Abstract
Ku80 forms a heterodimer with Ku70, called Ku, that repairs DNA double-strand breaks (DSBs) via the nonhomologous end joining (NHEJ) pathway. As a consequence of deleting NHEJ, Ku80-mutant cells are hypersensitive to agents that cause DNA DSBs like ionizing radiation. Here we show that Ku80 deletion also decreased resistance to ROS and alkylating agents that typically cause base lesions and single-strand breaks (SSBs). This is unusual since base excision repair (BER), not NHEJ, typically repairs these types of lesions. However, we show that deletion of another NHEJ protein, DNA ligase IV (Lig 4), did not cause hypersensitivity to these agents. In addition, the ROS and alkylating agents did not induce γ-H2AX foci that are diagnostic of DSBs. Furthermore, deletion of Ku80, but not Lig 4 or Ku70, reduced BER capacity. Ku80 deletion also impaired BER at the initial lesion recognition/strand scission step; thus, involvement of a DSB is unlikely. Therefore, our data suggests that Ku80 deletion impairs BER via a mechanism that does not repair DSBs.
Keywords: nonhomologous end joining, base excision repair, double strand breaks, single strand breaks, base lesions
1. Introduction
NHEJ repairs DNA DSBs and utilizes at least seven proteins in mammals: Ku80, Ku70, DNA-PKCS, Artemis, Xrcc4, DNA ligase IV (Lig4) and Cernunnos-Xlf [1]. Ku80 and Ku70 form a heterodimer called Ku that binds to DNA ends [2]. These ends are processed by DNA-PKCS and ligated by the Xrcc4-Lig4 heterodimer in a complex with Cernunnos-Xlf [3, 4]. Mice deleted for Ku or Xrcc4/Lig4 exhibit a phenotype that results from defective repair of general DNA DSBs such as hypersensitivity to clastogenic agents and chromosomal rearrangements [1]. In addition, these mice are immunosuppressed since NHEJ is essential for repairing site specific DSBs that initiate V(D)J [Variable (Diverse) Coding] recombination [5]. Thus, NHEJ deletion disables repair of both general and site specific DNA DSBs.
Most biological data confirm this classical NHEJ pathway with regard to Ku80 and Ku70. For example, mice deleted for either Ku80 or Ku70 or both exhibited the same phenotype when controlled for environment and genetic background levels [6]. These cohorts presented with early aging and low cancer levels. In addition, both cohorts were immunosuppressed due to defective V(D)J recombination and cells deleted for either Ku80 or Ku70 were very sensitive to γ-radiation-induced DSBs and exhibit chromosomal rearrangements [7–11]. Therefore, these observations suggest Ku80 and Ku70 function only within the Ku heterodimer to repair DNA DSBs that occur in response to DNA damaging agents or during V(D)J recombination as would be predicted by classical NHEJ.
However, this orthodox viewpoint did not hold true when analyzing ku70−/− and ku80−/− mice in a p53-mutant background [12]. For this comparison ku70−/− p53−/− mice lived longer than ku80−/− p53−/− mice because the former exhibited less pro-B cell lymphoma, a form of cancer caused by an IgH/c-myc translocation [9, 13]. This observation suggests that either Ku70 or Ku80 function outside the Ku heterodimer. It is possible that the Ku subunits function independent of the heterodimer since some Ku70 remains in the absence of Ku80 [8] and vice versa [11]. Thus, we predicted that Ku80, in the absence of Ku70, inhibited IgH/c-myc translocations that induce pro-B cell lymphoma since deleting Ku80 in ku70−/− p53−/− mice recapitulated the ku80−/− p53−/− phenotype. In support of this possibility, an alternate form of Ku80 is used for DNA end-binding in mammalian mitochondria [14]. Thus, Ku80 may influence DNA repair when it is not equimolar to Ku70.
In addition to the mouse phenotype, dermal fibroblasts derived from ku70−/− p53−/− mice and ku80−/− p53−/− mice were hypersensitive to streptonigrin and paraquat [12]. Interestingly, these genotoxins cause single strand breaks and base lesions, damage that BER, not NHEJ, typically repairs. Furthermore, the ku80−/− p53−/− fibroblasts were more sensitive to these agents than the ku70−/− p53−/− fibroblasts suggesting that either Ku70 or Ku80 or both function outside of the Ku heterodimer. Thus, it is possible that deletion of either Ku80 or Ku70 inhibits BER in addition to NHEJ.
BER acts upon a broad spectrum of lesions and is composed of multiple sub-pathways. To reconcile these diverse pathways, Almeida and Sobol presented a unified BER model that divided these sub-pathways into three functional processes: lesion recognition/strand scission, gap tailoring and DNA synthesis/ligation [15]. A simplified version of these sub-pathways is presented here (Fig. 1A), for a detailed description please refer to Almeida and Sobol [15]. For the first functional process a base lesion may be recognized by a specific DNA glycosylase [16]. For example 8-oxoguanosine-glycosylase 1 (OGG1) recognizes 8-oxoG (ROS induced damage). Glycosylases remove the damaged base to generate an apurinic/apyridimic (AP)-site. AP endonuclease (APE1) generates a nick 5′ to the AP-site, generating a 5′-dRP (5′-deoxyribose phosphate) intermediate and a one base gap that is then ready for the second functional step. For the second functional process Poly(ADP-ribose) polymerase-1 (PARP-1) coordinates or stimulates a variety of enzymatic BER components and in the third functional process polymerase β (pol β) repairs the intermediate structure using both polymerase and 5′dRP-lyase activities. Its polymerase activity fills in the missing nucleotide while its 5′dRP-lyase activity generates a 5′ phosphorylated DNA strand by excising the 5′ terminal dRP residue so that DNA ligase may repair the nick. Thus, deletion of Ku80 or Ku70 may impair the BER pathway at any of these functional steps to cause hypersensitivity to streptonigrin and paraquat.
Fig. 1.
Models that account for impaired BER observed in ku80−/− cells. (A) Simplified BER model showing three functional activities [15]. (B) The classical NHEJ model. Classical NHEJ repairs DSBs are they are generated when replication forks collide with SSBs or base lesions. Alternatively, DSBs may form as BER intermediates. (C) The nonclassical NHEJ model. The Ku heterodimer either repairs or protects DSBs without Lig 4. (D) The Ku80 independent function model. Ku80, independent of Ku70, facilitates repair at the base lesion (red star) or at an intermediate step (not shown) either by itself or in association with other proteins (box labeled ?). (E) The Ku70 independent function model. Ku70, independent of Ku80, interferes with BER by associating with the base lesion (red star) or an intermediate step (not shown) either by itself or in association with other proteins.
Here we show Ku80 deletion impairs BER through a mechanism that is not classical NHEJ. We show that ku80−/− cells, but not lig4−/− cells, were hypersensitive to a variety of agents that cause base lesions and SSBs. Most of these agents failed to elicit a DSB repair response suggesting that damage is largely restricted to a single DNA strand. In addition, ku80−/−, but not lig4−/− or ku70−/− cellular extracts exhibited diminished capacity to perform BER. Finally, ku80−/− cells were defective for BER at multiple functional stages including base lesion recognition. Thus, Ku80 deletion impairs BER at multiple functional stages via a mechanism independent of classical NHEJ.
2. Materials and Methods
2.1 Generation of Cell Lines and Culture conditions
The Ku80-mutant MEFs [9] and the Lig4-mutant MEFs [17] are in a p53-mutant background [18] otherwise these cells would undergo premature replicative senescence. These MEFs were passaged 40 times by a modified 3T3 method to immortalize the cells [9]. MEFs were maintained in M10: high glucose DMEM supplemented with 10% fetal bovine serum, 1mM glutamine, 3mg/ml penicillin, 5mg/ml streptomycin and grown on plastic in an incubator at 5% CO2, 37°C at atmospheric O2.
