Abstract
Background
In severe acquired aplastic anemia, hematopoietic failure is the result of immune mediated destruction of bone marrow stem and progenitor cells. Immunosuppressive therapy with antithymocyte globulin (ATG) plus cyclosporine is an effective alternative to stem cell transplantation and improves blood counts and survival. While horse ATG is standard, rabbit ATG is more potent at depleting peripheral blood lymphocytes and is preferred in other clinical circumstances.
Methods
From December 2005 to July 2010, we performed a randomized trial comparing these two different ATG formulations at conventional regimens. Patients were treated at a single government facility. Primary outcome was hematologic response at 6 months, as determined by blood counts. The study was designed to accrue 60 patients per arm and powered to detect a 25% difference in response rate.
Results
There was a large, unexpected difference in hematologic responses at 6 months in favor of horse ATG (68%; 95% confidence interval (CI), 56%–80%) compared to rabbit ATG (37%; 95% CI, 24%–49%; p<0.001). Overall survival at 3 years also differed, with 96% (95% CI, 90%–100%) surviving in the horse ATG group compared to 76% (95% CI, 61%–95%; p=0.04) in the rabbit ATG group when stem cell transplantation was censored, and 94% (95% CI, 88%–100%) for horse ATG and 70% (95% CI, 56%–86%; p=0.008) for rabbit ATG when stem cell transplantation events were not censored.
Conclusions
In a randomized study, rabbit ATG was markedly inferior to horse ATG as first treatment in severe aplastic anemia as measured by hematologic response and survival.
Introduction
Acquired aplastic anemia in its severe form is fatal without treatment. The disease is characterized pathologically by an “empty” bone marrow, in which hematopoietic precursor cells are replaced by fat, resulting in pancytopenia.1 Severe aplastic anemia was first definitively treated with the development of stem cell transplantation in the 1970s. The serendipitous observation of autologous marrow reconstitution in a few patients who rejected their donor grafts suggested that the conditioning agents required for transplant might themselves be therapeutic.2 Purposeful immunosuppression based on infusion of polyclonal antibodies generated in animals by inoculation with human thymocytes, or antithymocyte globulin (ATG), proved to be effective, producing approximately long-term survival equivalent to the results of stem cell transplantation from a histocompatible sibling.3, 4 An immune mechanism of hematopoietic cell destruction was inferred from the success of ATG, and subsequent research in the laboratory and in animal models confirmed that progenitor and stem cells were targeted by immune effector cells and cytokines.1 Consistent with this pathophysiology, cyclosporine added to ATG improved the response rate and survival compared to ATG alone.5
In the 1980s and 1990s, most formal studies of efficacy in severe aplastic anemia conducted in Europe, Japan and the United States, utilized horse ATG with hematologic responses observed in about two-thirds of cases.6–9 From 1999, an ATG made in rabbits (Thymoglobulin®) has been available in the United States after its approval for the treatment of acute renal allograft rejection. In our experience, horse ATG yielded hematologic responses of 60–65% in severe aplastic anemia as first therapy10–12, and we wished to improve this rate. We hypothesized that rabbit ATG, as compared to horse ATG, would produce a higher response rate as first treatment, for several reasons. First, in direct comparison trials, rabbit ATG was superior to horse ATG in preventing and reversing acute renal allograft rejection.13, 14 Second, in severe aplastic anemia, rabbit ATG has been effective in salvaging patients who are refractory or relapse after initial therapy with horse ATG.15, 16 Third, in comparison to horse ATG, rabbit ATG more efficiently depletes lymphocytes in vivo and is more cytotoxic on a weight basis in vitro.17 Fourth, and possibly relevant to its mechanism of activity, rabbit ATG but not horse ATG induces regulatory T cell development from normal T cells in tissue culture, which should be beneficial in suppressing a harmful immune mediated or autoimmune pathophysiology.18, 19 Based on these observations, rabbit ATG has been administered as first therapy in severe aplastic anemia with the premise that it be a comparable (or superior) regimen. However, the effectiveness of rabbit ATG in this setting has not been prospectively studied. Here we report a randomized trial directly comparing horse ATG to rabbit ATG in treatment-naïve severe aplastic anemia.
