Abstract
Introduction
Adenoid cystic carcinoma is an uncommon type of breast cancer. There are limited data about its epidemiology, tumor characteristics, and outcomes. Using a large, population-based database, this study aims to identify specific characteristics of patients with adenoid cystic breast cancer, investigate its natural history, and determine its long-term prognosis.
Methods
The California Cancer Registry (CCR), a population-based registry, was reviewed from the years 1988 to 2006. The data were analyzed with relation to patient age, tumor size and stage, and overall survival. Relative cumulative actuarial survival was determined using the Berkson-Gage life table method.
Results
A total of 244 cases of invasive adenoid cystic cancer were identified in women during this time period. The patients’ median age was 61.9 years. Most cases were diagnosed in non-Hispanic White women (82%, n=200), followed by African-American (6%, n=15), Asian/Pacific-Islander (5.7%, n=14) and Hispanic women (4.4%, n=12). The remainder of the patients was of unknown or other ethnicity. Tumors were between 1 and 140 mm in size. At the time of diagnosis, 92% (n=225) of patients had localized disease, 5% (n=12) of patients had regional disease, and even fewer (n=7) had either distant or unknown staged disease. Lymph node involvement was not present in any tumors smaller than 1.4 cm. The relative cumulative survival of patients with adenoid cystic breast carcinoma was 95.6% at 5 years and 94.9% at 10 years.
Conclusion
Adenoid cystic carcinoma of the breast is a rare disease with an overall good prognosis. Knowing that this cancer usually presents as localized disease, with lymph node involvement seen only with larger tumors, can help clinicians plan the operative management of these tumors.
Keywords: Adenoid Cystic Breast Carcinoma, Incidence, Prognosis, Survival, Treatment
Introduction
Adenoid cystic carcinoma (ACC) of the breast is a rare entity accounting for 0.1% of all breast cancers 1, 2. ACC has been described in women between 30 and 90 years of age, but is most common in the fifth and sixth decades of life. Patients typically present with a subareolar mass or breast pain. Patients with ACC have a better prognosis compared to other breast malignancies. Metastasis to non-nodal sites at any point in the disease process is rare, but will occur at times without evidence of prior lymph node involvement 1, 3. Treatment with mastectomy or breast conservation therapy is typically followed by prolonged survival 4. At this time, we have minimal data regarding the need for axillary staging. In contrast to extramammary ACC, those arising in the breast have an excellent prognosis.
Histologically ACC of the breast is characterized by small basaloid cells with a solid cribiform pattern or tubular growth patterns enclosing pseudoglandular spaces with minimal eosinophilic material 5. ACC has been seen in multiple areas of the body including the breast, vulva, skin, salivary glands, lacrimal glands, and the upper respiratory tract 6. The cell of origin is unknown, but many feel it may arise from the ductal epithelium and myoepithelium. This malignancy also has biphasic cellularity with myoepitheial cells mixed with other cell types. These tumors tend to be estrogen receptor (ER) and progesterone receptor (PR) negative 7.
Materials and Methods
Data from the California Cancer Registry (CCR) was evaluated from the years 1988 to 2005. The CCR is a population-based database established in the late 1940s to report a 10% sample of the incident cancer cases in the State. Mandatory reporting of all incident cancers (excluding squamous cell and basal cell skin cancers, and later, cervical CIS, and “intermediate” ovarian cystadenocarcinomas) began in 1988. The database contains information about the type of cancer (histology), patient demographics, disease stage at diagnosis and survival. For breast cancers, stage is recorded within the CCR (as in other total-population based registries in the US and Canada) as local disease, regional (with lymph node involvement and/or direct extension into adjacent structures), or distant disease. There is no distinction between lymph node involvement and direct extension into adjacent structures in the CCR. A limitation in reporting from the CCR is we are required by CCR policies (and thus, by the laws of California) not to report the actual numbers in “cells” (groups) of five or less to avoid any possibility of identification of a patient.
The Berkson-Gage life table method was used to calculate relative cumulative survival of patients diagnosed with ACC of the breast. Relative survival rates compare the mortality of a group of patients to that of the general population of the same age, sex, and ethnic distribution. Tumor characteristics, patient demographics, and survival rates of ACC of the breast were compared to those of all types of breast carcinoma other than ACC.