2.2 Dose response assays
These dose response assays were performed as previously described for HeLa cells using the genotoxin screen [19]. Briefly, this assay is a cellular proliferation assay that determines the dose-response to a wide range of agents that damage DNA. This assay is performed on a 24-well plate and 1000 cells are seeded per well (day 0). Twenty-four hours later the genotoxin is added in one ml of M10. For the zero dose control, the solvent used for the genotoxin (DMSO for ENU and Streptonigrin; H2O for MMS and Paraquat; M10 media for H2O2) is added at the same concentration as the highest concentration of agent. Cells (not colonies) are counted 6 days later using a hemacytometer.
2.3 Immunocytochemistry
Detection of γ-H2AX by immunocytochemistry was performed as previously described [20]. For PARP-1 PAR activity, 1×105 cells/well were seeded in a chamber slide, H2O2 was added the next day at different concentrations and incubated at 37°C for 10minutes. These cells were washed with phosphate buffered saline (PBS)(GIBCO), cells fixed by incubation at 4°C for 15 minutes in PBS buffer containing 3% paraformaldehyde (Sigma). For permeation, cells were incubated at room temperature for 15minutes in PBS buffer containing 0.4% Triton X-100 (Sigma). Cells were blocked at room temperature for one hour in PBS buffer containing 1% non fat milk, 0.1% Tween-20, incubated anti-PAR (500X in blocking buffer) overnight at 4°C. The following day, cells were washed once using blocking buffer and incubated with Alex 488 anti-mouse IgG (200X dilution in blocking buffer) at room temperature in the dark for one hour. Finally, nuclei were stained with DAPI for 10 minutes before observing cells under a fluorescent microscope (Axioplan 2 Imaging, Zeiss).
2.4 In vitro spBER and sp/lpBER assays
The short patch BER (spBER) assay was performed as described [21]. Nuclear extracts were prepared using the NucBuster nuclear protein extraction reagent (Novagen) and dialyzed in buffer containing 50mM HEPES (pH 7.5), 100mM KCl, 0.5mM EDTA, 20% Glycerol and 1mM DTT. Bio-Rad protein assay reagents determined protein concentration in 16μl. Control and mutant cell extracts (20μg) were incubated with 52bp duplex DNA oligonucleotide substrate (100nM) containing uracil (U) at position 23 (U strand: 5′-GCTTGCATGCCTGCAGGTCTGAUTCTAGAGGATCCCCGGGTACCGAGCTCGA-3′; Template strand: 5′-TCGAGCTCGGTACCCGGGGATCCTCTAGAGTCAGACCTGCAGGCATGCAAGC-3′) in a reaction mixture [50mM HEPES (pH 7.5), 100mM KCl, 0.5 mM EDTA, 5mM DTT, 10mM MgCl2 4mM ATP, 2μM dCTP and 0.3μM of [α-32P]dCTP (3000Ci/mmol, Amersham Biosciences] at 37°C. Aliquots (4μl) were removed at 1, 5, and 10 minutes separately. An equal volume (4μl) of DNA gel-loading buffer (95% formamide, 20mM EDTA, 0.02% bromophenol blue and 0.02% xylene cyanol) was added to terminate the reaction. After incubation at 75°C for 2minutes, the reaction products were separated by electrophoresis in a 15% polyacrylamide gel containing 7M urea in 89mM Tris HCl (pH 8.8), 89mM boric acid and 2mM EDTA. For quantitation, using a PhosphorImager, the gel was scanned and bands quantified with Image Quant 5.1 software (Molecular Dynamic). Relative percentage of every band was calculated by dividing absolute number of every sample to wild type at the same time point. The experiment was repeated three times with two Ku80-mutant clones.
The short patch/long patch (sp/lp) BER assay was performed as described [22] with modifications. Nuclear extracts were prepared with the NucBuster (Novagen). Bio-Rad protein assay reagents determined protein concentration in 20μl. Cell extracts (10μg) were incubated with the 35bp duplex DNA oligonucleotide substrate (250nM) containing uracil (U) at position 15 (U strand: 5′-GCCCTGCAGGTCGA UTCTAGAGGATCCCCGGGTAC-3′; Template strand: 5′-GTACCCGGGGATCCTCTAGA GTCGACCTGCAGGGC-3′) for 5minutes at room temp in a BER reaction mixture [50mM HEPES (pH 7.5), 0.5mM EDTA, 2mM DTT, 20mM KCl, 4mM ATP, 5mM phosphocreatine, 100μg/ml phosphocreatine kinase, 0.5mM NAD and 100μM ddTTP]. The repair reaction was initiated by adding 10mM MgCl2 and 2μl of [α-32P]dCTP (3000Ci/mmol, Amersham Biosciences) at 37°C. Aliquots (4μl) were removed at the 1, 5 and 10minutes separately. The reaction products were handled as described above except 5μl of DNA gel loading buffer was added to stop the reaction and a 25% polyacrylamide gel was used.
2.5 Plasmid expression vectors and transfection conditions
hPARP-1 and hOOG-1 cDNAs were cloned into pcDNA3 after RT-PCR amplification from total RNA extracted from HeLa cells using a sense primer from the initiation ATG and an antisense primer from the stop codon. These cDNA inserts were sequenced to ensure fidelity. Overexpressing cell lines were generated as follows: 2 × 105 cells were seeded into 60mm dishes and incubated for 24 to 30hours, the expression plasmids were co-transfected with pCMV/Bsd (Invitrogen, for the purpose of selection) using FuGene 6 Transfection Reagent (Roche Diagnostic Corp.) according to the manufacturer’s instructions. Stable cell lines were selected in 500μg/mL Blasticidin (Invitrogen) for 2weeks, individual clones were amplified, and whole cell extracts were analyzed by immunoblotting for the over-expression of the BER proteins. Two individual clones were analyzed for each expression vector.
2.6 Luciferase assay
Generation of damaged DNA and luciferase assay was performed as described previously [23]. Control and ku80−/− MEFs (2×105) were seeded 24hours before transfection in a 6 well plate. Two luciferase reporter vectors pGL3-CMV and pRL3-CMV were a generous gift from Dr. X Lu (Baylor College of Medicine, Texas). pGL3-CMV DNA was incubated for 6hours with heat (70°C) and acid (0.01M Sodium citrate + 0.1M KCL, pH 5) before co-transfection with untreated pRL3-CMV (internal transfection control) into MEFs. Luciferase activities were measured by Dual-Luciferase Reporter Assay system (Promega), quantified data using Luminoskan Ascent (Thermo). All the experiments were performed in duplicated wells and repeats 3 times independently.
3. Results
3.1 Four models
Deletion of Ku80 or Ku70 caused different levels of hypersensitivity to streptonigrin and paraquat [12]; therefore, we hypothesized that deletion of the Ku subunits diminished BER. Four models will be tested to investigate this hypothesis (Fig 1B–E). First, the classical NHEJ model proposes classical NHEJ repairs a DSB that arises when a base lesion or single strand break collides with a replication fork or perhaps during an intermediate BER step. Second, the nonclassical NHEJ model proposes that a nonclassical form of NHEJ repairs a DSB. Third: the Ku80 independent function model proposes Ku80, independent of Ku70, facilitates repair of base lesions/SSBs in competition with BER or perhaps facilitates BER. Fourth, the Ku70 independent function model proposes Ku70, independent of Ku80, interferes with base lesion/SSB repair. Thus, DSBs are central to the first and second models while base lesions/SSBs are central to the third and fourth models. To differentiate between these models, our first step is to perform a genotoxic screen [19].