Methods
Study patients
Consecutive patients older than two years of age with severe aplastic anemia were enrolled from December 2005 to July 2010 at the Mark O. Hatfield Clinical Research Center of the National Institutes of Health in Bethesda, Maryland (registered at www.clinicaltrials.gov as NCT00260689). Patients (or legal guardians) signed informed consent according to a protocol approved by the Institutional Review Board of the National Heart, Lung and Blood Institute. The study was monitored by an external Data Safety and Monitoring Board (for details, see Methods section in Supplementary Appendix).
Study design
This original study design is shown as Figure 1 in Supplementary Appendix. Assignment of treatment was done using a 1:1 block randomization scheme with the assignment probability remaining fixed over the course of the trial; construction of the randomization schedule was based on a table of random numbers and conducted by the Pharmacy Department at the Clinical Center.
The protocol’s primary endpoint was hematologic response at 6 months, defined as no longer meeting criteria for severe aplastic anemia; this endpoint strongly correlates with transfusion-independence and long-term survival.6, 12 Secondary endpoints included robustness of hematologic recovery, relapse, response rate at three months and yearly, clonal evolution to myelodysplasia and overall survival. Relapse was defined as any requirement for further immunosuppression (cyclosporine or another course of ATG) for decreased blood counts.12 Clonal evolution was defined as a new clonal cytogenetic abnormality or characteristic dysplastic changes in the bone marrow.
Immunosuppressive regimens
Horse ATG (ATGAM®; Pfizer) was administered at 40 mg/kg/day for 4 days and rabbit ATG (Thymoglobulin®; Genzyme) at 3.5 mg/kg/day for 5 days as previously described.12, 16 Cyclosporine at 10 mg/kg/day (15 mg/kg/day for children under 12) in divided doses every 12 hours was administered from day one and continued for at least 6 months in both arms, with dosing adjusted to maintain drug trough levels of 200–400 ng/ml (for details, see Methods section in Supplementary Appendix).
Statistical methods
Sample size was calculated based on the 6 month response rate (primary endpoint) of 60% for standard horse ATG plus cyclosporine (control arm).12 Based on a group sequential trial design with a two-sided test at 5% significance level, 80% power and one interim analysis (when half of the accrual per arm were evaluable for the primary endpoint), 60 patients per arm were required to detect a 25% difference between groups for the 6-month response rate (for details, see Methods section in Supplementary Appendix).
Results
The study completed accrual after 120 consecutive patients, ages 2–77 years, were randomized between horse and rabbit ATG (60 in each arm; Figure 2 in Supplementary Appendix). Patient characteristics are shown in Table 1; there were no significant differences in demographic and clinical features between the groups. Serious adverse events are summarized in Table 1 in Supplementary Appendix; as expected in this population, the most prevalent complications were infectious. Two patients in the horse ATG arm and nine in the rabbit ATG arm were not evaluable at 6 months due to death or progressive disease (Figure 2 in Supplementary Appendix). Median follow-up for all patients was 839 days (range, 2–1852) and for surviving patients 891 days (range, 185–1852).
Table 1.
Patient characteristics
| Characteristic | Horse ATG (n=60) | Rabbit ATG (n=60) | P-value |
|---|---|---|---|
| Age, yrs | 37.4 ± 2.7 | 31.2 ± 2.6 | 0.09 |
| Age <18 yrs, number (%) | 12 (20) | 18 (30) | 0.20 |
| Male sex, number (%) | 34 (57) | 37 (62) | 0.58 |
| Etiology, number (%) | |||
| Idiopathic | 58 (97) | 55 (92) | 0.24 |
| Post-hepatitis | 2 (3) | 5 (8) | 0.24 |
| Blood counts (per μL) | |||
| ARC | 22,100 ± 2,584 | 18,072 ± 2,283 | 0.24 |
| ALC | 1,291 ± 71 | 1,220 ± 79 | 0.50 |
| ANC | 408 ± 50 | 356 ± 46 | 0.44 |
| ANC <200, number (%) | 23 (38) | 26 (43) | 0.58 |
| Platelets | 16,317 ± 4,689 | 12,650 ± 1,138 | 0.45 |
| PNH clone < 1% | 35 (58) | 41 (68) | 0.25 |
| PNH clone ≥ 1% | 25 (42) | 19 (32) | 0.25 |
Plus-minus values are mean ± SD.
ARC, absolute reticulocyte count; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; ATG, anti-thymocyte globulin; PNH, paroxysmal nocturnal hemoglobinuria.
