Abstract
Context
Critically short telomeres produce apoptosis, cell senescence and chromosomal instability in tissue culture and animal models. Variations in telomere length have been reported in severe aplastic anemia (SAA) but their clinical significance is unknown.
Objective
To investigate the relationship between telomere length and clinical outcomes in SAA.
Design and Setting
Single institution analysis of SAA patients treated in sequential prospective protocols at NIH from 2000 to 2008.
Patients
We retrospectively analyzed pre-treatment leukocyte age-adjusted telomere length in 183 patients with SAA consecutively enrolled into immunosuppression protocols with anti-thymocyte globulin plus cyclosporine for correlation with clinical outcomes.
Main Outcomes Measures
The outcomes studied were hematologic response, relapse, clonal evolution and survival.
Results
There was no relationship between hematologic response and telomere length with response rates of 56.5%, 54.3%, 60%, and 56.5% in the first (n=46), second (n=46), third (n=45), and fourth quartiles (n=46), respectively. In multivariate analysis, telomere length was associated with relapse, clonal evolution, and mortality. Evaluated as a continuous variable, telomere length inversely correlated with the probability of hematologic relapse (HR=0.16; 95% CI, 0.03–0.69; p=0.01). The rate of clonal evolution was higher in patients in the first quartile (24.5%; 95% CI, 8.7%–37.5%) compared to quartiles 2–4 (8.4%; 95% CI, 3.2%–13.3%; p=0.009), and evolution to monosomy 7 or complex cytogenetics was more common in the first quartile (18.8%; 95% CI, 3.5%–31.6%) compared to quartiles 2–4 (4.5%; 95% CI, 0.5%–8.2%; p=0.002). Survival between these two groups differed, with 66% (95% CI, 52.9%–82.5%) surviving 6 years in the first quartile compared to 83.8% (95% CI, 77.3%–90.9%) in quartiles 2–4 (p=0.008).
Conclusion
In a cohort of patients with severe aplastic anemia receiving immunosuppressive therapy, telomere length was unrelated to response, but was associated with risk of relapse, clonal evolution, and overall survival.
Introduction
Severe aplastic anemia (SAA) is characterized by life-threatening cytopenias and a profound diminution of bone marrow progenitor cells. Clinical and laboratory evidence implicate SAA as an immune mediated disorder where oligoclonal cytotoxic T-cells target and destroy hematopoietic progenitor cells resulting in profound marrow failure.1 SAA can be cured by hematopoietic stem cell transplantation but in older patients and when a histocompatible sibling donor is unavailable, immunosuppressive therapy with anti-thymocyte globulin (ATG) plus cyclosporine is effective.1 The majority of patients, 60–70%, respond with hematologic improvement to immunosuppression. However, relapses occur in about one-third of responders and clonal evolution is observed in 10–15% of cases, which manifest late as myelodysplasia.1
While immune destruction of hematopoietic cells is the proximate cause of SAA, recently target cell abnormalities have been identified as risk factors in bone marrow failure. Mutations in telomerase complex genes resulting in extremely short telomeres have been described in some patients with apparently acquired SAA.2–5 Telomeres are nucleotide repeats at the end of the chromosomes which function as protective caps to prevent erosion of genomic DNA during cell division. Telomeric DNA can be elongated by the telomerase complex which is comprised of a reverse transcriptase catalytic subunit (encoded by TERT), an RNA template (encoded by TERC), and associated proteins.2 In order to determine the effect of telomere attrition in acquired SAA, we measured telomere length pre-treatment in consecutive patients at our institution that had received ATG plus cyclosporine since 2000 and analyzed its relationship with hematologic recovery, relapse, clonal evolution and survival.
Patient and methods
Patient and treatment details
Patients were enrolled into three sequential treatment-naïve SAA protocols from November 2000 to May 2008 at the National Institutes of Health in Bethesda, MD. All consecutive patients treated with ATG plus cyclosporine for whom sufficient samples were available for testing were included. A total of 248 SAA patients enrolled into treatment protocols during the analysis period: 19 were not treatment-naïve, 16 received alternative immunosuppression other than ATG plus cyclosporine, and in 30 sufficient (or adequate) sample was not available for analysis. In total 183 patients were included in the analysis. Patients (or legal guardians) signed informed consent according to approved protocols by the Institutional Review Board of the National, Heart, Lung, and Blood Institute. For protocol entry purposes, SAA was defined as bone marrow cellularity of less than 30% and severe pancytopenia with at least two of the following peripheral blood count criteria: 1) absolute neutrophil count < 500/μL; 2) absolute reticulocyte count < 60,000/μL; 3) platelet count < 20,000/μL.6 Chromosomes were assayed after in vitro exposure of lymphocytes to diepoxybutane and in some cases also to mitomycin C to exclude Fanconi anemia. Patients with inherited SAA or evidence of a clonal hematologic disorder as inferred from bone marrow cytogenetics were excluded from enrolling into these treatment protocols.
