.0b013e3283535790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JS, Jr, Furman BD, Zeitler E, et al. Genetic and cellular evidence of decreased inflammation associated with reduced post-traumatic arthritis in MRL/MpJ mice. Arthritis Rheum. 2012;66:660–670. doi: 10.1002/art.37796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Mohan S, Gu W, Baylink DJ. Differential protein profile in the ear-punched tissue of regeneration and non-regeneration strains of mice: a novel approach to explore the candidate genes for soft-tissue regeneration. Biochim. Biophys. Acta. 2000;1524:102–109. doi: 10.1016/s0304-4165(00)00118-5. [DOI] [PubMed] [Google Scholar]
- Li X, Gu W, Masinde G, et al. Genetic control of the rate of wound healing in mice. Heredity (Edinb) 2001a;86(Pt 6):668–674. doi: 10.1046/j.1365-2540.2001.00879.x. [DOI] [PubMed] [Google Scholar]
- Li X, Mohan S, Gu W, Baylink DJ. Analysis of gene expression in the wound repair/regeneration process. Mamm. Genome. 2001b;12:52–59. doi: 10.1007/s003350010230. [DOI] [PubMed] [Google Scholar]
- Lin TW, Cardenas L, Soslowsky LJ. Biomechanics of tendon injury and repair. J. Biomech. 2004;37:865–877. doi: 10.1016/j.jbiomech.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Lin TW, Cardenas L, Glaser DL, Soslowsky LJ. Tendon healing in interleukin-4 and interleukin-6 knockout mice. J. Biomech. 2006;39:61–69. doi: 10.1016/j.jbiomech.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Liu W, Wang DR, Cao YL. TGF-beta: a fibrotic factor in wound scarring and a potential target for anti-scarring gene therapy. Curr. Gene Ther. 2004;4:123–136. doi: 10.2174/1566523044578004. [DOI] [PubMed] [Google Scholar]
- Liu J, Johnson K, Li J, et al. Regenerative phenotype in mice with a point mutation in transforming growth factor beta type I receptor (TGFBR1) Proc. Natl Acad. Sci. USA. 2011;108:14560–14565. doi: 10.1073/pnas.1111056108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longaker MT, Whitby DJ, Adzick NS, et al. Studies in fetal wound healing, VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J. Pediatr. Surg. 1990;25:63–68. doi: 10.1016/s0022-3468(05)80165-4. discussion 68–9. [DOI] [PubMed] [Google Scholar]
- Longaker MT, Whitby DJ, Ferguson MW, Lorenz HP, Harrison MR, Adzick NS. Adult skin wounds in the fetal environment heal with scar formation. Ann. Surg. 1994;219:65–72. doi: 10.1097/00000658-199401000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin. Sports Med. 2003;22:675–692. doi: 10.1016/s0278-5919(03)00004-8. [DOI] [PubMed] [Google Scholar]
- Majewski M, Ochsner PE, Liu F, Fluckiger R, Evans CH. Accelerated healing of the rat Achilles tendon in response to autologous conditioned serum. Am. J. Sports Med. 2009;37:2117–2125. doi: 10.1177/0363546509348047. [DOI] [PubMed] [Google Scholar]
- Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Masinde G, Li X, Baylink DJ, Nguyen B, Mohan S. Isolation of wound healing/regeneration genes using restrictive fragment differential display-PCR in MRL/MPJ and C57BL/6 mice. Biochem. Biophys. Res. Commun. 2005;330:117–122. doi: 10.1016/j.bbrc.2005.02.143. [DOI] [PubMed] [Google Scholar]
- McBrearty BA, Clark LD, Zhang XM, Blankenhorn EP, Heber-Katz E. Genetic analysis of a mammalian wound-healing trait. Proc. Natl Acad. Sci. USA. 1998;95:11792–11797. doi: 10.1073/pnas.95.20.11792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33:381–394. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- Monroe DG, McGee-Lawrence ME, Oursler MJ, Westendorf JJ. Update on Wnt signaling in bone cell biology and bone disease. Gene. 2012;492:1–18. doi: 10.1016/j.gene.2011.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh YS, Thomson LE, Fishbein MC, Berman DS, Sharifi B, Chen PS. Scar formation after ischemic myocardial injury in MRL mice. Cardiovasc. Pathol. 2004;13:203–206. doi: 10.1016/j.carpath.2004.03.610. [DOI] [PubMed] [Google Scholar]