3.2 Ku80-mutant cells are hypersensitive to genotoxins that generate base lesions and SSBs
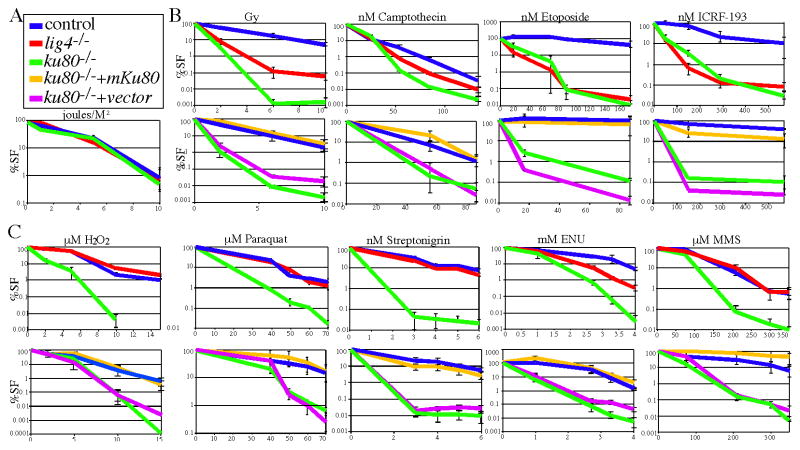
Ku80 is well known for repairing DNA DSBs via the NHEJ pathway. However, we wanted to determine if Ku80 could function independent of classical NHEJ since ku80−/− p53−/− mice exhibited a different phenotype than ku70−/− p53−/− mice and since cells derived from these mice are hypersensitive to streptonigrin and paraquat [12]. Therefore, we performed a genotoxic screen since it tests many pathways without investigator bias. This screen is a 6-day cell proliferation assay that measures the dose-response to a variety of agents that cause many different types of DNA alterations and challenges a full complement of chromatin metabolism pathways [19]. We compared control MEFs to ku80−/− [9] and lig4−/− MEFs [17]. We tested Lig4 because it is absolutely required for V(D)J recombination and has no known function other than classical NHEJ [24]. Two clones of ku80−/−, lig4−/−, and control MEFs were evaluated in a p53-mutant background [18]. p53 must be deleted in ku80−/− and lig4−/− MEFs to allow for proliferation since NHEJ-deletion causes premature replicative senescence [9, 17]. Therefore, all clones, including controls are deleted for p53.
The genotoxic screen includes multiple agents that serve as controls. For example both ku80−/− and lig4−/− MEFs should not be hypersensitive to an agent that causes helix-distorting lesions (UV-C) while both should be hypersensitive to agents that cause DSBs (γ-radiation, camptothecin, etoposide, ICRF-193). As expected these results were observed for both classes of agents (Fig. 2A, B). To validate the screen, ectopic expression of either human or mouse Ku80 corrected hypersensitivity to the DSB-inducing agents (Fig. 2B, bottom panel, only mouse Ku80 is shown). Nevertheless, there were some unusual observations since ku80−/− MEFs exhibited a greater degree of hypersensitivity than lig4−/− MEFs to γ-radiation and camptothecin suggesting Ku80 plays a more important role than Lig 4 for repairing damage caused by these two agents.
Fig. 2.
A genotoxic screen in ku80−/− and lig4−/− cells. Top panel, comparison of control cells to ku80−/− and lig4−/− cells. Bottom panel, comparison of control and ku80−/− cells to ku80−/− cells that express mouse Ku80 cDNA or empty vector. Human Ku80 cDNA also rescued hypersensitivity (not shown). % survival fraction (%SF). (A) UV light (joules/M2). (B) Agents that generate DSBs: ionizing radiation (Gray, Gy) and topoisomerase inhibitors (camptothecin, etoposide, ICRF-193). (C) ROS (H2O2), ROS-inducing (paraquat, streptonigrin) and alkylating (ENU, MMS) agents.
To our surprise ku80−/−, but not lig4−/− MEFs, were hypersensitive to a variety of agents that generate base lesions and SSBs (Fig. 2C, top panel). These agents include a ROS (H2O2) [25], ROS-inducing agents (streptonigrin and paraquat) [26, 27] and an alkylating agent (methyl methane sulfonate, MMS) [25]. ku80−/− MEFs also exhibited a greater degree of hypersensitivity than lig4−/− MEFs to another alkylating agent, N-ethyl-N-nitrosourea (ENU) [25]. The effect of Ku80-deletion was dramatic since it increased sensitivity by orders of magnitude as measured by cell number. Furthermore, both mouse and human Ku80 cDNA stably expressed in ku80−/− MEFs, restored wild type levels of resistance to these agents (Fig. 2C, bottom panel, only mouse cDNA is shown). Therefore, Ku80-deletion impaired correction of ROS and alkylator-induced base lesions and SSBs which corresponds to our observation that ku80−/− MEFs are more sensitive than lig4−/− MEFs to γ-radiation and camptothecin since these agents also generate base lesions and SSBs in addition to DSBs, respectively [25, 28]. These data show that Ku80 deletion, but not Lig4 deletion, influenced repair of ROS and alkylator induced DNA damage. Thus, classical NHEJ shown in figure 1B is not important for repairing these lesions. Yet, nonclassical NHEJ (without Lig 4) or a Ku80/Ku70 independent function could be responsible for repairing these lesions.
3.3 Most of the ROS and alkylating agents fail to induce a DSB response
ROS and alkylating agents are not known to directly cause DSBs [25], although DSBs could be generated if two SSBs oppose each other on complementary strands or could occur as secondary lesions at the replication fork or during an intermediate step in a repair process. Therefore, we investigated whether or not these agents induce a DSB repair response by immunostaining for the formation of phosphorylated H2AX (γ-H2AX) foci since an antibody to γ-H2AX can detect the many molecules of γ-H2AX that recognize a DNA DSB [29]. The type 1 topoisomerase inhibitor, camptothecin, was used as a positive control since it generates DNA DSBs during S phase [30] and because both ku80−/− and lig4−/− MEFs were hypersensitive to this agent (Fig. 2B). MEFs were exposed to equivalently toxic doses as judged by dose response curves; we used the concentration of agent that reduces wild type cell number by 0% (dose 0, solvent only), 90% (dose 1), 99% (dose 2), or ~100% (dose 3). We find that exposure to these ROS and alkylating agents did not increase the levels of γ-H2AX foci compared to similarly toxic doses of camptothecin (Fig. 3). There were comparatively few γ-H2AX foci after exposure to most of these agents compared to camptothecin, even when comparing dose 2 or 3 to dose 1 for camptothecin. However, exposure to H2O2 also generated γ-H2AX foci that progressively increase with each dose, albeit not as much as compared to equivalently toxic doses of camptothecin. Thus, most of these agents fail to elicit a DSB repair response, even at high concentrations suggesting that DSBs are not generated at sufficient levels to contribute to the cytotoxicity shown in figure 2C. In addition, ku80−/− MEFs do not exhibit increased levels of γ-H2AX foci as compared to control MEFs with the exception of camptothecin dose 1. Based on these results the nonclassical NHEJ model seems unlikely for all agents with the possible exception of H2O2. Therefore, we wanted to test NHEJ mutant cells for BER capacity.
Fig. 3.

