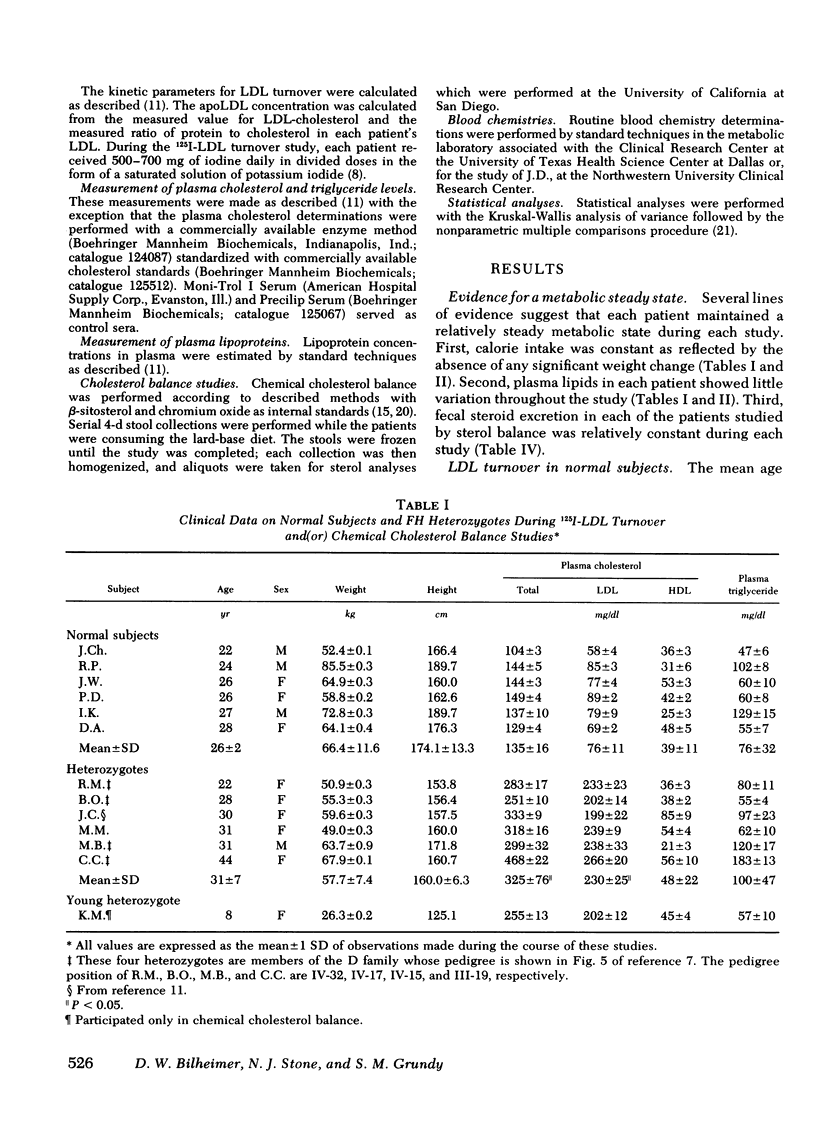
Abstract
To investigate the gene-dosage effect in familial hypercholesterolemia (FH), metabolic studies were conducted in a group of well-characterized patients with either heterozygous (n = 7) or homozygous (n = 7) FH and the results were compared to those obtained in normal subjects (n = 6). The turnover of 125I-labeled low-density lipoprotein (LDL) was measured in all of the normals, all but one of the FH heterozygotes, and in all of the homozygotes. Chemical cholesterol balance was performed simultaneously with the 125I-LDL turnover in all seven of the homozygotes.