Hematologic response and relapse
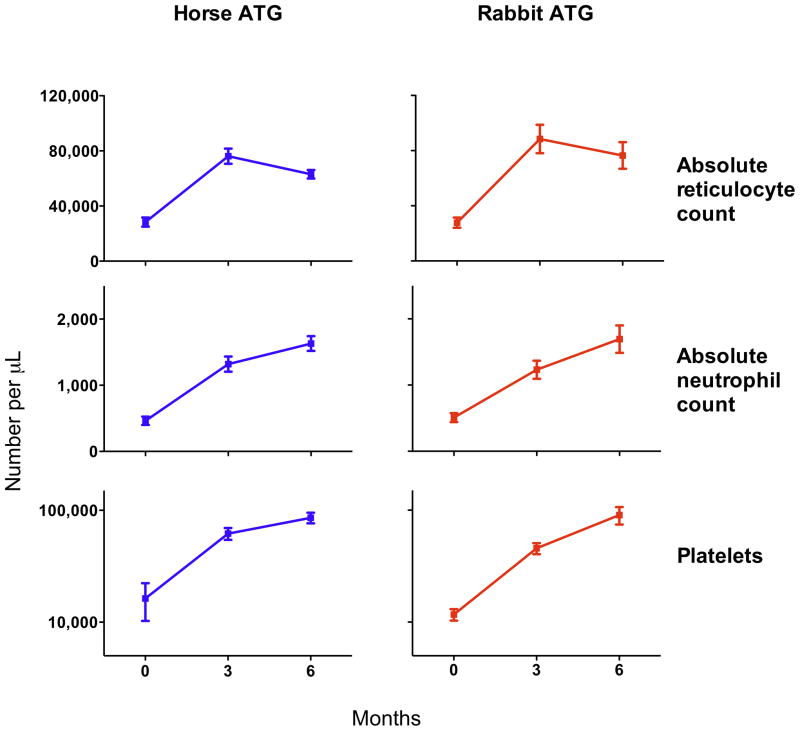
The hematologic response rate at 6 months for horse ATG was 68% (95% CI, 56%–80%) and for rabbit ATG 37% (95% CI, 24%–49%; p<0.001; Table 2). The response rate at 3 and 6 months to horse ATG plus cyclosporine observed in this study is in accord with our large historical experience with this regimen at our institution.6, 10–12 The majority of patients responded by 3 months, and only 4 patients in the horse ATG and 2 in the rabbit ATG arm recovered between 3 and 6 months. For responders, increments in blood counts were comparable between groups (Figure 1). When only evaluable patients at 6 months were analyzed, the response rate for horse ATG was 71% (95% CI, 59%–83%) compared to 43% (95% CI, 29%–57%; p=0.003) for rabbit ATG. The disposition among non-responders in each arm is shown as Figure 3 in Supplementary Appendix.
Table 2.
Hematologic response at 3 and 6 months to horse and rabbit ATG
| Response | Horse ATG | 95% CI | Rabbit ATG | 95% CI | P-value |
|---|---|---|---|---|---|
| 3 months | 37/60 (62%) | 49, 74 | 20/60 (33%) | 21, 46 | 0.002 |
| 6 months | 41/60 (68%) | 56, 80 | 22/60 (37%) | 24, 49 | < 0.001 |
Figure 1. Increase in blood counts in patients with hematologic improvement after ATG.
Among responders to immunosuppression (n=41 for horse ATG and n=22 for rabbit ATG), there were comparable increments in blood counts at 3 and 6 months between the two groups. The mean ± standard error of mean is shown.
To date, the cumulative incidence of relapse at 3 years did not appear to differ significantly between the two arms: 28% (95% CI, 9%–43%) for horse ATG and 11% (95% CI, 0%–25%; p=0.35) for rabbit ATG (the relatively small number of responders, especially in the rabbit ATG group, resulted in wide confidence intervals and loss of statistical power; Figure 4A in Supplementary Appendix). All 9 patients in the horse ATG and the 2 in the rabbit ATG arm who relapsed received more immunosuppression.