Response to DNA DSBs as measured by γ-H2AX positivity. Control and ku80−/− MEFs were exposure to streptonigrin (STP), paraquat (PQ), ENU, MMS, H2O2 and camptothecin (CPT). All cells with one or more foci are counted as positive. Cells were exposed to a concentration of agent that reduces cell number by either 0% (dose 0, solvent only), 90% (dose 1), 99% (dose 2), or ~100% (dose 3) as judged by the dose-response curves shown in figure 2. There is no value recorded for control and ku80−/− MEFs exposed to 100 mM ENU and for ku80−/− MEFs exposed to 100 nM STP since these cells were dead or detaching from the plate. Between 138–732 cells were counted for each recorded value. Control, closed box; ku80−/−, open box. Dose 1: 4 nM STP, 50 μM PQ, 2.7 mM ENU, 210 μM MMS, 2 μM H2O2 and 25 nM CPT. Dose 2:10 nM STP, 70 μM PQ, 4 mM ENU, 350 μM MMS, 10 μM H2O2 and 85 nM CPT. Dose 3: 100 nM STP, 1 mM PQ, 100 mM ENU, 1 mM MMS, 1 mM H2O2 and 1 μM CPT.
3.4 Cellular extracts deleted for Ku80, but not Lig4 or Ku70, exhibited reduced BER capacity
We tested BER capacity for ku80−/−, ku70−/− and lig4−/− cellular extracts by measuring correction of a U/G mismatched oligonucleotide. In genomic DNA, the U/G mismatch mostly occurs from cytosine deamination and is repaired by BER. There are two U/G mismatch assays that test short patch BER (spBER, a.k.a. single nucleotide BER) and long patch BER (lpBER) [15]. The length of the intermediate single-strand gap defines these sub-pathways: spBER repairs a gap of only a single base while lpBER repairs a gap of more than one base [31]. The first assay tested spBER [21] (Fig. 4A) while the second assay tested both spBER and lpBER (Fig. 4B). The latter assay can distinguish between spBER and lpBER by differentiating between the incorporation of either one nucleotide (spBER) or 2 or more nucleotides (lpBER) [22]. By the first assay, ku80−/− nuclear extracts are ~35% deficient in repairing the U/G mismatch (Fig. 4A, C, E) and by the second assay ku80−/− nuclear extracts are ~50% deficient in repairing the U/G mismatch by both spBER and lpBER (Fig. 4B, D, E). Expression of mouse Ku80 cDNA rescued this deficiency. By comparison extracts generated for cells deleted for pol β exhibited about a 70% reduction (not shown). However, by the spBER pathway lig4−/− cellular extracts are undiminished for repairing the U/G mismatch compared to control. In addition, by either assay, ku70−/− nuclear extracts exhibited the same BER capacity as control extracts. Thus, Ku80-deletion impaired U/G mismatch repair demonstrating a direct defect in BER that is independent of Lig4 and Ku70. These data do not support the nonclassical NHEJ model but instead the Ku80/Ku70 independent function models.
Fig. 4.
ku80−/− cells are defective for repairing a U/G mismatch in vitro. (A) Experimental design for short patch assay: a 52 bp oligonucleotide duplex substrate containing a U/G mismatch at position 23 is incubated with [32P]dCTP + ddTTP and cell extracts are generated at 1, 5 and 10 minutes. (B) Experimental design for short patch – long patch assay: a 35 bp oligonucleotide duplex substrate containing a U/G mismatch at position 15 is incubated with [32P]dCTP + ddTTP and cell extracts are generated at 1, 5 and 10 minutes. BER products: 35-mer (ligated spBER), 16-mer (2-nuclotide addition, lpBER) and 15-mer (1 nucleotide addition, either spBER or lpBER). (C) Phosphorimage of polyacrylamide gel showing in vitro short patch BER products. Control (Con), lig4−/− and ku80−/− clones are shown plus ku80−/− MEFs that express mouse Ku80 cDNA or vector alone. In addition, ku70−/− MSFs are shown with its control. (D) Phosphorimage of polyacrylamide gel showing in vitro short patch BER products. Control (Con) and ku80−/− clones 1 & 2 (1, 2) are shown plus ku80−/− MEFs that express mouse Ku80 cDNA or vector alone. In addition, ku70−/− MSFs are shown with its control. (E) Summary and quantization for spBER (left, the 52mer from the short patch assay), spBER (center, the 35mer from the short patch – long patch assay) and lpBER (right, the 16mer from the short patch – long patch assay). Statistically significant differences are show (Student’s t test).
3.5 Ku80 deleted cells were defective for BER at the lesion recognition/strand scission functional process
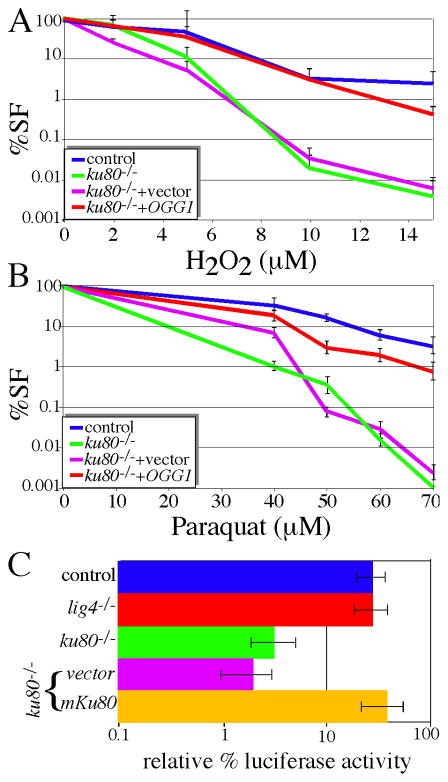
To test lesion recognition, we overexpressed the OGG1 glycosylase in control and ku80−/− MEFs. OGG1 recognizes 8-oxoG (ROS-induced base lesion) [16]. If Ku80 or Ku70 act directly upon the base lesion, then glycosylase overexpression would ameliorate sensitivity to ROS and alkylating agents as predicted by the Ku80/Ku70 independent function models. However, if Ku80 deletion disrupts the second or third functional processes, then glycosylase overexpression would exacerbate sensitivity to ROS and alkylating agents much like AAG enhanced sensitivity of pol β−/− cells to alkylating agents [32]. Control and ku80−/− MEFs that overexpress human OGG1 were tested for sensitivity to ROS agents that generates base lesions recognized by hOGG1 (H2O2, paraquat). We found that hOGG1 overexpression fully rescued ku80−/− MEFs hypersensitivity to both ROS agents (Fig. 5A, B). Furthermore, hOGG1 overexpression did not rescue sensitivity to camptothecin-induced DSBs (not shown). These data suggest that Ku80 or Ku70 impacts the lesion recognition/strand scission stage by acting directly on ROS induced base lesions (specifically 8-oxoG). Therefore, hOGG1 rescue supports the Ku80/Ku70 independent function models, but not the nonclassical NHEJ model.
Fig. 5.
ku80−/− cells are defective for the lesion recognition/strand scission functional process. Overexpression of OGG1 rescues hypersensitivity of ku80−/− cells to (A) H2O2 and (B) paraquat. (C) The luciferase assay measures AP-site repair. Note log scale.
We tested the capacity of ku80−/− MEFs to correct AP-sites using a luciferase assay with two luciferase expression vectors, firefly and Renilla, that emit light at different wave-lengths [23]. Glycosylases remove the damaged base to generate an AP-site. AP endonuclease (APE1) makes a nick 5′ to the AP-site to generate a 5′-dRP (deoxyribose phosphate) intermediate and a one base gap. AP-sites are generated in the firefly luciferase expression plasmid by acid treatment at 70°C for 6 hours and then neutralized while Renilla luciferase is untreated and serves as a baseline transfection control. These luciferase vectors are co-transfected into cells and 24 hours later luciferase activity is measured. We find that deletion of Ku80, but not Lig4, impaired the correction of these AP-sites (Fig. 5C) and expression of mouse Ku80 cDNA corrected this defect. These data suggest that ku80−/− MEFs are impaired for the lesion recognition/strand scission stage due to defective AP-site repair in support of the Ku80/Ku70 independent function models, but not the nonclassical NHEJ model.