Hematologic response, defined as no longer meeting criteria for SAA, was determined at six months following ATG and, for the current analysis, adopted as the criterion for hematologic recovery.6 Patients who relapsed by definition required reinstitution of immunosuppression.6 Clonal evolution was defined as the appearance of a new cytogenetic abnormality on bone marrow cytogenetics after immunosuppressive therapy. All patients were tested for mutations in the telomerase complex genes TERT and TERC, as previously described.3
Patients underwent one of four regimens: three were based on horse ATG plus cyclosporine and one rabbit ATG plus cyclosporine regimen.6–9 The three horse ATG regimens were standard horse ATG plus cyclosporine, horse ATG/cyclosporine/mycophenolate mofetil and horse ATG/cyclosporine/sirolimus and have been described previously in detail.6–8 There was no difference in clinical outcome among the three horse ATG regimens, which were combined for this analysis. Cyclosporine was discontinued after six months in all but 47 responders to horse ATG plus cyclosporine who had their cyclosporine dose tapered after 6 months.
Telomere length measurement
Telomere length of pre-treatment peripheral blood leukocytes was assessed by quantitative polymerase chain reaction (PCR) as previously described.10, 11 Total leukocytes were separated by ammonium-based lysis of red blood cells and DNA extracted using the DNeasy Blood kit (Qiagen, Maryland). PCRs were performed in a 7500 Real Time PCR System (Applied Biosystems, Foster City, CA). Each sample’s telomere length (x) was based on the telomere to single copy gene ratio (T/S ratio) and based on the calculation of the ΔCt [Ct(telomeres)/Ct(single gene)]. Telomere length was expressed as relative T/S ratio, which was normalized to the average T/S ratio of reference sample [2−(ΔCtx− ΔCtr) = 2−ΔΔCt], used for the standard curve, as reference sample, and as validation sample. In order to make comparable the results from different plate runs, the results of each plate were approved only if the relative T/S ratio of the validation reference sample fell within 3% variation. Laboratory personnel conducting the telomere length assay were blinded to patients’ clinical outcomes prior to statistical analysis.
Statistical methods
Age-adjusted telomere length for each subject was computed by subtracting the subject’s linear predicted telomere length from the observed telomere length. Nonparametric Cox regression based on splines with continuous age-adjusted telomere length was used as an exploratory analysis for the probability distributions of time to relapse, time to evolution and overall survival. Based on estimated log-hazard curves, we evaluated the effects of age-adjusted telomere length quartiles on the event probabilities of these clinical outcomes using the Cox proportional hazard model. Based on the survival curves per quartile for each of the clinical events, quartiles with similar event probabilities were grouped in further analysis. Consequently, for analysis of time-to-clonal evolution and survival, patients in the first quartile formed a distinct group, and patients in the second, third and fourth quartiles were combined due to their similar clinical outcomes. As the relationship between telomere length and relapse was more linear, there was no apparent threshold that discriminated those at higher risk for this outcome. Summary statistics (means, proportions and standard deviations) stratified by age-adjusted telomere quartiles were used to describe patients’ age, sex, and other baseline characteristics. P-values based on multi-sample tests for proportions and the analysis of variance tests were used to compare patients’ baseline characteristics. P-values from the log-rank tests were used to evaluate the overall covariate effects in the univariate and multivariate Cox proportional hazard models. Numerical results were computed using the S-PLUS software package (TIBCO Software Inc., Palo Alto, CA). Two-sided P-value was used throughout and considered statistically significant if <0.05.
Results
Telomere length was measured in pre-treatment leukocytes in a total of 183 patients who received initial therapy at our institution and categorized in quartiles after age adjustment (Table 1). Age distribution among all four quartiles was similar (Table 1 and eFigure 1). Median follow-up in all patients was 55 (range, 0.1–116) months and for surviving patients 64 (range, 6–116) months.
Table 1.