Clonal evolution and survival
To date, the cumulative incidences of clonal evolution at 3 years (in all patients, responders and non-responders) in the horse ATG arm was 21% (95% CI, 7%–33%) and in the rabbit ATG arm 14% (95% CI, 1%–25%; p=0.69; Figure 4B in Supplementary Appendix). Among patients treated with horse ATG, evolutions were deletion 3 (1), deletion 5q (1), deletion 13q (1), deletion 20q (1), leukemia (1) and monosomy 7 was observed in 4 patients (in two patients monosomy 7 was preceded by t(12;13) and deletion 13q). In the rabbit arm, 5 evolutions events were monosomy 7 and one deletion 13q.
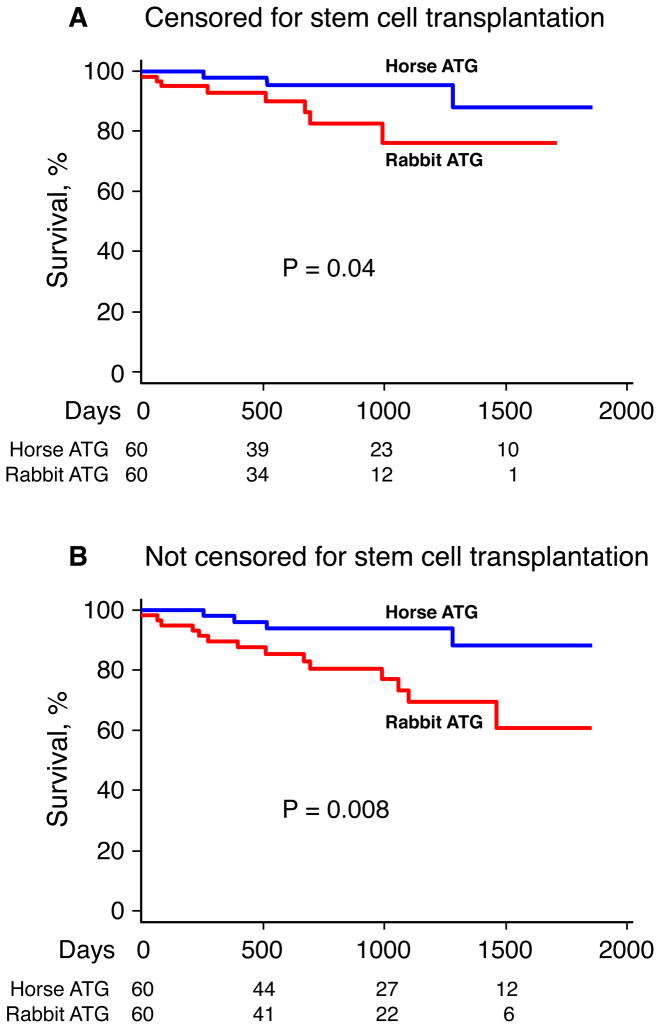
Overall survival between the two regimens differed: 96% (95% CI, 90%–100%) survived 3 years in the horse ATG group compared to 76% (95% CI, 61%–95%; p=0.04) in the rabbit ATG group, when stem cell transplantation was censored (Figure 2A), and 94% (95% CI, 88%–100%) in the horse ATG group and 70% (95% CI, 56%–86%; p=0.008) in the rabbit ATG group when time to stem cell transplantation was not censored (Figure 2B). Of the 4 deaths in the horse ATG arm, one resulted from intracranial hemorrhage, one occurred after stem cell transplantation (from a sibling donor), one from sepsis, and one from lung cancer. Of the 14 deaths in the rabbit ATG arm, 2 resulted from intracranial hemorrhage, 3 from infection (1 pneumonia, 1 septicemia, 1 necrotizing fasciitis), 6 occurred after stem cell transplantation (3 from a sibling and 3 from an unrelated donor), 1 patient died in a traffic accident, and in 2 mortality was from unknown causes.
Figure 2. Kaplan-Meier curves of overall survival.
Patients were censored at time of stem cell transplantation in Panel A while stem cell transplantation events were ignored in Panel B. Numbers at the bottom of the graph indicate patients at risk for each time point.
In vivo alterations in lymphocyte number and subpopulations
As polyclonal sera, ATGs contain antibodies that recognize a variety of different antigens present on cell surface membranes, and they are cytotoxic to lymphocytes in vitro. Rapid lymphodepletion occurred in both regimens but lymphopenia was more protracted following rabbit ATG, consistent with previous reports (Figure 3A).17 There were strikingly lower levels of CD4+ T cells for virtually the entire 6 months prior to the primary endpoint in patients treated with rabbit ATG, compared to patients who received horse ATG (Figure 3C, D). Numbers of regulatory T cells (defined as CD4+CD25+CD127− for this analysis)20 were much lower in the weeks following rabbit ATG, as would be anticipated by the markedly lower CD4+ T cells in this group (Figures 3E, F). There was less dramatic difference in the kinetics of CD8+ T cell depletion and reconstitution (Figure 3G, H).