- Panetta NJ, Gupta DM, Longaker MT. Bone regeneration and repair. Curr. Stem Cell Res. Ther. 2010;5:122–128. doi: 10.2174/157488810791268618. [DOI] [PubMed] [Google Scholar]
- Perlman R, Schiemann WP, Brooks MW, Lodish HF, Weinberg RA. TGF-beta-induced apoptosis is mediated by the adapter protein Daxx that facilitates JNK activation. Nat. Cell Biol. 2001;3:708–714. doi: 10.1038/35087019. [DOI] [PubMed] [Google Scholar]
- Reinke JM, Sorg H. Wound repair and regeneration. Eur. Surg. Res. 2012;49:35–43. doi: 10.1159/000339613. [DOI] [PubMed] [Google Scholar]
- Ricchetti ET, Reddy SC, Ansorge HL, et al. Effect of interleukin-10 overexpression on the properties of healing tendon in a murine patellar tendon model. J Hand Surg Am. 2008;33:1843–1852. doi: 10.1016/j.jhsa.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology (Oxford) 2004;43:131–142. doi: 10.1093/rheumatology/keg448. [DOI] [PubMed] [Google Scholar]
- Robey TE, Murry CE. Absence of regeneration in the MRL/MpJ mouse heart following infarction or cryoinjury. Cardiovasc. Pathol. 2008;17:6–13. doi: 10.1016/j.carpath.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt JM, Hwang K, Winn SR, Hollinger JO. Bone morphogenetic proteins: an update on basic biology and clinical relevance. J. Orthop. Res. 1999;17:269–278. doi: 10.1002/jor.1100170217. [DOI] [PubMed] [Google Scholar]
- Seifert AW, Kiama SG, Seifert MG, Goheen JR, Palmer TM, Maden M. Skin shedding and tissue regeneration in African spiny mice (Acomys) Nature. 2012;489:561–565. doi: 10.1038/nature11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereysky JB. New York, NY: Mount Sinai School of Medicine; 2011. An analysis of the structure, function, and biological response of tendon during overuse and repair using two genetic backgrounds, Doctoral dissertation. [Google Scholar]
- Sereysky JB, Andarawis-Puri N, Ros SJ, Jepsen KJ, Flatow EL. Automated image analysis method for quantifying damage accumulation in tendon. J. Biomech. 2010;43:2641–2644. doi: 10.1016/j.jbiomech.2010.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereysky JB, Andarawis-Puri N, Jepsen KJ, Flatow EL. Structural and mechanical effects of in vivo fatigue damage induction on murinetendon. J. Orthop. Res. 2012;30:965–972. doi: 10.1002/jor.22012. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereysky JB, George NS, Laudier D, Flatow EL, Andarawis-Puri N. Murphy Roths Large Mice Exhibit Improved Healing After Laceration and Fatigue Loading. San Antonio, TX: Transactions of the 59th Meeting of the Orthopaedic Research Society; 2013. [Google Scholar]
- Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J. Cell Sci. 1995;108(Pt 3):985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- Smith RK, Birch HL, Goodman S, Heinegard D, Goodship AE. The influence of ageing and exercise on tendon growth and degeneration–hypotheses for the initiation and prevention of strain-induced tendinopathies. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002;133:1039–1050. doi: 10.1016/s1095-6433(02)00148-4. [DOI] [PubMed] [Google Scholar]