Patient characteristics
| Factor | All Patients | 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | P-value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| N(%) | Mean | SD | N(%) | Mean | SD | N(%) | Mean | SD | N(%) | Mean | SD | N(%) | Mean | SD | ||
| Total | 183 | 46(25) | 46(25) | 45(25) | 46(25) | |||||||||||
| Telomere length (T/S) (range) | 183 | 1.23(0.53–1.93) | 46 | 0.86(0.53–1.17) | 46 | 1.09(0.78–1.41) | 45 | 1.33(1.04–1.67) | 46 | 1.64(1.26–1.93) | ||||||
| Age | 183 | 35 | 1.5 | 46 | 37 | 3.3 | 46 | 34 | 3 | 45 | 34 | 2.9 | 46 | 34 | 3.1 | 0.89 |
| Sex | ||||||||||||||||
| Male | 105(57) | 3.7 | 29(63) | 7.2 | 26(57) | 7.4 | 25(56) | 7.5 | 25(54) | 7.4 | 0.84 | |||||
| Female | 78(43) | 3.7 | 17(37) | 7.2 | 20(44) | 7.4 | 20(44) | 7.5 | 21(47) | 7.4 | ||||||
| Immunosuppression | ||||||||||||||||
| Horse ATG/CsA | 70(38) | 3.6 | 16(35) | 7.1 | 15(33) | 7 | 23(51) | 7.5 | 16(35) | 7.1 | 0.24 | |||||
| Horse ATG/CsA/MMF | 48(26) | 3.3 | 17(37) | 7.2 | 9(20) | 5.9 | 11(24) | 6.5 | 11(24) | 6.4 | 0.26 | |||||
| Horse ATG/CsA/Rapa | 35(19) | 2.9 | 5(11) | 4.6 | 11(24) | 6.4 | 8(18) | 5.8 | 11(24) | 6.4 | 0.33 | |||||
| Rabbit ATG/CsA | 30(16) | 2.7 | 8(17) | 5.7 | 11(24) | 6.4 | 3(7) | 3.8 | 8(17) | 5.7 | 0.16 | |||||
| Ethnicity | ||||||||||||||||
| White | 91(50) | 3.7 | 23(50) | 7.5 | 24(52) | 7.4 | 25(56) | 7.5 | 19(41) | 7.3 | 0.57 | |||||
| Black | 45(25) | 3.2 | 9(20) | 5.9 | 12(26) | 6.5 | 7(16) | 5.5 | 17(37) | 7.2 | 0.09 | |||||
| Hispanic | 35(19) | 2.9 | 11(24) | 6.4 | 7(15) | 5.4 | 8(18) | 5.8 | 9(20) | 5.9 | 0.34 | |||||
| Asian | 12(7) | 1.8 | 3(7) | 3.7 | 3(7) | 3.7 | 5(11) | 4.7 | 1(2) | 2.2 | 0.40 | |||||
| Blood counts (/−L) | ||||||||||||||||
| ARC | 20464 | 1371 | 24352 | 2820 | 22181 | 3265 | 20158 | 2474 | 15159 | 2177 | 0.12 | |||||
| ALC | 1312 | 48 | 1328 | 121 | 1285 | 92 | 1322 | 71 | 1313 | 96 | 0.89 | |||||
| ANC | 363 | 22 | 456 | 46 | 382 | 48 | 346 | 35 | 266 | 39 | 0.02 | |||||
| ANC < 200 | 67(37) | 3.6 | 11(24) | 6.4 | 17(37) | 7.2 | 16(36) | 7.2 | 23(50) | 7.5 | 0.08 | |||||
| Platelet | 10852 | 1423 | 9304 | 923 | 9848 | 886 | 14911 | 5519 | 9435 | 1157 | 0.67 | |||||
Telomere length is depicted by the quartiles. ATG, anti-thymocyte globulin; CsA, cyclosporine; MMF, mycophenolate mofetil; Rapa, sirolimus; ARC, absolute reticulocyte count; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; SD, standard deviation;. P-values were based on the F-statistics for comparing all four quartiles. Log-transformed ARC, ALC, ANC and platelet count were used for the analysis of variance models.
Multivariate analysis of telomere length on the rate of response, relapse, clonal evolution and survival
In multivariate logistic regression, telomere length was not associated with response at six months. Reported covariates predictive of response included the reticulocyte and lymphocyte counts.12 In a multivariate Cox proportional hazard model, shorter telomeres were associated with relapse, clonal evolution, and mortality (Table 2). Evaluated as a continuous variable, telomere length inversely associated with relapse; for clonal evolution and mortality, those in the first quartile had a rate about 1/3 compared to quartiles 2–4 (Table 2).
Table 2.
Multivariate Cox proportion hazard model for relapse, clonal evolution and survival
| Relapse | Clonal Evolution | Survival | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Risk factor | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Telomere length | 0.16 | 0.03, 0.69 | 0.01 | 0.29 | 0.11, 0.76 | 0.01 | 0.35 | 0.17, 0.73 | 0.005 |
| Age (years) | 1.03 | 1.01, 1.05 | 0.005 | 1.03 | 1.00, 1.05 | 0.01 | 1.03 | 1.02, 1.05 | <0.001 |
| ARC (/μL) | 0.99 | 0.63, 1.55 | 0.96 | 1.10 | 0.55, 2.19 | 0.79 | 0.63 | 0.41, 0.97 | 0.03 |
| ALC (/μL) | 1.31 | 0.55, 3.10 | 0.54 | 1.16 | 0.51, 2.66 | 0.72 | 0.89 | 0.48, 1.66 | 0.72 |
| ANC (/μL) | 1.20 | 0.67, 2.16 | 0.54 | 0.70 | 0.38, 1.27 | 0.24 | 0.95 | 0.65, 1.38 | 0.78 |
| Platelet (/μL) | 0.80 | 0.46, 1.38 | 0.42 | 0.76 | 0.45, 1.28 | 0.30 | 1.11 | 0.73, 1.67 | 0.63 |
Continuous telomere length was used for relapse due to the linear relationship between relapse and telomere length. For clonal evolution and survival, short telomere was defined as an age-adjusted telomere length < first quartile and long telomere as an age-adjusted telomere length > first quartile. Natural log-transformed ARC, ALC, ANC and platelet count were used to reduce the skewness of these variables. ARC, absolute reticulocyte count, ALC, absolute lymphocyte count, ANC, absolute neutrophil count; HR; hazard ratio; 95% CI, 95% Confidence Interval.