3.6 Ku80 deleted cells were defective for BER at the gap tailoring and DNA synthesis/ligation functional process
PARP-1 is directly involved in multiple BER sub-pathways at the gap tailoring and strand synthesis stages perhaps by detecting DNA interruptions and coordinating or stimulating enzymatic BER components including XRCC1 and Pol β for efficient processing of these anomalies in a coordinated manner [15]. Poly (ADP-ribose) (PAR) likely recruits XRCC1 to BER intermediates since DNA damage induces PAR sites and since XRCC1 foci subsequently appear at those sites [33]. Therefore, we compared PAR synthesis in MEFs deleted for Ku80, Lig4 and Brca2 exon 27; Brca2 is a tumor suppressor important for repairing DNA DSBs by homologous recombination [34]. Rates of PAR synthesis correlate in a linear manner to the number of enzymatically active PARP-1 molecules [35]. We find that PAR synthesis was increased in ku80−/− MEFs compared to these other cells after exposure to 0.1 or 1.0 mM H2O2 while cells from all genotypes exhibited elevated PAR synthesis after exposure to 10 mM H2O2 (Fig. 6A, B). Thus, increased PAR synthesis at low dose is specific to deletion of Ku80 and does not reflect a general decline in DSB repair since deletion of NHEJ (lig4−/− MEFs) and impairment of HR (Brca2 exon 27 deleted MEFs) failed to increase PAR. In addition we tested if human PARP-1 can rescue sensitivity of ku80−/− MEFs to alkylating and ROS agents. We observed that PARP-1 over-expression restores resistance of ku80−/− MEFs to H2O2 (Fig. 6C) but not to MMS and ENU (not shown). We also found PARP-1 defective for DNA binding (PARP-1C21G/C125G) [36] and an enzymatic mutant (PARP-1E988K) [37] failed to rescue. Thus, increased PAR synthesis reflects a response to compensate for Ku80-deletion and fully functional PARP-1 is essential to rescue sensitivity of ku80−/− MEFs to H2O2. Recently OGG1 was shown to associate with PARP-1 and oxidative stress enhanced this association [38]. OGG1 stimulated PARP-1’s poly(ADP-ribosyl)ation activity and ogg1−/− cells exhibited reduced PAR levels and were hypersensitive to PARP-1 inhibitors. Furthermore, defects in BRCA2-mediated DSB enhanced the cytotoxicity of PARP-1 inhibitors [39]. Therefore, ku80−/− cells could exhibit altered sensitivity to PARP-1 inhibitors: increased sensitivity would reflect defective DSB while no change or decreased sensitivity would reflect increased PAR synthesis. We found that ku80−/− cells exhibited the same level of sensitivity to the PARP-1 inhibitors 3-aminobenzamide [40] and 4-amino-1,8-naphthalimide [41] as control cells (not shown) supporting the notion that ku80−/− cells have elevated PAPP-1 function. Therefore, our observations suggest that Ku80 deletion negatively impacts multiple BER functional processes to reduce BER capacity since OGG1 and PARP-1 rescue hypersensitivity to ROS agents in ku80−/− cells indicating defective lesion recognition/strand scission and defective gap tailoring, DNA synthesis/ligation, respectively. These observations support the Ku80/Ku70 independent function models, but not the nonclassical NHEJ model.
Fig. 6.
Ku80 and PARP-1 interaction after H2O2 exposure. (A, B) Deletion of Ku80 increases PAR activity after exposure to low H2O2 levels. (A) Immunofluorescence for PAR activity after exposure to 0.1 and 10 mM H2O2. Cells deleted for Brca2 exon 27 (called brca2−/−, previously called brca2lex1/lex2) [61]. (B) Graph summarizing % of cells showing PAR activity after exposure to 0.1, 1.0 or 10 mM H2O2. Cells without exposure to H2O2 were used to establish an exposure time that does not detect positive cells; this exposure time is used for all exposed cells. (C) Overexpression of PARP-1, but not PARP-1C21G/C125G or PARP-1E988K, rescues hypersensitivity to H2O2 for Ku80-mutant cells.
4. Discussion
Here we show ku80−/− cells are impaired for BER at multiple functional stages via a defect that is not classical NHEJ. First, we show ku80−/− cells, but not lig4−/− cells, are hypersensitive to ROS and alkylating agents suggesting a defect in a DNA repair pathway that does not require Lig4. Second, we show that most of the ROS and alkylating agents fail to induce a response to DSBs as measured by γ-H2AX formation suggesting pathways that repair DSBs are not required to repair ROS and alkylator induced DNA damage. Third, we show ku80−/− cell extracts, but not lig4−/− or ku70−/− cell extracts, are defective for spBER and lpBER. Once again this is not NHEJ. Fourth, we show that hOGG-1 overexpression rescues hypersensitivity to H2O2 and paraquat and ku80−/−, but not lig4−/− cells, are defective for repairing AP-sites demonstrating Ku80 deletion impacts the initial strand lesion recognition/strand scission stage. Fifth, we show ku80−/− cells, but not lig4−/− and Brca2 exon 27 deleted cells, exhibit increased PAR synthesis after H2O2 exposure and that hPARP-1 overexpression rescues H2O2 hypersensitivity demonstrating Ku80 deletion impacts the gap tailoring or DNA synthesis/ligation stages. Thus, a variety of approaches all come to the same conclusion: Ku80-deleted cells, through an NHEJ-independent mechanism, are impaired for BER at multiple functional stages.
4.1 Could defective NHEJ cause these results?
An NHEJ defect is unlikely to cause this phenotype for the following reasons. 1) Ku80-deleted cells, but not Lig4-deleted cells were hypersensitive to ROS and alkylating agents (Fig. 2), had reduced capacity for repairing a U/G mismatch by an in vitro assay (Fig. 4), displayed reduced capacity for repairing AP-sites on a luciferase reporter (Fig. 5C), and exhibited increased PAR synthesis (Fig. 6). Lig4-deleted cells would show the same phenotype as Ku80-deleted cells if defective classical NHEJ were responsible for these phenotypes since Lig4 is essential for this pathway and has no other known function [24]. It is important to note that ku80−/− cells shared a common phenotype to lig4−/− cells when it comes to NHEJ: both were hypersensitive to clastogenic agents (Fig. 2B) and were defective for V(D)J recombination [7, 17]. In addition, the ku80−/− cells were more sensitive to γ-radiation than the lig4−/− cells. This seems odd since both are defective for repairing γ-radiation-induced DSBs. However, the difference is understandable since γ-radiation also causes ROS damage and only the ku80−/− cells are hypersensitive to ROS. An analogous argument can be made for camptothecin and SSBs. 2) With the exception of H2O2, the ROS and alkylating agents did not induce a DNA DSB repair response as measure by the formation of γ-H2AX foci (Fig. 3). Even though H2O2 induced a DSB repair response, it is lower than the response induced by equivalently toxic doses of camptothecin: an agent that generates DSBs repaired by NHEJ. Thus, DSBs were likely to account for only part of the cytotoxicity to H2O2. In addition, DSBs were unlikely to cause hypersensitivity since this phenotype was rescued by OGG1 overexpression; thus, implicating 8oxoG as the toxic lesion. 3) OGG1 overexpression rescued hypersensitivity to the ROS and alkylating agents, yet OGG1 overexpression was unable to rescue hypersensitivity to an agent that generates DSBs repaired by NHEJ, camptothecin. OGG1 is important for BER, but not NHEJ. Furthermore, OGG1 is specialized such that it could not easily be adapted to other repair pathways like NHEJ and OGG1 drives the BER pathway to single strand break (SSB)-gap intermediates that would exacerbate hypersensitivity to ROS if the Ku heterodimer functioned at a downstream BER step much like AAG enhanced sensitivity of pol β−/− cells to alkylating agents [32]. 4) Cell extracts deleted for Ku80, but not Lig4 or Ku70, were defective for repairing the U/G mismatch (Fig. 4). This result implies that deletion of the Ku heterodimer was not responsible for this repair defect. Thus, Ku80-deletion impaired BER by an NHEJ independent mechanism.