Figure 3. Lymphodepletion after ATG administration.
(A) The initial decrease in total absolute lymphocyte count was similar between the two ATGs, but lymphocyte counts remained lower longer after rabbit ATG. (B) T cells (CD3+CD45+) decreased rapidly with both ATGs with reconstitution in subsequent weeks. (C, D) There was a large difference between the kinetics of CD4+ T cell depletion after horse and rabbit ATG, with a much lower frequency and absolute numbers after rabbit ATG. (E, F) The frequency of regulatory T cells (Tregs; defined as CD4+CD25+CD127− for this analysis) was higher after rabbit ATG, but absolute numbers were markedly lower due to more potent depletion of CD4+ T cells, as compared to horse ATG. (G, H) The difference in depletion kinetics of CD8+ T cells was less striking between the two ATGs when compared to CD4+ T cells. The mean ± standard error of mean is shown. All 120 patients are depicted in panel A. Fourteen patients are depicted in panels B–H (7 from each ATG group). Differences for each time point that are statistically significant (p<0.05) are denoted by an asterisk (paired t-test). For details on flow cytometric analysis, see Methods section in Supplementary Appendix.
Discussion
Immunosuppression with ATG and cyclosporine is often administered as first therapy in severe aplastic anemia, since most patients lack a histocompatible sibling donor or are not suitable candidates for stem cell transplantation due to age, comorbidities, or lack of access to this treatment modality. Most published experience is with the horse formulation of the polyclonal antibody. In the past decade, rabbit ATG plus cyclosporine has gained in popularity due to its activity in relapsed and refractory severe aplastic anemia. In some centers in the US, rabbit ATG has been used as first therapy, and in Europe, Japan and Latin America, rabbit ATG is the only formulation currently available.
The reported experience with rabbit ATG (Thymoglobulin®) plus cyclosporine as initial therapy in severe aplastic anemia is limited to retrospective studies with conflicting results. In a small phase II study in the US of 13 patients with severe aplastic anemia, response to rabbit ATG was observed in 12 (92%) at about 3 months after therapy.21 In contrast, a retrospective analysis conducted in Brazil of 71 patients found a higher response rate at 6 months in those who had received horse ATG (60%) compared to rabbit ATG (35%), with a survival benefit noted for the former. In addition, rabbit ATG was an independent predictor of mortality in multivariate analysis in this study.22 In another recent and also retrospective report from Europe, there was no difference in overall response between horse (49%) and rabbit ATG (45%) when administered as first line therapy; 23 but the response rate to horse ATG of 49% was markedly lower than reported response rates of 60–70% with this regimen in large prospective studies in the US, Europe, and Japan.1
In the current work, in which a randomized prospective protocol with good matching of patients in randomization arms was executed to completion, rabbit ATG plus cyclosporine was inferior to horse ATG plus cyclosporine when administered as first line treatment. The hematologic response rate for rabbit ATG was about half that of horse ATG, which translated into about a 25% worse survival at 3 years. Despite a relative short period of follow-up, the rate of relapse and clonal evolution do not appear to differ between the 2 arms.
These results were unanticipated, given the success of rabbit ATG in treating relapsed and refractory severe aplastic anemia and the superiority of this regimen in protecting kidney allografts.13–16 The introduction of this regimen as first line in severe aplastic anemia was logical due to its greater immunosuppressive properties17, and a higher response rate and improved survival anticipated. Our study was originally designed to test this hypothesis and powered to detect a 25% difference between 2 groups. Due to the large clinical difference between the 2 arms, the response rate and survival crossed statistical boundaries of significance at the conclusion of the study with confidence intervals between groups that did not overlap for hematologic response and survival.