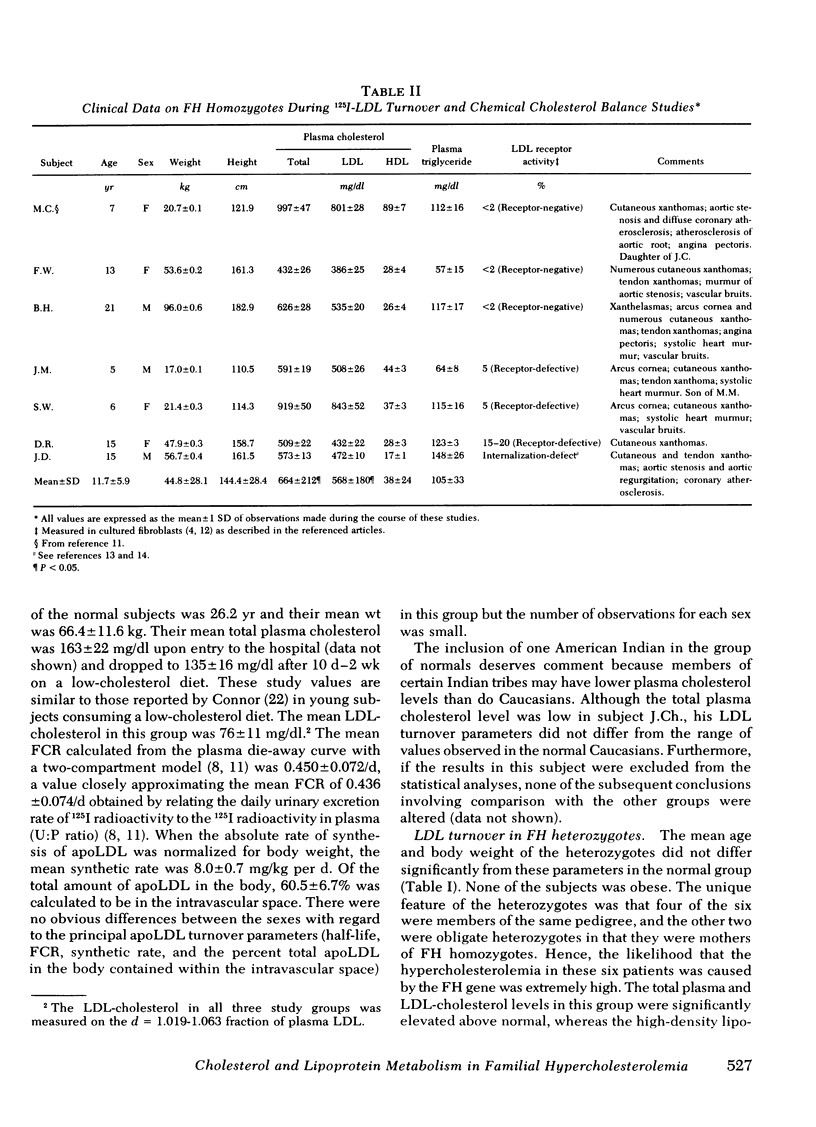
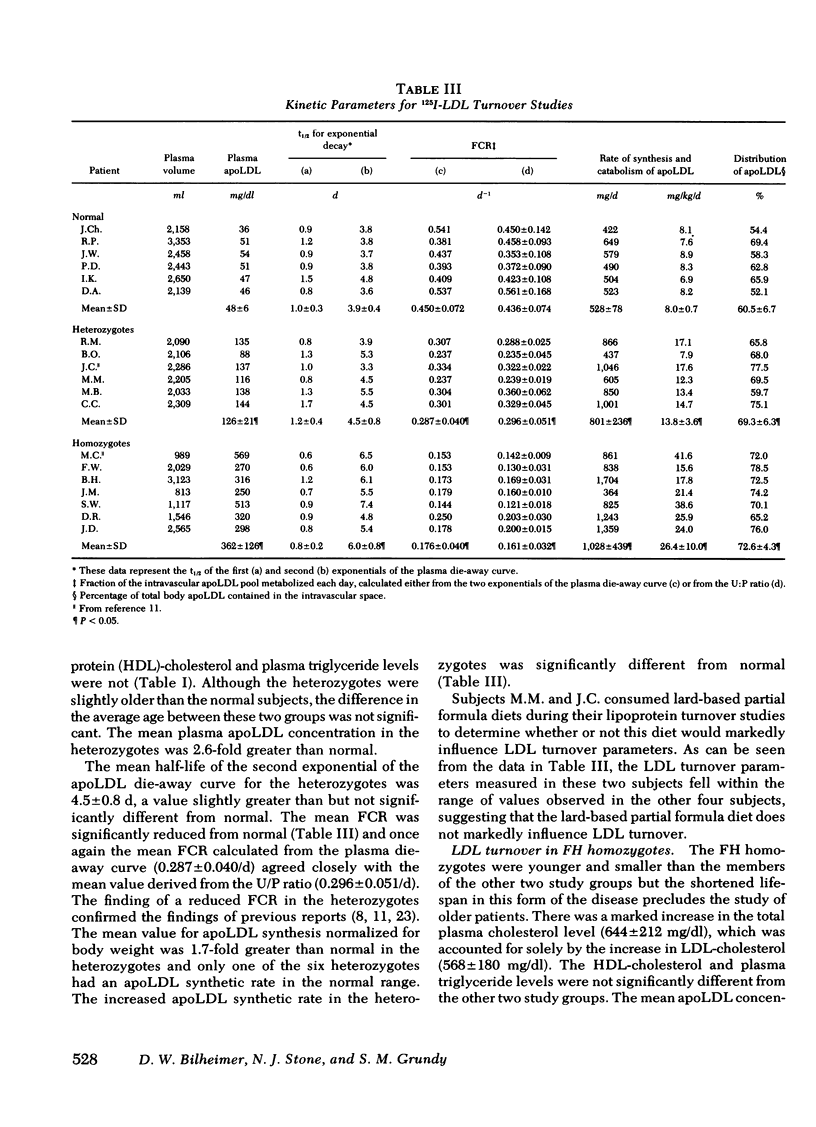
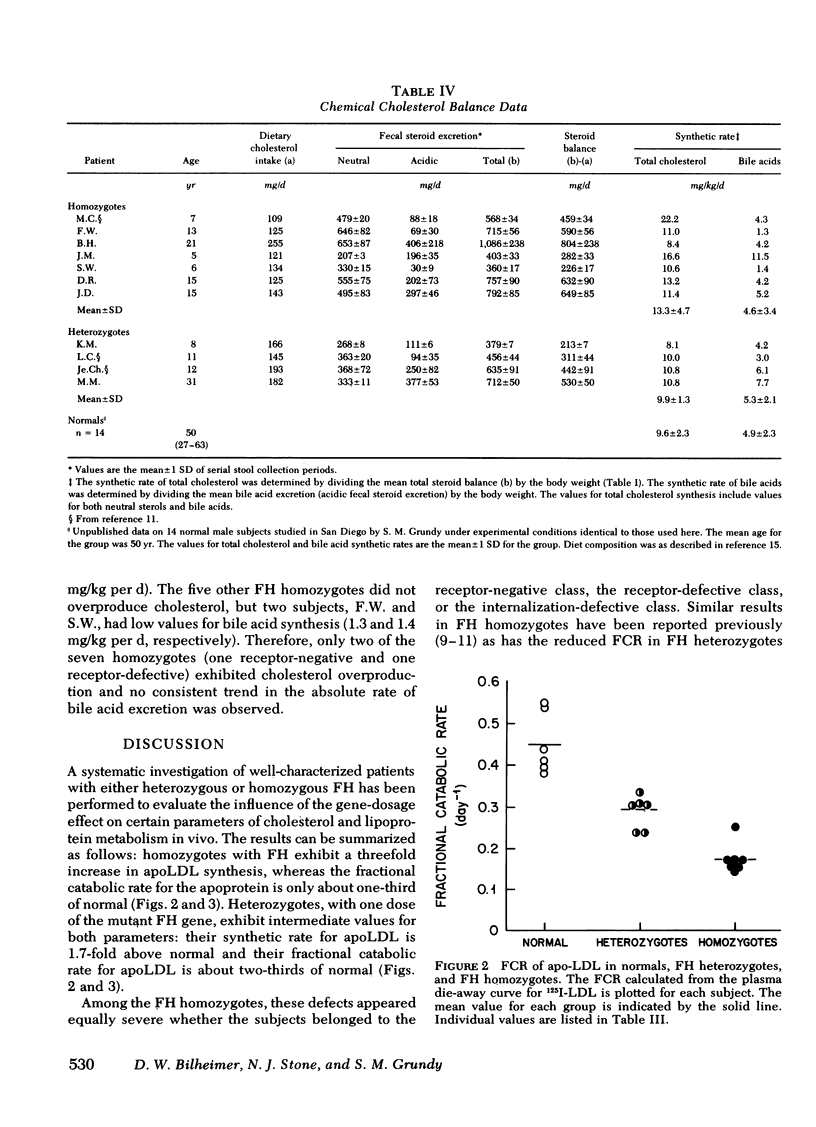
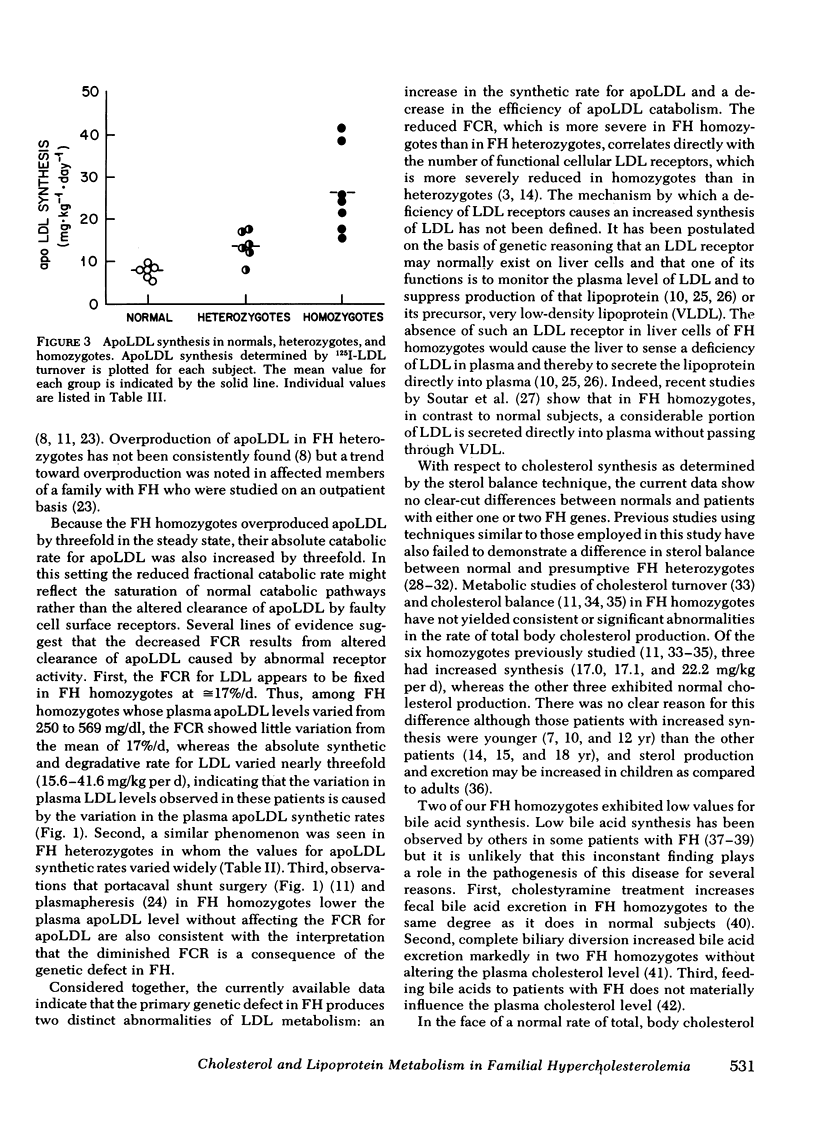
With regard to 125I-LDL turnover, FH homozygotes, who possess two doses of the mutant FH gene, exhibited a threefold increase in the rate of apoLDL synthesis while the fractional catabolic rate (FCR) for the apoprotein was only about one-third of normal. Heterozygotes, who have only one dose of the mutant FH gene, exhibited intermediate values for both parameters; that is, the FCR was two-thirds of normal and the apoLDL synthetic rate was 1.7-fold greater than normal.
The data indicate that the single gene defect in FH produces two distinct abnormalities of LDL metabolism: (a) an increase in the synthetic rate for apoLDL and (b) a decrease in the efficiency of apoLDL catabolism. Both defects are more severe in FH homozygotes than in heterozygotes.