4.2 Why are ku80−/− cells impaired for BER?
BER is complicated, composed of multiple sub-pathways that utilize components that are unique to only a subset of these pathways coupled with components shared between many sub-pathways [15]. These sub-pathways are selective for repairing the wide range of DNA damage caused by ROS and alkylating agents. This selectivity is exemplified by the large variety of DNA glycosylases specific for only a subset of base lesions [16]. In addition, Pol β repairs only a subset of damage induced by alkylating agents (MMS but not ENU) [42, 43]. Data presented here corroborates these results. Thus, it is curious that ku80−/− cells are hypersensitive to all five agents tested showing that the BER defect is more comprehensive for ku80−/− cells than pol β−/− cells. Perhaps Ku80-deletion impairs BER by a single mechanism. However, this seems unlikely since multiple BER components rescued ku80−/− cells that function at different steps in the BER pathway: OGG1 rescues at the first step of lesion recognition/strand scission and is specific for 8oxoG lesions while PARP-1 follows this step for gap tailoring and DNA synthesis/ligation [15]. Thus, there are potentially multiple mechanisms to explain these observations. We propose four models in figure 1. However, our data does not support the classical and nonclassical NHEJ models for the reasons mentioned above. Thus, Ku70 and/or Ku80 likely influence BER independent of the Ku heterodimer. It is possible that either Ku70 or Ku80 has no impact on BER. If Ku70 has no impact, then Ku80 enables the repair of ROS/alkylator-induced damage (Ku80 independent function model). By contrast, if Ku80 has no impact, then Ku70 interferes with the repair of these lesions (Ku70 independent function model.
The Ku80 independent function model proposes that Ku80, in the absence of Ku70, actively repairs the base lesion or SSB (or gap of one or more nucleotides) in competition with other BER components (or facilitates these components) such that Ku80 deletion reduces capacity for multiple BER sub-pathways. The exact mode of Ku80 action is not known. The possibility that Ku80 associates with DNA lesions, independent of Ku70, comes from our observations that ku70−/− p53−/− mice exhibited a milder phenotype than ku80−/− p53−/− mice since the former cohort lived longer and displayed lower levels of pro-B cell lymphoma [12]. We suggested that Ku80, independent of Ku70, inhibited pro-B cell lymphoma by interfering with the cancer causing IgH/c-myc translocation since deleting Ku80 from ku70−/− p53−/− mice elevated pro-B cell lymphoma levels similar to ku80−/− p53−/− mice. Thus, Ku80 appears to influence illegitimate DSB repair. In support, an alternate form of Ku80 is required for mitochondrial DNA end-binding [14]. Similarly, Ku80 (perhaps an alternate form) could directly associate with 8oxoG or a SSB or a gap to enable repair. If so, then Ku80-deletion would diminish both spBER and lpBER. In the absence of Ku80, BER capacity is reduced as long as the other competing BER components are insufficient to account for the deficit. Thus, over-expression of BER components would correct the agent-induced hypersensitivities in a lesion specific manner and ku80−/− cells, but not ku70−/− cells, would be defective for repairing the U/G mismatch. This model fits all these data.
The Ku70 independent function model proposes that Ku70, in the absence of Ku80, disrupts the repair of a base lesion or SSB (or gap of one or more nucleotides) by forming nonproductive intermediates in competition with multiple BER components. Thus, Ku80 deletion impairs multiple BER sub-pathways by unleashing Ku70 and both spBER and lpBER would be affected. Like for Ku80, the exact mode of action is not known. However, Ku70 independent of Ku80, associates with DNA [44–47]. Ku70, in the absence of Ku80, also associates with chromatin structure proteins TRF1, TRF2 and Hp1α [48–50] and with the DNA repair protein MRE11 [51]. In addition, nuclear Ku70, but not Ku80, increases in response to γ radiation [52]; an agent that causes ROS-induced damage [25]. Thus, a multitude of data shows that Ku70 may disable BER without Ku80. This model also fits all data presented here since over-expression of BER components would out compete these nonproductive intermediates and ku70−/− cell extracts would not exhibit defective repair of the U/G mismatched substrate.
4.3 Does Ku80 or Ku70 influence BER in vivo?
Are these results a laboratory artifact or do either Ku70 or Ku80 influence BER in nature? There are several points of data that support the possibility that these tissue culture studies reflect in vivo function since Ku70 and Ku80 exist without the other and function independent of the Ku heterodimer. For example Ku70 is unilaterally sequestered or degraded; therefore, free Ku80 may exist in a natural setting. A variety of proteins unilaterally sequester Ku70 including chromatin structure proteins (TRF1, TRF2 and Hp1α) [48–50], a DNA repair protein (MRE11) [51], cell death proteins (Bax and p18-cyclin E) [53, 54] and other proteins [55, 56]. Furthermore, Granzyme A unilaterally degrades Ku70 in cytotoxic T lymphocytes and natural killer cells as a defense against viruses and tumors [57]. In addition to unilateral sequestration and degradation, evidence shows that Ku70 and Ku80 function independent of the Ku heterodimer since each subunit enters the nucleus through a different nuclear localization signal [58, 59] and since Ku70 levels increase in response to γ-radiation without Ku80 [52]. Furthermore, the different phenotypes displayed in p53−/− mice deleted for either Ku80 or K70 confirm that at least one of the Ku subunits is active outside the heterodimer in vivo. Finally, variant forms of Ku80 have been described specific to mammalian mitochondria [14] and normal human peripheral B-lymphocytes [60]. Thus, Ku70 and Ku80 likely exist and function independent of the Ku heterodimer in vivo.
Highlights.
Ku80-deleted cells are hypersensitive to ROS and alkylating agents.
Cells deleted for Ku80, but not Ku70 or Lig 4, have reduced BER capacity.
OGG1 rescues hypersensitivity to H2O2 and paraquat in Ku80-mutant cells.
Cells deleted for Ku80, but not Lig 4, are defective at repairing AP sites.
Cells deleted for Ku80, but not Lig 4 or Brca2 exon 27, exhibit increased PAR.
Acknowledgments
We thank Dr. Fred Alt for the gift of DNA Ligase 4−/− MEFs and Dr. X Lu for the luciferase reporters (pGL3-CMV and pRL3-CMV). This work was supported by UO1 ES11044, R01 CA76317-05A1 and P01 AG17242 to PH.