Results
Among all breast cancers for 1988-2006 in the CCR, adenoid cystic carcinoma accounted for 0.058%. ACC of the breast accounted for for 11.5% of all sites of orgin of ACC and was the third most common after salivary gland and head and neck cancers. Women (N=244) who were identified as having invasive ACC of the breast between 1988 and 2005 were included in this analysis. The median age at diagnosis was 61.9 years old with a range from 30 to 99 years and a standard error of 0.849. The majority of the patients were non-Hispanic White women (82%, n=200) followed by African-American women (6%, n=15), Asian/Pacific-Islanders (5.7%, n=14) and Hispanic women (4.4%, n=12), the remainder of the patients were of unknown ethnicity (Table 1). In addition to these 244 women, there were 5 cases of adenoid cystic breast carcinoma-in-situ, all in women, and fewer than 5 cases of invasive adenoid cystic carcinoma in men. We could show no statistically significant predilection toward ACC being located in the nipple, areola, or centrally in the breast compared to all other types of breast cancer.
Table 1.
Patient Demographics: Breakdown of ethnicity of adenoid cystic carcinoma of the breast and in the California breast cancer population as a whole.
| Ethnicity | Number of cases | Percent of cases | % of all breast cancer (CCR) in women ≥ 50 years of age |
|---|---|---|---|
| Non-Hispanic White | 200 | 82.0 | 78.1 |
| African-American | 15 | 6.1 | 5.1 |
| Asian/Pacific Islander | 14 | 5.7 | 6.0 |
| Hispanic | 12 | 4.4 | 10.0 |
| Other | < 5 | < 2 | 0.9 |
Chi square = 7.81, 4 df, p = 0.09
At the time of diagnosis, 92.2% of the patients (n=225) had localized disease. Regional disease was present in 4.9% (n=12). Seven patients presented with distant metastasis or unspecified stage (Table 2).
Table 2.
Stage. Breakdown of the number of cases of adenoid cystic carcinoma of the breast by stage.
| N (Number of cases) |
% (Percentage of cases) |
|
|---|---|---|
| Localized | 225 | 92.2 |
| Regional | 12 | 4.9 |
| Distant + Unspecified | 7 | 2.9 |
Tumors varied in size from 1 mm to 140 mm at the time of excision (Figure 1). The majority of the tumors were 16-20 mm in size. The eight cases with lymph node involvement had tumors ranging from 14 to 70 mm. Of the 38 cases of ACC that were 13 mm or smaller, none had lymph node metastasis. Of the 144 patients with ACC for whom both the nodal status and tumor size were known (59% of the total), eight patients had positive lymph nodes (as above). These patients had a mean tumor size of 36.6 mm (range 14 to 70 mm). For the 136 patients who had negative lymph nodes the mean tumor size was 22.0 mm (range 0.1 to 80.0 mm). Although this difference did not reach statistical significance (z = 1.86, p = 0.06), there is a trend toward smaller tumors being less likely to have lymph node metastasis; a larger sample size may confirm this.
Figure 1.
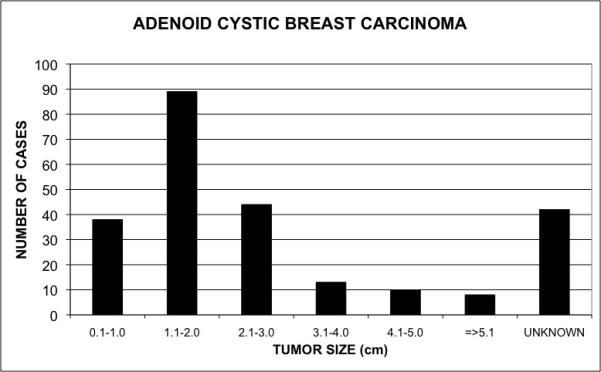
Distribution of adenoid cystic carcinoma of the breast by tumor size. This graph shows the number of cases of adenoid cystic carcinoma of the breast by tumor size (cm).