Our data raise questions as to the mechanism by which hematopoiesis is restored following ATG administration in severe aplastic anemia. Despite undergoing apparently similar manufacturing processes, there are marked differences in vitro and in vivo between the horse and rabbit preparations of ATG. In human peripheral blood mononuclear cells co-cultured with different ATGs, expansion of regulatory T cells was observed with rabbit ATG but not with horse ATG.18 Furthermore, there was a marked difference in gene expression profile in human cells cultured with either horse or rabbit ATG.18 In humans, more prolonged lymphopenia follows rabbit ATG administration, and patterns of viral reactivations have been shown to differ between these two agents.17
Lot-to-lot variability among ATGs is unlikely to explain the observed large differences in outcomes. First, laboratory testing has not disclosed significant or consistent dissimilarity in cytotoxicity or antigen binding specificities among multiple horse and rabbit ATG lots24, 25 nor among different commercially available ATGs.26 Second, in our clinical experience over several decades, the response rate to horse ATG in sequential protocols for treatment-naïve aplastic anemia has been nearly identical, at an average of 62% (including the current study).10–12 Responses to rabbit ATG in refractory severe aplastic anemia have appeared stable in separate studies conducted over 10 years at our institution, at about 33%.16, 27 Third, as many more animals contribute to the preparation of rabbit ATG, less variability would be expected with this formulation. Fourth, the kinetics of lymphocyte depletion with either agent was consistent in patients. Finally, secular trends in the response observed in the current study remained steady, nor were there significant differences in response rates among patients treated with different lots of ATG in this study (data not shown).
Other, more plausible explanations likely account for our results. Both ATGs led to similar depletion of CD8+ cytotoxic T cells, but there was more profound CD4+ T cell depletion after rabbit ATG (Figure 3). One possible inference is that CD8+ T cell depletion is linked to success of horse and rabbit ATG, as expected from the pathophysiology of aplastic anemia, but that loss of CD4+ T cells after rabbit ATG may be detrimental. The CD4 cell compartment is phenotypically and functionally heterogeneous. Contained within the large CD4 cell population are regulatory T cells, which regulate immune response. In the current study, the frequency of regulatory T cells was higher after rabbit ATG (as predicted from tissue culture experiments)18, 19, but this effect was negated by the more potent deletion of CD4+ T cells compared to horse ATG (Figure 3E, F). CD4 cells have other positive effects on hematopoiesis and they may be important for hematologic recovery as well as in promoting tolerance in this setting (as after stem cell transplantation).28 In addition, horse serum might contribute to recovery of hematopoiesis by stimulatory effects in the bone marrow.29, 30 More prolonged lymphopenia after rabbit ATG might impair marrow recovery as stimulatory cytokines derived from T-cells are depleted.31
It is unclear if further intensification of immunosuppression will yield superior outcomes in severe aplastic anemia.32 The addition of mycophenolate mofetil10 and sirolimus11 added to horse ATG plus cyclosporine has not achieved this goal, and now the addition of more potent lymphocytotoxic agents (rabbit ATG and alemtuzumab) in place of horse ATG has yielded inferior results. Nevertheless, horse ATG plus cyclosporine should remain the immunosuppressive regimen of choice in severe aplastic anemia as first line therapy. As horse ATG is not available in Europe and Japan, these data have implications for the treatment of aplastic anemia worldwide, as well as to the mechanism of action of polyclonal antisera in general and in particular in this disease.
Supplementary Material
Acknowledgments
We are grateful to Hematology Branch attending physicians for their attentive care of patients: A. John Barrett, Cynthia Dunbar, Richard Childs, Adrian Wiestner, Georg Aue, John Tisdale, Matthew Hsieh, among others. We thank Drs. J. Philip McCoy Jr. and Xingming Feng for help in discussion of the flow cytometric data. This research was supported by the Intramural Research Program of the National Institutes of Health, National Heart, Lung and Blood Institute.
Footnotes
Potential Conflict of Interest
The authors have no conflicts to disclose.