Footnotes
Conflict of interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burma S, Chen BP, Chen DJ. Role of non-homologous end joining (NHEJ) in maintaining genomic integrity. DNA Repair (Amst) 2006;5:1042–1048. doi: 10.1016/j.dnarep.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Walker JR, Corpina RA, Goldberg J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature. 2001;412:607–614. doi: 10.1038/35088000. [DOI] [PubMed] [Google Scholar]
- 3.Buck D, Malivert L, de Chasseval R, Barraud A, Fondaneche MC, Sanal O, Plebani A, Stephan JL, Hufnagel M, le Deist F, Fischer A, Durandy A, de Villartay JP, Revy P. Cernunnos, a novel nonhomologous end-joining factor, is mutated in human immunodeficiency with microcephaly. Cell. 2006;124:287–299. doi: 10.1016/j.cell.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 4.Ahnesorg P, Smith P, Jackson SP. XLF Interacts with the XRCC4-DNA Ligase IV Complex to Promote DNA Nonhomologous End-Joining. Cell. 2006;124:301–313. doi: 10.1016/j.cell.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 5.Lieber MR, Ma Y, Pannicke U, Schwarz K. The mechanism of vertebrate nonhomologous DNA end joining and its role in V(D)J recombination. DNA Repair (Amst) 2004;3:817–826. doi: 10.1016/j.dnarep.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Vogel H, Holcomb VB, Gu Y, Hasty P. Deletion of Ku70, Ku80, or both causes early aging without substantially increased cancer. Mol Cell Biol. 2007;27:8205–8214. doi: 10.1128/MCB.00785-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu C, Bogue MA, Lim DS, Hasty P, Roth DB. Ku86-deficient mice exhibit severe combined immunodeficiency and defective processing of V(D)J recombination intermediates. Cell. 1996;86:379–389. doi: 10.1016/s0092-8674(00)80111-7. [DOI] [PubMed] [Google Scholar]
- 8.Nussenzweig A, Chen C, da Costa Soares V, Sanchez M, Sokol K, Nussenzweig MC, Li GC. Requirement for Ku80 in growth and immunoglobulin V(D)J recombination. Nature. 1996;382:551–555. doi: 10.1038/382551a0. [DOI] [PubMed] [Google Scholar]
- 9.Lim DS, Vogel H, Willerford DM, Sands AT, Platt KA, Hasty P. Analysis of ku80-mutant mice and cells with deficient levels of p53. Mol Cell Biol. 2000;20:3772–3780. doi: 10.1128/mcb.20.11.3772-3780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nussenzweig A, Sokol K, Burgman P, Li L, Li GC. Hypersensitivity of Ku80-deficient cell lines and mice to DNA damage: the effects of ionizing radiation on growth, survival, and development. Proc Natl Acad Sci U S A. 1997;94:13588–13593. doi: 10.1073/pnas.94.25.13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu Y, Jin S, Gao Y, Weaver DT, Alt FW. Ku70-deficient embryonic stem cells have increased ionizing radiosensitivity, defective DNA end-binding activity, and inability to support V(D)J recombination. Proc Natl Acad Sci U S A. 1997;94:8076–8081. doi: 10.1073/pnas.94.15.8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Choi YJ, Hanes MA, Marple T, Vogel H, Hasty P. Deleting Ku70 is milder than deleting Ku80 in p53-mutant mice and cells. Oncogene. 2009;28:1875–1878. doi: 10.1038/onc.2009.57. [DOI] [PubMed] [Google Scholar]
- 13.Difilippantonio MJ, Zhu J, Chen HT, Meffre E, Nussenzweig MC, Max EE, Ried T, Nussenzweig A. DNA repair protein Ku80 suppresses chromosomal aberrations and malignant transformation [see comments] Nature. 2000;404:510–514. doi: 10.1038/35006670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coffey G, Campbell C. An alternate form of Ku80 is required for DNA end-binding activity in mammalian mitochondria. Nucleic Acids Res. 2000;28:3793–3800. doi: 10.1093/nar/28.19.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almeida KH, Sobol RW. A unified view of base excision repair: lesion-dependent protein complexes regulated by post-translational modification. DNA Repair (Amst) 2007;6:695–711. doi: 10.1016/j.dnarep.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parsons JL, Elder RH. DNA N-glycosylase deficient mice: a tale of redundancy. Mutat Res. 2003;531:165–175. doi: 10.1016/j.mrfmmm.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Frank KM, Sekiguchi JM, Seidl KJ, Swat W, Rathbun GA, Cheng HL, Davidson L, Kangaloo L, Alt FW. Late embryonic lethality and impaired V(D)J recombination in mice lacking DNA ligase IV. Nature. 1998;396:173–177. doi: 10.1038/24172. [DOI] [PubMed] [Google Scholar]
- 18.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 19.Marple T, Li H, Hasty P. A genotoxic screen: rapid analysis of cellular dose-response to a wide range of agents that either damage DNA or alter genome maintenance pathways. Mutat Res. 2004;554:253–266. doi: 10.1016/j.mrfmmm.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Yaneva M, Li H, Marple T, Hasty P. Non-homologous end joining, but not homologous recombination, enables survival for cells exposed to a histone deacetylase inhibitor. Nucleic Acids Res. 2005;33:5320–5330. doi: 10.1093/nar/gki821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singhal RK, Prasad R, Wilson SH. DNA polymerase beta conducts the gap-filling step in uracil-initiated base excision repair in a bovine testis nuclear extract. J Biol Chem. 1995;270:949–957. doi: 10.1074/jbc.270.2.949. [DOI] [PubMed] [Google Scholar]
- 22.Harrigan JA, Wilson DM, 3rd, Prasad R, Opresko PL, Beck G, May A, Wilson SH, Bohr VA. The Werner syndrome protein operates in base excision repair and cooperates with DNA polymerase beta. Nucleic Acids Res. 2006;34:745–754. doi: 10.1093/nar/gkj475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Offer H, Wolkowicz R, Matas D, Blumenstein S, Livneh Z, Rotter V. Direct involvement of p53 in the base excision repair pathway of the DNA repair machinery. FEBS Lett. 1999;450:197–204. doi: 10.1016/s0014-5793(99)00505-0. [DOI] [PubMed] [Google Scholar]
- 24.Rooney S, Chaudhuri J, Alt FW. The role of the non-homologous end-joining pathway in lymphocyte development. Immunol Rev. 2004;200:115–131. doi: 10.1111/j.0105-2896.2004.00165.x. [DOI] [PubMed] [Google Scholar]
- 25.Friedberg EC, Walker GC, Siede W. DNA repair and mutagenesis. American Society of Microbiology; Washington, D. C: 1995. [Google Scholar]
- 26.Bolzan AD, Bianchi MS. Genotoxicity of streptonigrin: a review. Mutat Res. 2001;488:25–37. doi: 10.1016/s1383-5742(00)00062-4. [DOI] [PubMed] [Google Scholar]
- 27.Bus JS, Gibson JE. Paraquat: model for oxidant-initiated toxicity. Environ Health Perspect. 1984;55:37–46. doi: 10.1289/ehp.845537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plo I, Liao ZY, Barcelo JM, Kohlhagen G, Caldecott KW, Weinfeld M, Pommier Y. Association of XRCC1 and tyrosyl DNA phosphodiesterase (Tdp1) for the repair of topoisomerase I-mediated DNA lesions. DNA Repair (Amst) 2003;2:1087–1100. doi: 10.1016/s1568-7864(03)00116-2. [DOI] [PubMed] [Google Scholar]
- 29.Pilch DR, Sedelnikova OA, Redon C, Celeste A, Nussenzweig A, Bonner WM. Characteristics of gamma-H2AX foci at DNA double-strand breaks sites. Biochem Cell Biol. 2003;81:123–129. doi: 10.1139/o03-042. [DOI] [PubMed] [Google Scholar]