Response and relapse according to telomere length
One hundred four (57%) patients responded to immunosuppressive therapy. There was no correlation between telomere length at first presentation and the probability of response. The response rate for patients in the first quartile was 56.5% (95% confidence interval [CI], 41.6%–71.4%), in the second quartile 54.3% (95% CI, 39.4%–69.3%), in the third quartile 60% (95% CI, 45.1–74.9%), and in the fourth quartile 56.5% (95% CI, 41.6–71.4%). Of the 59 unresponsive patients with telomere length in quartiles 2–4, 37 underwent second course of immunosuppression and 13 responded; of the 20 patients in the first quartile, 10 underwent second course of immunosuppression and two responded.
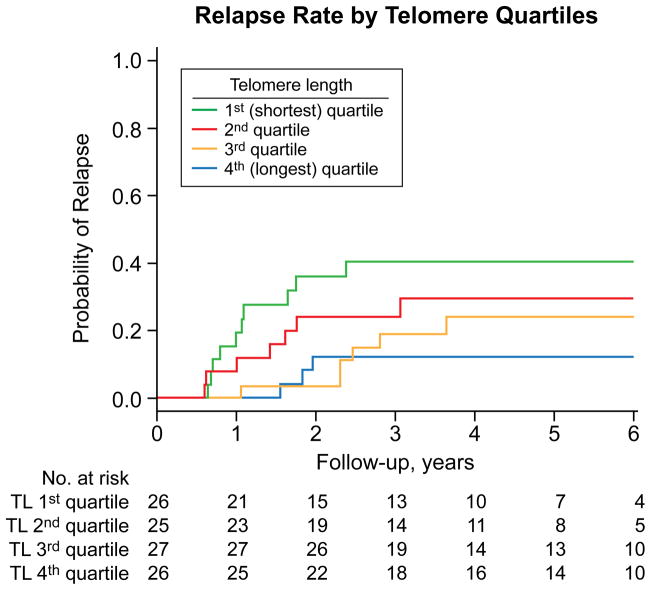
Twenty-six of the 104 patients who responded to a first course of immunosuppressive therapy later relapsed with a 6-year relapse rate of 26.3% (95% CI, 17%–34.5%). In three additional patients, a cytogenetic abnormality was identified at the time of their relapse and considered to have evolved for the purpose of analysis. Among responders, the rate of relapse inversely correlated with telomere length; risk for this outcome increased with shorter telomere length (Figure 1). Overall log-rank p-value for difference in relapse among quartiles was not significant (p=0.08; Figure 1, only responding patients were at risk for relapse, reducing statistical power). However, when telomere length was evaluated as a continuous variable, the rate of relapse increased as telomere length shortened (HR=0.16; p=0.01; Table 2). Among the 9 relapsed patients in quartiles 3–4, eight were treated with immunosuppression, and of the seven patients who are evaluable to date, six have responded; and among the 17 in quartiles 1–2, 16 were treated with immunosuppression, and of the 15 evaluable patients to date, 12 have responded. Thus, shorter telomeres were associated with greater risk of relapse but did not preclude hematologic recovery with further immunosuppression.
Figure 1.
Cumulative incidence of relapse according to pre-treatment telomere length. Incidence of relapse per quartile. A greater rate of relapse was observed with each quartile as the telomere length shortened from the fourth to the first quartile.