This is a drastic difference when the proportion of positive lymph nodes is compared to size of tumor in all other invasive breast cancers. In tumors of other types, those of < 20mm had a 23% rate of positive lymph nodes, 21-30mm had 47% positive, 31-40mm had 57% positive, and when a patient has a tumor greater than 40mm, 67% of those patients had positive lymph nodes at the time of resection.
The relative cumulative survival of all women with invasive ACC was 95.5% at five years and 93.5% at 10 years (Figure 2). Compared to other types of invasive breast carcinoma, ACC of the breast offers a relative cumulative survival benefit of approximately 20% at ten years (z = 3.34;P < 0.001).
Figure 2.
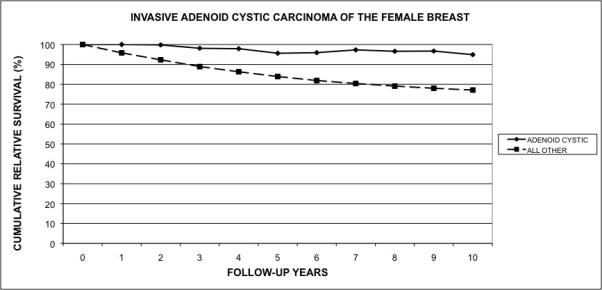
Cumulative relative survival of adenoid cystic carcinoma of the breast. The graph compares the cumulative relative survival of patients with adenoid cystic carcinoma of the breast when compared to the relative survival of patients with all other types of breast cancer.
As seen in Figure 3, the 10 year relative cumulative survival for patients with negative lymph nodes was 101.8% (se = 5.37), while that for patients with positive nodes was 32.7% (se = 19.55). In spite of the large standard errors, the difference in survival of the two groups is significant (z = 3.41, P < 0.001). Interestingly, the 10 year relative cumulative survival for patients whose nodal status is unknown is 97.8%, which is statistically no different from the group with negative nodes (z = 0.286, P = 1).
Figure 3.
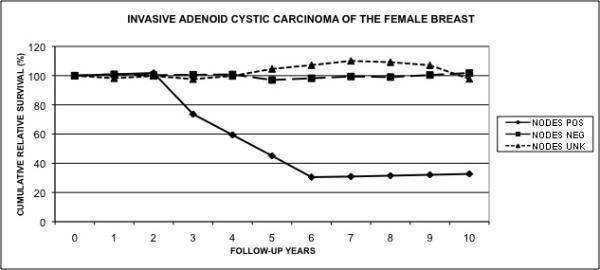
Cumulative relative survival of invasive adenoid cystic carcinoma of the female breast according to lymph node status. The graph compares the relative survival of patients with invasive adenoid cystic carcinoma of the breast according to their lymph node status.
The authors also conducted an analysis of the National Cancer Institute’s SEER data to determine if there were any differences from the CCR data. This analysis of the non-California cases of adenoid cystic carcinoma of the breast in SEER showed only minor, statistically non-significant differences from the results of the California data analysis8.
Discussion
Adenoid cystic carcinoma of the breast is rare and was first described as “cylindroma” by Billroth 9. It presents with a breast mass, typically subareolar, or breast pain. Although this malignancy has a predilection for a location near the areola, nipple discharge is a rare symptom. The pain that patients experience with adenoid cystic carcinoma of the salivary glands is due to perineural invasion. In ACC of the breast perineural invasion is rare, so it is uncertain why patients experience tenderness as their dominant symptom. Diagnosis typically occurs from symptomatic complaint as imaging tends to be non-specific. Mammography most commonly reveals a lobulated nodule. The masses tend to be ill defined with rare calcifications.
ACC has had various growth patterns described: glandular, tubular and solid. The characteristic histologic pattern that includes small basaloid cells with a solid cribiform pattern or tubular growth patterns enclosing pseudoglandular spaces with minimal eosinophilic material is noted on fine needle aspiration 5. Ro et al. 10 correlated histologic grade with survival in patients with ACC of the breast. The grade increases with the proportion of solid elements. Grade I (no solid element), Grade 2 (<30% solid elements) and Grade 3 (≥30% solid elements). They propose treatment based on grade: local excision for grade 1, simple mastectomy for grade 2, and mastectomy with axillary node dissection for grade 3 tumors.