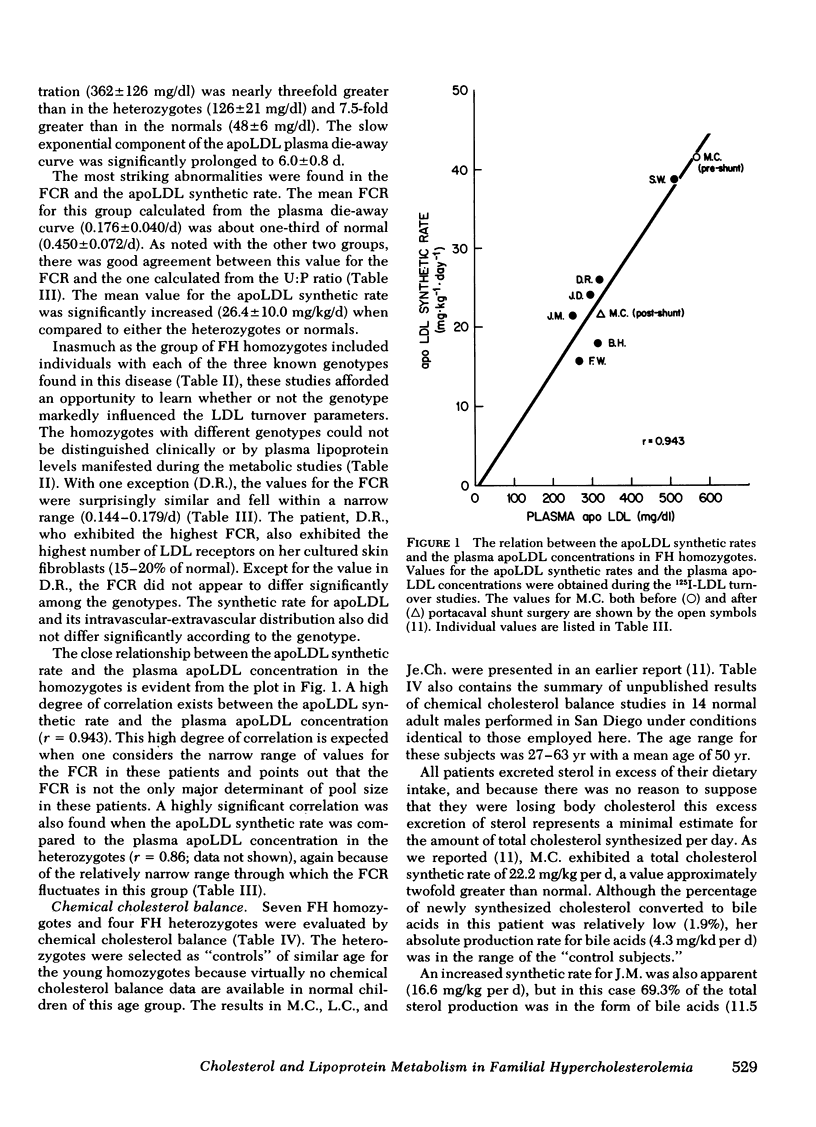
The FCR for apoLDL in the homozygotes appeared to be fixed at ≅ 17%/d whereas the plasma LDL level varied about twofold. These findings suggest that the twofold variation in plasma LDL levels observed in these seven patients is caused by variation in the plasma apoLDL synthetic rates. Consistent with this conclusion was the finding that the correlation between the plasma LDL level and the apoLDL synthetic rates in the seven FH homozygotes was 0.943.
The rate of total body cholesterol synthesis determined by chemical cholesterol balance did not appear to clearly differ between normals and patients with either one or two mutant FH genes. Two of the youngest FH homozygotes exhibited cholesterol overproduction but the other five did not. No consistent abnormality of bile acid metabolism was observed in these patients. Because the daily plasma flux of cholesterol on LDL is about threefold greater than the amount of cholesterol produced per day, a significant amount of the cholesterol liberated from LDL degradation must be reused.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilheimer D. W., Goldstein J. L., Grundy S. M., Brown M. S. Reduction in cholesterol and low density lipoprotein synthesis after portacaval shunt surgery in a patient with homozygous familial hypercholesterolemia. J Clin Invest. 1975 Dec;56(6):1420–1430. doi: 10.1172/JCI108223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilheimer D. W., Ho Y. K., Brown M. S., Anderson R. G., Goldstein J. L. Genetics of the low density lipoprotein receptor. Diminished receptor activity in lymphocytes from heterozygotes with familial hypercholesterolemia. J Clin Invest. 1978 Mar;61(3):678–696. doi: 10.1172/JCI108980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilheimer D. W. Needed: new therapy for hypercholesterolemia. N Engl J Med. 1977 Mar 3;296(9):508–510. doi: 10.1056/NEJM197703032960909. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Feb 10;249(3):789–796. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Analysis of a mutant strain of human fibroblasts with a defect in the internalization of receptor-bound low density lipoprotein. Cell. 1976 Dec;9(4 Pt 2):663–674. doi: 10.1016/0092-8674(76)90130-6. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Familial hypercholesterolemia: A genetic defect in the low-density lipoprotein receptor. N Engl J Med. 1976 Jun 17;294(25):1386–1390. doi: 10.1056/NEJM197606172942509. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Familial hypercholesterolemia: genetic, biochemical and pathophysiologic considerations. Adv Intern Med. 1975;20:273–296. [PubMed] [Google Scholar]
- Deckelbaum R. J., Lees R. S., Small D. M., Hedberg S. E., Grundy S. M. Failure of complete bile diversion and oral bile acid therapy in the treatment of homozygous familial hypercholesterolemia. N Engl J Med. 1977 Mar 3;296(9):465–470. doi: 10.1056/NEJM197703032960901. [DOI] [PubMed] [Google Scholar]