Clonal evolution and survival according to telomere length
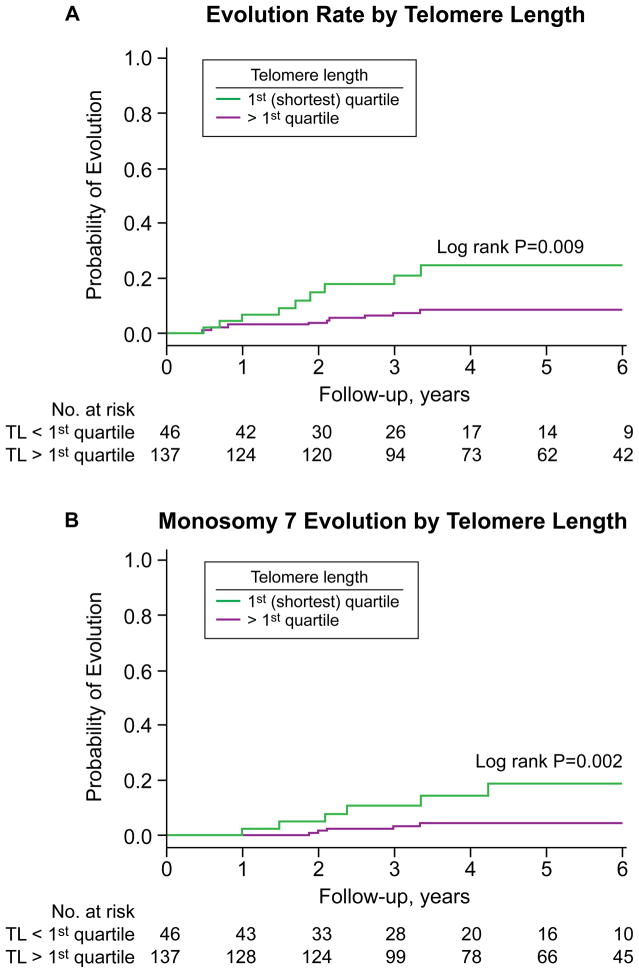
When the incidence for clonal evolution and survival initially were analyzed per quartile, a higher rate for these outcomes was observed for those with shorter telomeres (overall log-rank p=0.02 and 0.06, respectively, for differences among all quartiles; data not shown). In univariate analysis, those in quartiles 2–4 clustered as more favorable compared to those with a telomere length in the first quartile who had a higher risk of clonal evolution and mortality (Figures 2A and 3A). When the relationship between telomere length and clonal evolution and survival were analyzed using the estimated log-hazard curve, differences in outcome were observed for those in first quartile (data not shown). When the quartiles according to the estimated log-hazard curve were grouped, the cumulative incidence of clonal evolution was 8.4% (95% CI, 3.2%–13.3%) for those in quartiles 2–4 compared to 24.5% (95% CI, 8.7%–37.5%) in those with shortest telomeres in the first quartile (p=0.009, log-rank; Figure 2A). Of the ten patients who evolved in the longer telomere group, four had monosomy 7 (one patient developed t(12;13) prior to evolving to monosomy 7), one deletion 13q, one loss of chromosome Y, one loss of chromosome 18, one t(6;14), one deletion of chromosome 3, and one had complex cytogenetic abnormalities. Of the nine patients in the shortest telomere group (first quartile) who evolved, six had monosomy 7 (two patients developed deletion 13q prior to evolving to monosomy 7), one had deletion 13q, one had t(9;19), and one evolved to complex cytogenetics (which followed a loss of chromosome Y abnormality). More evolution to monosomy 7 or complex cytogenetics was observed in the shortest (first quartile) telomere group (18.8%; 95% CI, 3.5%–31.6%) compared to those with longer telomeres (4.5%; 95% CI, 0.5%–8.2%; p=0.002, log-rank; Figure 2B).
Figure 2.
Cumulative incidence of clonal evolution according to pre-treatment telomere length. (A) Incidence of clonal evolutions in the first quartile (shortest) compared to quartiles 2–4. (B) Incidence of monosomy 7 or complex cytogenetics in the first quartile (shortest) compared to quartiles 2–4.
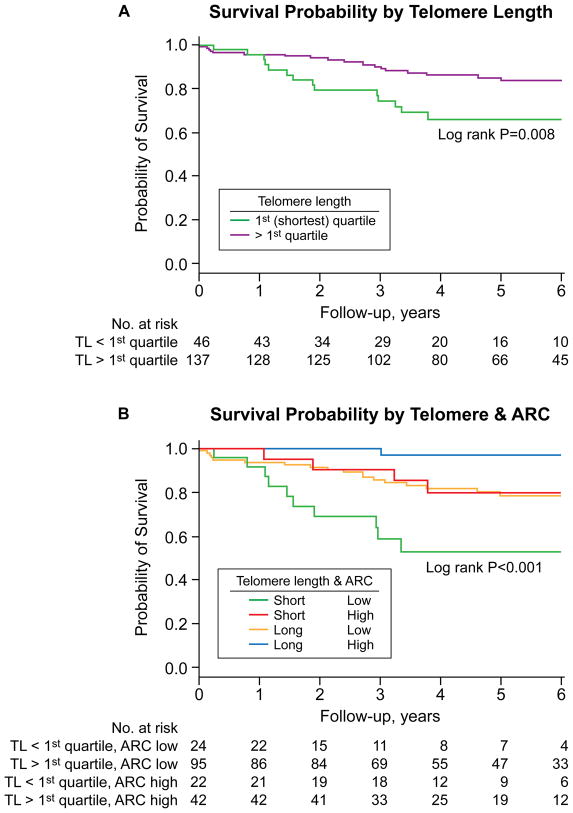
Figure 3.