At the time of diagnosis, metastasis to the lymph nodes is a rare finding 4. The majority of the patients have tumor confined to the breast. Our cohort of patients from the CCR has the same findings. Due to this, the treatment compared to other invasive breast carcinomas should vary. While breast conservation surgery with sentinel node biopsy is the standard operation for most invasive carcinomas found at an early stage, ACC poses a difficult decision. The indolent process of ACC may allow the surgeon to perform a surgical resection of the primary tumor without lymph node sampling. As the size of the tumor increased, the incidence of positive lymph nodes did as well. Thus, a surgeon may decide to perform a sentinel node biopsy or axillary lymph node dissection depending on original tumor size. With such a small incidence of positive lymph nodes in small tumors, the morbidity of axillary lymph node dissection may outweigh the benefits.
Axillary lymph node dissection is not without morbidity. Up to 60% of patients have the risk of lymphedema, bleeding, shoulder restriction, weakness, pain and permanent nerve damage 11, 12. Due to these risks, the decision to proceed with a lymph node sampling must be taken cautiously. There have been several randomized prospective trials comparing sentinel node biopsy to axillary dissection, and although the morbidity associated with sentinel node biopsy is lower than a full dissection, there are still risks associated with it. Both studies demonstrated that there is still around a five percent risk of lymphedema in patients who undergo a sentinel node biopsy 13-15. Therefore, surgeons must also consider this risk when recommending even a sentinel node biopsy in patients. In the case of a small ACC tumor, the risk of lymphedema may be higher than the chance of identifying a positive node.
A consensus for the treatment of ACC of the breast has not been reached partially because of the rarity of this malignancy. Case reports 1-4 have been published ranging from a simple local excision to radical mastectomy. The indolent course, rare axillary node involvement or metastasis, and favorable prognosis make ACC an ideal malignancy to optimize treatment 7. A prospective, randomized trial would be ideal for answering this question, but due to the rarity of the malignancy this would prove to be difficult.
It is doubtful that there is an “in-situ” stage for adenoid cystic carcinoma of the breast. Rosen, in his treatise on tumors of the breast, describes “in-situ” ACC as a rare condition seen only at the periphery of invasive ACC16. Other such texts do not even mention the possibility of ACC-in-situ17, 18. We do not know what the sections of the 5 alleged “CIS” actually showed, but they were so interpreted by the involved pathologist.
There are limitations to the California Cancer Registry data. The diagnoses are based on the evaluation from each institution’s pathologist. Furthermore, it would be exceedingly difficult and expensive to review individual cases by a central pathologist. However, this series remains the largest analysis of this rare tumor type and therefore, it is helpful for clinicians to gain knowledge on how to treat this rare tumor type.
Conclusions
In summary, the women with “unknown” ACC lymph node status have the same excellent ten-year relative cumulative survival as do the women with known negative lymph nodes. Thus, the findings from this analysis suggest that unless there are palpable lymph nodes or other reasons to suspect nodal involvement, routine axillary lymph node biopsy or excision may not be warranted especially in T1 tumors..
Acknowledgements
This study was supported by funding from the National Cancer Institute’s grants R25CA65745 and Cancer Center Core grant #5 P30CA023100-22, the National Institute’s of Health’s Divisional of National Center for Minority Health Disparities EXPORT grant P60MD00220; and the Minority Institution/Cancer Center Partnership Program grants #US56 CA92079/U56 CA93081 and U54CA132379/U54CA132384.
Footnotes
Disclaimer: The collection of cancer incidence data used in this study was supported by the California Department of Health Services as part of the statewide cancer reporting program mandated by the California Health and Safety Code Section 103885, the National Cancer Institute’s Surveillance, Epidemiology and End Results Program, and the Centers for Disease Control and Prevention Program of Cancer Registries. The ideas and opinions expressed herein are those of the authors and endorsement by the State of California, Department of Health Services, the National Cancer Institute and the Centers for Disease Control and Prevention is not intended nor should be inferred.