References
- 1.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509–19. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathe G, Amiel JL, Schwarzenberg L, et al. Bone marrow graft in man after conditioning by antilymphocytic serum. Br Med J. 1970;2:131–6. doi: 10.1136/bmj.2.5702.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speck B, Gratwohl A, Nissen C, et al. Treatment of severe aplastic anaemia with antilymphocyte globulin or bone-marrow transplantation. Br Med J (Clin Res Ed) 1981;282:860–3. doi: 10.1136/bmj.282.6267.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation (EBMT) Haematologica. 2007;92:11–8. doi: 10.3324/haematol.10075. [DOI] [PubMed] [Google Scholar]
- 5.Frickhofen N, Kaltwasser JP, Schrezenmeier H, et al. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. The German Aplastic Anemia Study Group. N Engl J Med. 1991;324:1297–304. doi: 10.1056/NEJM199105093241901. [DOI] [PubMed] [Google Scholar]
- 6.Rosenfeld SJ, Kimball J, Vining D, Young NS. Intensive immunosuppression with antithymocyte globulin and cyclosporine as treatment for severe acquired aplastic anemia. Blood. 1995;85:3058–65. [PubMed] [Google Scholar]
- 7.Bacigalupo A, Bruno B, Saracco P, et al. Antilymphocyte globulin, cyclosporine, prednisolone, and granulocyte colony-stimulating factor for severe aplastic anemia: an update of the GITMO/EBMT study on 100 patients. European Group for Blood and Marrow Transplantation (EBMT) Working Party on Severe Aplastic Anemia and the Gruppo Italiano Trapianti di Midolio Osseo (GITMO) Blood. 2000;95:1931–4. [PubMed] [Google Scholar]
- 8.Kojima S, Hibi S, Kosaka Y, et al. Immunosuppressive therapy using antithymocyte globulin, cyclosporine, and danazol with or without human granulocyte colony-stimulating factor in children with acquired aplastic anemia. Blood. 2000;96:2049–54. [PubMed] [Google Scholar]
- 9.Fuhrer M, Burdach S, Ebell W, et al. Relapse and clonal disease in children with aplastic anemia (AA) after immunosuppressive therapy (IST): the SAA 94 experience. German/Austrian Pediatric Aplastic Anemia Working Group. Klin Padiatr. 1998;210:173–9. doi: 10.1055/s-2008-1043875. [DOI] [PubMed] [Google Scholar]
- 10.Scheinberg P, Nunez O, Wu C, Young NS. Treatment of severe aplastic anaemia with combined immunosuppression: anti-thymocyte globulin, ciclosporin and mycophenolate mofetil. Br J Haematol. 2006;133:606–11. doi: 10.1111/j.1365-2141.2006.06085.x. [DOI] [PubMed] [Google Scholar]
- 11.Scheinberg P, Wu CO, Nunez O, Boss C, Sloand EM, Young NS. Treatment of severe aplastic anemia with a combination of horse antithymocyte globulin and cyclosporine, with or without sirolimus: a prospective randomized study. Haematologica. 2009;94:348–54. doi: 10.3324/haematol.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130–5. doi: 10.1001/jama.289.9.1130. [DOI] [PubMed] [Google Scholar]
- 13.Brennan DC, Flavin K, Lowell JA, et al. A randomized, double-blinded comparison of Thymoglobulin versus Atgam for induction immunosuppressive therapy in adult renal transplant recipients. Transplantation. 1999;67:1011–8. doi: 10.1097/00007890-199904150-00013. [DOI] [PubMed] [Google Scholar]
- 14.Gaber AO, First MR, Tesi RJ, et al. Results of the double-blind, randomized, multicenter, phase III clinical trial of Thymoglobulin versus Atgam in the treatment of acute graft rejection episodes after renal transplantation. Transplantation. 1998;66:29–37. doi: 10.1097/00007890-199807150-00005. [DOI] [PubMed] [Google Scholar]
- 15.Di Bona E, Rodeghiero F, Bruno B, et al. Rabbit antithymocyte globulin (r-ATG) plus cyclosporine and granulocyte colony stimulating factor is an effective treatment for aplastic anaemia patients unresponsive to a first course of intensive immunosuppressive therapy. Gruppo Italiano Trapianto di Midollo Osseo (GITMO) Br J Haematol. 1999;107:330–4. doi: 10.1046/j.1365-2141.1999.01693.x. [DOI] [PubMed] [Google Scholar]