Overall survival according to pre-treatment telomere length and absolute reticulocyte count (ARC). (A) Overall survival according to pre-treatment telomere length. A superior 6-year survival was observed in those in quartiles 2–4 compared to those in the first (shortest) quartile. (B) Overall survival according to pre-treatment telomere length and ARC. A favorable outcome was observed in patients with both a high ARC and longer telomeres (quartiles 2–4) compared to those with a low ARC and with the shortest telomeres (first quartile). An intermediate group with either a low ARC and longer telomere (quartiles 2–4) or a high ARC and with the shortest telomere length (first quartile) was observed. A high ARC was defined as ≥ 25,000/μL and a low ARC < 25,000/μL.12
Worse survival was observed in patients with the shortest telomere length (first quartile) while the curves for the remaining quartiles clustered to yield a similar survival rate. The survival proportions between the two groups differed significantly, with 66% (95% CI, 52.9%–82.5%) in the shortest telomere (first quartile) group surviving 6 years compared to 83.8% (95% CI, 77.3%–90.9%) in those with longer telomeres in quartiles 2–4 (p=0.008, log-rank; Figure 3A). In the longer telomere group, twelve died from complications of pancytopenia, three died after hematopoietic stem cell transplantation, two from progression to myelodysplasia, one from heart failure, and one in a traffic accident. In the shortest telomere group, three died from complications of pancytopenia, five died after stem cell transplantation, three died after clonal evolution, one from failure to thrive, one from a traffic accident, and one from unknown causes. Among the deaths in longer telomere group (n=19), three occurred after relapse or clonal evolution, and among the deaths in the first quartile (n=14), five occurred after relapse or clonal evolution.
Comment
When first observed, short telomeres of leukocytes in acquired SAA was presumed to be secondary to hematopoietic “stress”.2 The discovery of loss-of-function mutations in genes of the telomerase complex (TERC, TERT) established a genetic etiology for telomere attrition in marrow failure. Telomerase mutations are etiologic in the constitutional marrow failure syndrome dyskeratosis congenita and are also found in a minority of patients with acquired aplastic anemia. However, in the present series, only one patient later tested positive for a TERT mutation (codon A202T; his leukocytes’ telomere length was below the tenth percentile). Therefore the current study describes a relationship between variations of telomere length within the normal range and SAA clinical outcomes in patients who (with a single exception) lacked known genetic explanations for shorter telomeres.
Telomere length was not associated with response to immunosuppression. Why some patients do not respond is unknown: insufficient number of hematopoietic stem cells, inadequate immunosuppression, and a nonimmune etiology for marrow failure each have been suggested.1 That shorter telomeres were not associated with unresponsiveness to immunosuppression indicates that this parameter does not distinguish a nonimmune etiology group. In our cohort, stem cell reserves appeared sufficient for recovery after therapy.
Clonal evolution to myelodysplasia is a major adverse event in SAA; it cannot be routinely predicted and usually signals a poor prognosis. In particular, the finding of monosomy 7 on bone marrow cytogenetics is associated with persistent pancytopenia unresponsive to immunosuppression and progression to myelodysplasia.13 The current work shows that telomere length relates to the development of abnormal marrow clones, in particular monosomy 7, with serious clinical consequences.
Prediction of important disease-related complications is critical to risk stratification and patient management. The major problems of immunosuppressive therapy in SAA are unresponsiveness, relapse, and clonal evolution. Recently, we reported that pre-treatment reticulocyte count was predictive of response to immunosuppression 12; however, there are no recognized predictors for relapse and clonal evolution. In the current study, we show that pre-treatment telomere length associated with relapse and clonal evolution, two serious late events in SAA. When absolute reticulocyte count and telomere length were combined in our cohort, three groups were observed: 1) a favorable group with high reticulocyte count and longer telomeres; 2) an intermediate group with high reticulocyte count and shorter telomeres or a low reticulocyte count and longer telomere length; 3) and a poor risk group with low reticulocyte count and shorter telomere length (Figure 3B). ATG plus cyclosporine may be adequate for the most favorable group; in contrast, better regimens are needed for the poor risk group. For example, androgen treatment offers a potential for in vivo modulation of telomere length,14 and for those at greater risk for late complications after immunosuppression, higher risk protocols such as stem cell transplantation in older patients and alternative source of stem cells might be considered earlier in younger patients.