- Einarsson K., Hellström K. The formation of bile acids in patients with three types of hyperlipoproteinaemia. Eur J Clin Invest. 1972 Jun;2(4):225–230. doi: 10.1111/j.1365-2362.1972.tb00648.x. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Stone N. J. Genetics of the LDL receptor: evidence that the mutations affecting binding and internalization are allelic. Cell. 1977 Nov;12(3):629–641. doi: 10.1016/0092-8674(77)90263-x. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Dana S. E., Brunschede G. Y., Brown M. S. Genetic heterogeneity in familial hypercholesterolemia: evidence for two different mutations affecting functions of low-density lipoprotein receptor. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1092–1096. doi: 10.1073/pnas.72.3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr, Davignon J. The interaction of cholesterol absorption and cholesterol synthesis in man. J Lipid Res. 1969 May;10(3):304–315. [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr Measurements of cholesterol turnover, synthesis, and absorption in man, carried out by isotope kinetic and sterol balance methods. J Lipid Res. 1969 Jan;10(1):91–107. [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr, Salen G. Interruption of the enterohepatic circulation of bile acids in man: comparative effects of cholestyramine and ileal exclusion on cholesterol metabolism. J Lab Clin Med. 1971 Jul;78(1):94–121. [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr, Salen G., Schreibman P. H., Nestel P. J. Mechanisms of action of clofibrate on cholesterol metabolism in patients with hyperlipidemia. J Lipid Res. 1972 Jul;13(4):531–551. [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr The effects of unsaturated dietary fats on absorption, excretion, synthesis, and distribution of cholesterol in man. J Clin Invest. 1970 Jun;49(6):1135–1152. doi: 10.1172/JCI106329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy S. M. Effects of polyunsaturated fats on lipid metabolism in patients with hypertriglyceridemia. J Clin Invest. 1975 Feb;55(2):269–282. doi: 10.1172/JCI107930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch F. T. Practical methods for plasma lipoprotein analysis. Adv Lipid Res. 1968;6:1–68. [PubMed] [Google Scholar]
- Ho Y. K., Brown S., Bilheimer D. W., Goldstein J. L. Regulation of low density lipoprotein receptor activity in freshly isolated human lymphocytes. J Clin Invest. 1976 Dec;58(6):1465–1474. doi: 10.1172/JCI108603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. K., Faust J. R., Bilheimer D. W., Brown M. S., Goldstein J. L. Regulation of cholesterol synthesis by low density lipoprotein in isolated human lymphocytes. Comparison of cells from normal subjects and patients with homozygous familial hypercholesterolemia and abetalipoproteinemia. J Exp Med. 1977 Jun 1;145(6):1531–1549. doi: 10.1084/jem.145.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. T., Rodriguez J. T., Woodward W. E., Nichols B. L. Comparison of patterns of fecal bile acid and neutral sterol between children and adults. Am J Clin Nutr. 1976 Nov;29(11):1196–1203. doi: 10.1093/ajcn/29.11.1196. [DOI] [PubMed] [Google Scholar]
- Kottke B. A. Difference in bile acid excretion. Primary hypercholesteremia compared to combined hypercholesteremia and hypertriglyceridemia. Circulation. 1969 Jul;40(1):13–20. doi: 10.1161/01.cir.40.1.13. [DOI] [PubMed] [Google Scholar]