References
- 1.Youk JH, Kim MJ, Kim EK, et al. Recurrence of adenoid cystic carcinoma in the breast after lumpectomy and adjuvant therapy. J Ultrasound Med. 2006;25(7):921–4. doi: 10.7863/jum.2006.25.7.921. [DOI] [PubMed] [Google Scholar]
- 2.Kasagawa T, Suzuki M, Doki T, et al. Two cases of adenoid cystic carcinoma: preoperative cytological findings were useful in determining treatment strategy. Breast Cancer. 2006;13(1):112–6. doi: 10.2325/jbcs.13.112. [DOI] [PubMed] [Google Scholar]
- 3.Muslimani AA, Ahluwalia MS, Clark CT, et al. Primary adenoid cystic carcinoma of the breast: case report and review of the literature. Int Semin Surg Oncol. 2006;3:17. doi: 10.1186/1477-7800-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Millar BA, Kerba M, Youngson B, et al. The potential role of breast conservation surgery and adjuvant breast radiation for adenoid cystic carcinoma of the breast. Breast Cancer Res Treat. 2004;87(3):225–32. doi: 10.1007/s10549-004-8693-z. [DOI] [PubMed] [Google Scholar]
- 5.Torrao MM, da Costa JM, Ferreira E, et al. Adenoid cystic carcinoma of the breast. Breast J. 2007;13(2):206. doi: 10.1111/j.1524-4741.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- 6.Alis H, Yigitbas H, Kapan S, et al. Multifocal adenoid cystic carcinoma of the breast: an unusual presentation. Can J Surg. 2008;51(2):E36–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Law YM, Quek ST, Tan PH, et al. Adenoid cystic carcinoma of the breast. Singapore Med J. 2009;50(1):e8–11. [PubMed] [Google Scholar]
- 8.Surveillance, Epidemiology, and End Results (SEER) Program (sss.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 17 Regs Limited-Use + Hurricane Katrina Impacted Louisiana Cases, Nov 2007 Sub (1973 - 2005 varying) - Linked to County Attributes - Total U.S., 1969 - 2005 Counties. National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; [released April 2008]. based on the November 2007 submission. [Google Scholar]
- 9.Billroth T. Untersuchunugen ueber die Entwicklung der Blutgefasse. G Reimer; Berlin: 1856. Die Cylindergeschwalst; pp. 55–69. [Google Scholar]
- 10.Ro JY, Silva EG, Gallager HS. Adenoid cystic carcinoma of the breast. Hum Pathol. 1987;18(12):1276–81. doi: 10.1016/s0046-8177(87)80413-6. [DOI] [PubMed] [Google Scholar]
- 11.Silberman AW, McVay C, Cohen JS, et al. Comparative morbidity of axillary lymph node dissection and the sentinel lymph node technique: implications for patients with breast cancer. Ann Surg. 2004;240(1):1–6. doi: 10.1097/01.sla.0000129358.80798.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sclafani LM, Baron RH. Sentinel lymph node biopsy and axillary dissection: added morbidity of the arm, shoulder and chest wall after mastectomy and reconstruction. Cancer J. 2008;14(4):216–22. doi: 10.1097/PPO.0b013e31817fbe5e. [DOI] [PubMed] [Google Scholar]
- 13.Mittendorf EA, Hunt KK. Lymphatic interrupted: do we really understand the risks and consequences? Ann Surg Oncol. 2009;16(7):1768–70. doi: 10.1245/s10434-009-0462-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25(24):3657–63. doi: 10.1200/JCO.2006.07.4062. [DOI] [PubMed] [Google Scholar]
- 15.Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8(10):881–8. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 16.Rosen P. Breast Pathology. Lippincott-Raven; Philadelphia and New York: 1996. [Google Scholar]
- 17.Tavisoli F. Pathology of the Breast. Appleton and Longo; Norwalk, CT: 1992. [Google Scholar]
- 18.Tavasoli Fa, D P. Tumors of the Breast and Female Genital Organs. WHO Classification of Tumors; Lyon: 2003. [Google Scholar]