Telomere attrition is not simply a biomarker; rather, a plausible mechanism for destabilization of the genome has been inferred from basic telomere biology. Ample in vitro and animal experimentation indicate that critical shortening of telomeres causes chromosome instability, tumor formation, and cancer progression.15–22 Normally, senescence would preclude cells with critically short telomeres from tumorigenesis23, but cells with malignant potential may escape by failure of mechanisms such as p53 signaling of DNA damage24 or activation of alternative modes of telomere maintenance.25 In telomerase “knockout” mice (Terc−/−), aneuploidy and end-to-end fusions were observed in fibroblasts after four generations, suggesting an inability to protect chromosome ends when telomerase was deficient.26 Furthermore, on breeding of telomerase-deficient knockout mice with short telomeres with wild-type mice with long telomeres, chromosome fusions and signal-free ends occurred preferentially on chromosomes with critically short telomeres.27
Clinically, telomere length has been associated with human cancer.28 In dyskeratosis congenita, the incidence of cancers was 11-fold higher compared to the general population, with high rates of tongue cancer and leukemia.29 In leukemia patients with no underlying telomerase disorder, hypomorphic mutations in TERT were three-fold more frequent in patients than in controls.30 Telomere attrition has been implicated in a variety of solid organ malignancies. In Barrett’s esophagus, there was an increased risk to develop esophageal adenocarcinoma in individuals who had shorter leukocyte telomere length at first clinical presentation.31 Furthermore, as in our study, telomere length was an independent predictor for progression to esophageal cancer after correction for other covariates.31 Premature shortening of telomeres of leukocytes and in colonic epithelia in a few patients has been correlated to cancer progression in ulcerative colitis.21, 32 Our current data are consistent with findings in other inflammatory diseases which predispose to cancer, in that the risk for clonal evolution was increased in those with pre-treatment, age-adjusted telomere length in the lower quartile. However, in contrast to many investigations of predisposition to gastrointestinal tumors, we determined telomere length months to years preceding malignant transformation, and in those hematopoietic cells directly subject to dysplasia and leukemia. Indeed, bone marrow cells from patients with short telomeres in vitro showed increased number of telomere-free chromosomal ends, aneuploidy, and chromosomal translocations not seen in cells of patients with longer telomeres (our unpublished data).
Our study has strengths and limitations. The strengths include the homogeneous cohort enrolled into our research protocols with specified diagnostic criteria, immunosuppressive drug administration, supportive care, and prospectively determined and defined clinical outcomes. We monitor our patients indefinitely at specified intervals, and our protocols require periodic assessment for adverse events like relapse and clonal evolution. Our study is limited due to the retrospective nature of the analysis and the relatively small number of patients, which did not allow for validation testing in a separate cohort or for reliable subgroup analysis. As a research facility and quaternary referral center, our patient population also may not be representative and our results not necessarily generalizable. Therefore, our results need to be replicated to validate the observed associations and to determine reliable telomere length thresholds that could be incorporated in treatment algorithms.
In conclusion, our data show that in a cohort of patients with SAA receiving immunosuppressive therapy, telomere length was not associated with response, but was associated with risk of relapse, clonal evolution, and overall survival.
Supplementary Material
Acknowledgments
Funding/Support: This research was supported by the Intramural Research Program of the NIH, National, Heart, Lung and Blood Institute. Cooper: NIH-Pfizer. His research year was made possible through the Clinical Research Training Program (CRTP), a public-private partnership supported jointly by the NIH and Pfizer Inc (via a grant to the Foundation for NIH from Pfizer Inc).
Role of the Sponsor: The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Dr. Scheinberg had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial disclosures: None reported.
Additional Contributions: We gratefully thank Olga Nuñez, RN and Barbara Weinstein, RN (both from the Hematology Branch, NHLBI) for patient care, sample collection and handling. They did not receive separate compensation for their contribution.