- Langer T., Strober W., Levy R. I. The metabolism of low density lipoprotein in familial type II hyperlipoproteinemia. J Clin Invest. 1972 Jun;51(6):1528–1536. doi: 10.1172/JCI106949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B., Myant N. B. Studies in the metabolism of cholesterol in subjects with normal plasma cholesterol levels and in patients with essential hypercholesterolaemia. Clin Sci. 1967 Apr;32(2):201–213. [PubMed] [Google Scholar]
- Miettinen T. A. New insights into cholesterol dynamics. Arch Surg. 1978 Jan;113(1):45–49. doi: 10.1001/archsurg.1978.01370130047007. [DOI] [PubMed] [Google Scholar]
- Miettinen T. A., Pelkonen R., Nikkilä E. A., Heinonen O. Low excretion of fecal bile acids in a family with hypercholesterolemia. Acta Med Scand. 1967 Nov;182(5):645–650. doi: 10.1111/j.0954-6820.1967.tb10890.x. [DOI] [PubMed] [Google Scholar]
- Moutafis C. D., Simons L. A., Myant N. B., Adams P. W., Wynn V. The effect of cholestyramine on the faecal excretion of bile acids and neutral steroids in familial hypercholesterolaemia. Atherosclerosis. 1977 Mar;26(3):329–334. doi: 10.1016/0021-9150(77)90085-5. [DOI] [PubMed] [Google Scholar]
- Noble R. P. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Res. 1968 Nov;9(6):693–700. [PubMed] [Google Scholar]
- Packard C. J., Third J. L., Shepherd J., Lorimer A. R., Morgan H. G., Lawrie T. D. Low density lipoprotein metabolism in a family of familial hypercholesterolemic patients. Metabolism. 1976 Sep;25(9):995–1006. doi: 10.1016/0026-0495(76)90129-3. [DOI] [PubMed] [Google Scholar]
- Quintão E., Grundy S. M., Ahrens E. H., Jr An evaluation of four methods for measuring cholesterol absorption by the intestine in man. J Lipid Res. 1971 Mar;12(2):221–232. [PubMed] [Google Scholar]
- Quintão E., Grundy S. M., Ahrens E. H., Jr Effects of dietary cholesterol on the regulation of total body cholesterol in man. J Lipid Res. 1971 Mar;12(2):233–247. [PubMed] [Google Scholar]
- Reichl D., Simons L. A., Myant N. B. The metabolism of low-density lipoprotein in a patient with familial hyperbetalipoproteinaemia. Clin Sci Mol Med. 1974 Dec;47(6):635–638. doi: 10.1042/cs0470635. [DOI] [PubMed] [Google Scholar]
- STEINFELD J. L., PATON R. R., FLICK A. L., MILCH R. A., BEACH F. E., TABERN D. L. Distribution and degradation of human serum albumin labeled with I 131 by different techniques. Ann N Y Acad Sci. 1957 Aug 30;70(1):109–121. doi: 10.1111/j.1749-6632.1957.tb35382.x. [DOI] [PubMed] [Google Scholar]
- Simons L. A., Reichl D., Myant N. B., Mancini M. The metabolism of the apoprotein of plasma low density lipoprotein in familial hyperbetalipoproteinaemia in the homozygous form. Atherosclerosis. 1975 Mar-Apr;21(2):283–298. doi: 10.1016/0021-9150(75)90087-8. [DOI] [PubMed] [Google Scholar]
- Soutar A. K., Myant N. B., Thompson G. R. Simultaneous measurement of apolipoprotein B turnover in very-low-and low-density lipoproteins in familial hypercholesterolaemia. Atherosclerosis. 1977 Nov;28(3):247–256. doi: 10.1016/0021-9150(77)90174-5. [DOI] [PubMed] [Google Scholar]
- Tangedahl T. N., Hofmann A. F., Kottke B. A. Biliary lipid secretion in hypercholesterolemia. J Lipid Res. 1979 Jan;20(1):125–133. [PubMed] [Google Scholar]
- Thompson G. R., Spinks T., Ranicar A., Myant N. B. Non-stedy-state studies of low-density-liproprotein turnover in familial hypercholesterolaemia. Clin Sci Mol Med. 1977 Apr;52(4):361–369. doi: 10.1042/cs0520361. [DOI] [PubMed] [Google Scholar]