- Soo C, Hu FY, Zhang X, et al. Differential expression of fibromodulin, a transforming growth factor-beta modulator, in fetal skin development and scarless repair. Am. J. Pathol. 2000;157:423–433. doi: 10.1016/s0002-9440(10)64555-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo C, Beanes SR, Hu FY, et al. Ontogenetic transition in fetal wound transforming growth factor-beta regulation correlates with collagen organization. Am. J. Pathol. 2003;163:2459–2476. doi: 10.1016/s0002-9440(10)63601-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HB, Li Y, Fung DT, Majeska RJ, Schaffler MB, Flatow EL. Coordinate regulation of IL-1beta and MMP-13 in rat tendons following subrupture fatigue damage. Clin. Orthop. Relat. Res. 2008;466:1555–1561. doi: 10.1007/s11999-008-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian RZ. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin. Sports Med. 2012;31:589–604. doi: 10.1016/j.csm.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv. Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J. Biomech. Eng. 2003;125:106–113. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- Thordarson DB, Shean CJ. Nerve and tendon lacerations about the foot and ankle. J. Am. Acad. Orthop. Surg. 2005;13:186–196. doi: 10.5435/00124635-200505000-00005. [DOI] [PubMed] [Google Scholar]
- Tredget EE, Nedelec B, Scott PG, Ghahary A. Hypertrophic scars, keloids, and contractures. The cellular and molecular basis for therapy. Surg. Clin. North Am. 1997;77:701–730. doi: 10.1016/s0039-6109(05)70576-4. [DOI] [PubMed] [Google Scholar]
- Tredget EE, Shankowsky HA, Pannu R, et al. Transforming growth factor-beta in thermally injured patients with hypertrophic scars: effects of interferon alpha-2b. Plast. Reconstr. Surg. 1998;102:1317–1328. doi: 10.1097/00006534-199810000-00001. discussion 1329–30. [DOI] [PubMed] [Google Scholar]
- Tucker B, Klassen H, Yang L, Chen DF, Young MJ. Elevated MMP expression in the MRL mouse retina creates a permissive environment for retinal regeneration. Invest. Ophthalmol. Vis. Sci. 2008;49:1686–1695. doi: 10.1167/iovs.07-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M, Lyons BL, Burzenski LM, et al. Accelerated wound healing of alkali-burned corneas in MRL mice is associated with a reduced inflammatory signature. Invest. Ophthalmol. Vis. Sci. 2005;46:4097–4106. doi: 10.1167/iovs.05-0548. [DOI] [PubMed] [Google Scholar]
- Varga J, Rosenbloom J, Jimenez SA. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem. J. 1987;247:597–604. doi: 10.1042/bj2470597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinarsky V, Atkinson DL, Stevenson TJ, Keating MT, Odelberg SJ. Normal newt limb regeneration requires matrix metalloproteinase function. Dev. Biol. 2005;279:86–98. doi: 10.1016/j.ydbio.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Vorotnikova E, McIntosh D, Dewilde A, et al. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol. 2010;29:690–700. doi: 10.1016/j.matbio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Ward BD, Furman BD, Huebner JL, Kraus VB, Guilak F, Olson SA. Absence of posttraumatic arthritis following intraarticular fracture in the MRL/MpJ mouse. Arthritis Rheum. 2008;58:744–753. doi: 10.1002/art.23288. [DOI] [PubMed] [Google Scholar]
- Whitby DJ, Ferguson MW. Immunohistochemical localization of growth factors in fetal wound healing. Dev. Biol. 1991;147:207–215. doi: 10.1016/s0012-1606(05)80018-1. [DOI] [PubMed] [Google Scholar]
- Williams-Boyce PK, Daniel JC., Jr Comparison of ear tissue regeneration in mammals. J. Anat. 1986;149:55–63. [PMC free article] [PubMed] [Google Scholar]
- Wong VW, Rustad KC, Akaishi S, et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat. Med. 2011;18:148–152. doi: 10.1038/nm.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]