References
- 1.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108(8):2509–2519. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008;111(9):4446–4455. doi: 10.1182/blood-2007-08-019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamaguchi H, Calado RT, Ly H, et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352(14):1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 4.Ball SE, Gibson FM, Rizzo S, Tooze JA, Marsh JC, Gordon-Smith EC. Progressive telomere shortening in aplastic anemia. Blood. 1998;91(10):3582–3592. [PubMed] [Google Scholar]
- 5.Brummendorf TH, Maciejewski JP, Mak J, Young NS, Lansdorp PM. Telomere length in leukocyte subpopulations of patients with aplastic anemia. Blood. 2001;97(4):895–900. doi: 10.1182/blood.v97.4.895. [DOI] [PubMed] [Google Scholar]
- 6.Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289(9):1130–1135. doi: 10.1001/jama.289.9.1130. [DOI] [PubMed] [Google Scholar]
- 7.Scheinberg P, Nunez O, Wu C, Young NS. Treatment of severe aplastic anaemia with combined immunosuppression: anti-thymocyte globulin, ciclosporin and mycophenolate mofetil. Br J Haematol. 2006;133(6):606–611. doi: 10.1111/j.1365-2141.2006.06085.x. [DOI] [PubMed] [Google Scholar]
- 8.Scheinberg P, Wu CO, Nunez O, Boss C, Sloand EM, Young NS. Treatment of severe aplastic anemia with a combination of horse antithymocyte globulin and cyclosporine, with or without sirolimus: a prospective randomized study. Haematologica. 2009;94(3):348–354. doi: 10.3324/haematol.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheinberg P, Nunez O, Young NS. Retreatment with rabbit anti-thymocyte globulin and ciclosporin for patients with relapsed or refractory severe aplastic anaemia. Br J Haematol. 2006;133(6):622–627. doi: 10.1111/j.1365-2141.2006.06098.x. [DOI] [PubMed] [Google Scholar]
- 10.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brouilette SW, Moore JS, McMahon AD, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369(9556):107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 12.Scheinberg P, Wu CO, Nunez O, Young NS. Predicting response to immunosuppressive therapy and survival in severe aplastic anaemia. Br J Haematol. 2009;144(2):206–216. doi: 10.1111/j.1365-2141.2008.07450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maciejewski JP, Risitano A, Sloand EM, Nunez O, Young NS. Distinct clinical outcomes for cytogenetic abnormalities evolving from aplastic anemia. Blood. 2002;99(9):3129–3135. doi: 10.1182/blood.v99.9.3129. [DOI] [PubMed] [Google Scholar]
- 14.Calado RT, Yewdell WT, Wilkerson KL, et al. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood. 2009;114(11):2236–2243. doi: 10.1182/blood-2008-09-178871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Artandi SE, Depinho RA. Telomeres and telomerase in cancer. Carcinogenesis. 2009 doi: 10.1093/carcin/bgp268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990;346(6287):866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- 17.Rossi D, Lobetti Bodoni C, Genuardi E, et al. Telomere length is an independent predictor of survival, treatment requirement and Richter’s syndrome transformation in chronic lymphocytic leukemia. Leukemia. 2009;23(6):1062–1072. doi: 10.1038/leu.2008.399. [DOI] [PubMed] [Google Scholar]
- 18.Svenson U, Ljungberg B, Roos G. Telomere length in peripheral blood predicts survival in clear cell renal cell carcinoma. Cancer Res. 2009;69(7):2896–2901. doi: 10.1158/0008-5472.CAN-08-3513. [DOI] [PubMed] [Google Scholar]
- 19.Engelhardt M, Drullinsky P, Guillem J, Moore MA. Telomerase and telomere length in the development and progression of premalignant lesions to colorectal cancer. Clin Cancer Res. 1997;3(11):1931–1941. [PubMed] [Google Scholar]
- 20.Meeker AK, Hicks JL, Platz EA, et al. Telomere shortening is an early somatic DNA alteration in human prostate tumorigenesis. Cancer Res. 2002;62(22):6405–6409. [PubMed] [Google Scholar]
- 21.O’Sullivan JN, Bronner MP, Brentnall TA, et al. Chromosomal instability in ulcerative colitis is related to telomere shortening. Nat Genet. 2002;32(2):280–284. doi: 10.1038/ng989. [DOI] [PubMed] [Google Scholar]
- 22.Counter CM, Avilion AA, LeFeuvre CE, et al. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11(5):1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldser DM, Greider CW. Short telomeres limit tumor progression in vivo by inducing senescence. Cancer Cell. 2007;11(5):461–469. doi: 10.1016/j.ccr.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Artandi SE, DePinho RA. Telomeres and telomerase in cancer. Carcinogenesis. 2010;31(1):9–18. doi: 10.1093/carcin/bgp268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrish TA, Greider CW. Short telomeres initiate telomere recombination in primary and tumor cells. PLoS Genet. 2009;5(1):e1000357. doi: 10.1371/journal.pgen.1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blasco MA, Lee HW, Hande MP, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91(1):25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 27.Hemann MT, Strong MA, Hao LY, Greider CW. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107(1):67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 28.Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361(24):2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in dyskeratosis congenita. Blood. 2009;113(26):6549–6557. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calado RT, Regal JA, Hills M, et al. Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia. Proc Natl Acad Sci U S A. 2009;106(4):1187–1192. doi: 10.1073/pnas.0807057106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Risques RA, Vaughan TL, Li X, et al. Leukocyte telomere length predicts cancer risk in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2649–2655. doi: 10.1158/1055-9965.EPI-07-0624. [DOI] [PubMed] [Google Scholar]
- 32.Risques RA, Lai LA, Brentnall TA, et al. Ulcerative colitis is a disease of accelerated colon aging: evidence from telomere attrition and DNA damage. Gastroenterology. 2008;135(2):410–418. doi: 10.1053/j.gastro.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.