- 16.Scheinberg P, Nunez O, Young NS. Retreatment with rabbit anti-thymocyte globulin and ciclosporin for patients with relapsed or refractory severe aplastic anaemia. Br J Haematol. 2006;133:622–7. doi: 10.1111/j.1365-2141.2006.06098.x. [DOI] [PubMed] [Google Scholar]
- 17.Scheinberg P, Fischer SH, Li L, et al. Distinct EBV and CMV reactivation patterns following antibody-based immunosuppressive regimens in patients with severe aplastic anemia. Blood. 2007;109:3219–24. doi: 10.1182/blood-2006-09-045625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng X, Kajigaya S, Solomou EE, et al. Rabbit ATG but not horse ATG promotes expansion of functional CD4+CD25highFOXP3+ regulatory T cells in vitro. Blood. 2008;111:3675–83. doi: 10.1182/blood-2008-01-130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez M, Clarkson MR, Albin M, Sayegh MH, Najafian N. A novel mechanism of action for anti-thymocyte globulin: induction of CD4+CD25+Foxp3+ regulatory T cells. J Am Soc Nephrol. 2006;17:2844–53. doi: 10.1681/ASN.2006050422. [DOI] [PubMed] [Google Scholar]
- 20.Banham AH. Cell-surface IL-7 receptor expression facilitates the purification of FOXP3(+) regulatory T cells. Trends Immunol. 2006;27:541–4. doi: 10.1016/j.it.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Garg R, Faderl S, Garcia-Manero G, et al. Phase II study of rabbit anti-thymocyte globulin, cyclosporine and granulocyte colony-stimulating factor in patients with aplastic anemia and myelodysplastic syndrome. Leukemia. 2009;23:1297–302. doi: 10.1038/leu.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atta E, Dias D, Marra V, de Azevedo A. Comparison between horse and rabbit antithymocyte globulin as first-line treatment for patients with severe aplastic anemia: a single-center retrospective study. Annals of Hematology. 89:851–9. doi: 10.1007/s00277-010-0944-y. [DOI] [PubMed] [Google Scholar]
- 23.Vallejo C, Montesinos P, Rosell A, et al. Comparison Between Lymphoglobuline-and Thymoglobuline-Based Immunosuppressive Therapy as First-Line Treatment for Patients with Aplastic Anemia. ASH Annual Meeting Abstracts. 2009;114:3194. [Google Scholar]
- 24.Raefsky EL, Gascon P, Gratwohl A, Speck B, Young NS. Biological and immunological characterization of ATG and ALG. Blood. 1986;68:712–9. [PubMed] [Google Scholar]
- 25.LaCorcia G, Swistak M, Lawendowski C, et al. Polyclonal rabbit antithymocyte globulin exhibits consistent immunosuppressive capabilities beyond cell depletion. Transplantation. 2009;87:966–74. doi: 10.1097/TP.0b013e31819c84b8. [DOI] [PubMed] [Google Scholar]
- 26.Ayuk F, Maywald N, Hannemann S, Larsen U, Zander A, Kroger N. Comparison of the cytotoxicity of 4 preparations of anti-T-cell globulins in various hematological malignancies. Anticancer Res. 2009;29:1355–60. [PubMed] [Google Scholar]
- 27.Scheinberg P, Wu CO, Scheinberg P, Nunez O, Sloand EM, Young NS. Alemtuzumab (Campath) Monotherapy for Severe Aplastic Anemia. ASH Annual Meeting Abstracts. 2010;116:1167. [Google Scholar]
- 28.Hanash AM, Levy RB. Donor CD4+CD25+ T cells promote engraftment and tolerance following MHC-mismatched hematopoietic cell transplantation. Blood. 2005;105:1828–36. doi: 10.1182/blood-2004-08-3213. [DOI] [PubMed] [Google Scholar]
- 29.Kawano Y, Nissen C, Gratwohl A, Wursch A, Speck B. Cytotoxic and stimulatory effects of antilymphocyte globulin (ALG) on hematopoiesis. Blut. 1990;60:297–300. doi: 10.1007/BF01736232. [DOI] [PubMed] [Google Scholar]
- 30.Barbano G, Schenone A, Roncella S, et al. Anti-lymphocyte globulin stimulates normal human T cells to proliferate and to release lymphokines in vitro. A study at the clonal level. Blood. 1988;72:956–63. [PubMed] [Google Scholar]
- 31.Naparstek E, Delukina M, Or R, et al. Engraftment of marrow allografts treated with Campath-1 monoclonal antibodies. Experimental Hematology. 1999;27:1210–8. doi: 10.1016/s0301-472x(99)00052-1. [DOI] [PubMed] [Google Scholar]
- 32.Passweg JR, Tichelli A. Immunosuppressive treatment for aplastic anemia: are we hitting the ceiling? Haematologica. 2009;94:310–2. doi: 10.3324/haematol.2008.002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.