- 30.Pommier Y. Diversity of DNA topoisomerases I and inhibitors. Biochimie. 1998;80:255–270. doi: 10.1016/s0300-9084(98)80008-4. [DOI] [PubMed] [Google Scholar]
- 31.Barnes DE, Lindahl T. Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annu Rev Genet. 2004;38:445–476. doi: 10.1146/annurev.genet.38.072902.092448. [DOI] [PubMed] [Google Scholar]
- 32.Trivedi RN, Almeida KH, Fornsaglio JL, Schamus S, Sobol RW. The role of base excision repair in the sensitivity and resistance to temozolomide-mediated cell death. Cancer Res. 2005;65:6394–6400. doi: 10.1158/0008-5472.CAN-05-0715. [DOI] [PubMed] [Google Scholar]
- 33.El-Khamisy SF, Masutani M, Suzuki H, Caldecott KW. A requirement for PARP-1 for the assembly or stability of XRCC1 nuclear foci at sites of oxidative DNA damage. Nucleic Acids Res. 2003;31:5526–5533. doi: 10.1093/nar/gkg761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.San Filippo J, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Annu Rev Biochem. 2008;77:229–257. doi: 10.1146/annurev.biochem.77.061306.125255. [DOI] [PubMed] [Google Scholar]
- 35.Kun E, Kirsten E, Bauer PI, Ordahl CP. Quantitative correlation between cellular proliferation and nuclear poly (ADP-ribose) polymerase (PARP-1) Int J Mol Med. 2006;17:293–300. [PubMed] [Google Scholar]
- 36.Gradwohl G, Menissier de Murcia JM, Molinete M, Simonin F, Koken M, Hoeijmakers JH, de Murcia G. The second zinc-finger domain of poly(ADP-ribose) polymerase determines specificity for single-stranded breaks in DNA. Proc Natl Acad Sci U S A. 1990;87:2990–2994. doi: 10.1073/pnas.87.8.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rolli V, O’Farrell M, Menissier-de Murcia J, de Murcia G. Random mutagenesis of the poly(ADP-ribose) polymerase catalytic domain reveals amino acids involved in polymer branching. Biochemistry. 1997;36:12147–12154. doi: 10.1021/bi971055p. [DOI] [PubMed] [Google Scholar]
- 38.Noren Hooten N, Kompaniez K, Barnes J, Lohani A, Evans MK. Poly(ADP-ribose) polymerase 1 (PARP-1) binds to 8-oxoguanine-DNA glycosylase (OGG1) J Biol Chem. 2011;286:44679–44690. doi: 10.1074/jbc.M111.255869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rios J, Puhalla S. PARP inhibitors in breast cancer: BRCA and beyond. Oncology (Williston Park) 2011;25:1014–1025. [PubMed] [Google Scholar]
- 40.Dona F, Chiodi I, Belgiovine C, Raineri T, Ricotti R, Mondello C, Scovassi AI. Poly(ADP-ribosylation) and neoplastic transformation: Effect of PARP inhibitors. Curr Pharm Biotechnol. 2012 doi: 10.2174/138920101405131111104642. [DOI] [PubMed] [Google Scholar]
- 41.Horton JK, Stefanick DF, Naron JM, Kedar PS, Wilson SH. Poly(ADP-ribose) polymerase activity prevents signaling pathways for cell cycle arrest after DNA methylating agent exposure. J Biol Chem. 2005;280:15773–15785. doi: 10.1074/jbc.M413841200. [DOI] [PubMed] [Google Scholar]
- 42.Fortini P, Pascucci B, Belisario F, Dogliotti E. DNA polymerase beta is required for efficient DNA strand break repair induced by methyl methanesulfonate but not by hydrogen peroxide. Nucleic Acids Res. 2000;28:3040–3046. doi: 10.1093/nar/28.16.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horton JK, Baker A, Berg BJ, Sobol RW, Wilson SH. Involvement of DNA polymerase beta in protection against the cytotoxicity of oxidative DNA damage. DNA Repair (Amst) 2002;1:317–333. doi: 10.1016/s1568-7864(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 44.Chou CH, Wang J, Knuth MW, Reeves WH. Role of a major autoepitope in forming the DNA binding site of the p70 (Ku) antigen. J Exp Med. 1992;175:1677–1684. doi: 10.1084/jem.175.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Dong X, Myung K, Hendrickson EA, Reeves WH. Identification of two domains of the p70 Ku protein mediating dimerization with p80 and DNA binding. J Biol Chem. 1998;273:842–848. doi: 10.1074/jbc.273.2.842. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Satoh M, Chou CH, Reeves WH. Similar DNA binding properties of free P70 (KU) subunit and P70/P80 heterodimer. FEBS Lett. 1994;351:219–224. doi: 10.1016/0014-5793(94)00863-9. [DOI] [PubMed] [Google Scholar]
- 47.Wang J, Satoh M, Pierani A, Schmitt J, Chou CH, Stunnenberg HG, Roeder RG, Reeves WH. Assembly and DNA binding of recombinant Ku (p70/p80) autoantigen defined by a novel monoclonal antibody specific for p70/p80 heterodimers. J Cell Sci. 1994;107(Pt 11):3223–3233. doi: 10.1242/jcs.107.11.3223. [DOI] [PubMed] [Google Scholar]
- 48.Hsu HL, Gilley D, Galande SA, Hande MP, Allen B, Kim SH, Li GC, Campisi J, Kohwi-Shigematsu T, Chen DJ. Ku acts in a unique way at the mammalian telomere to prevent end joining. Genes Dev. 2000;14:2807–2812. doi: 10.1101/gad.844000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song K, Jung D, Jung Y, Lee SG, Lee I. Interaction of human Ku70 with TRF2. FEBS Lett. 2000;481:81–85. doi: 10.1016/s0014-5793(00)01958-x. [DOI] [PubMed] [Google Scholar]
- 50.Song K, Jung Y, Jung D, Lee I. Human ku70 interacts with heterochromatin protein 1alpha. J Biol Chem. 2001;276:8321–8327. doi: 10.1074/jbc.M008779200. [DOI] [PubMed] [Google Scholar]
- 51.Goedecke W, Eijpe M, Offenberg HH, van Aalderen M, Heyting C. Mre11 and Ku70 interact in somatic cells, but are differentially expressed in early meiosis. Nat Genet. 1999;23:194–198. doi: 10.1038/13821. [DOI] [PubMed] [Google Scholar]
- 52.Brown KD, Lataxes TA, Shangary S, Mannino JL, Giardina JF, Chen J, Baskaran R. Ionizing radiation exposure results in up-regulation of Ku70 via a p53/ataxia-telangiectasia-mutated protein-dependent mechanism. J Biol Chem. 2000;275:6651–6656. doi: 10.1074/jbc.275.9.6651. [DOI] [PubMed] [Google Scholar]
- 53.Gomez JA, Gama V, Yoshida T, Sun W, Hayes P, Leskov K, Boothman D, Matsuyama S. Bax-inhibiting peptides derived from Ku70 and cell-penetrating pentapeptides. Biochem Soc Trans. 2007;35:797–801. doi: 10.1042/BST0350797. [DOI] [PubMed] [Google Scholar]
- 54.Mazumder S, Plesca D, Kinter M, Almasan A. Interaction of a Cyclin E Fragment with Ku70 Regulates Bax-mediated Apoptosis. Mol Cell Biol. 2007 doi: 10.1128/MCB.01448-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romero F, Dargemont C, Pozo F, Reeves WH, Camonis J, Gisselbrecht S, Fischer S. p95vav associates with the nuclear protein Ku-70. Mol Cell Biol. 1996;16:37–44. doi: 10.1128/mcb.16.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang CR, Yeh S, Leskov K, Odegaard E, Hsu HL, Chang C, Kinsella TJ, Chen DJ, Boothman DA. Isolation of Ku70-binding proteins (KUBs) Nucleic Acids Res. 1999;27:2165–2174. doi: 10.1093/nar/27.10.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu P, Zhang D, Chowdhury D, Martinvalet D, Keefe D, Shi L, Lieberman J. Granzyme A, which causes single-stranded DNA damage, targets the double-strand break repair protein Ku70. EMBO Rep. 2006;7:431–437. doi: 10.1038/sj.embor.7400622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koike M, Shiomi T, Koike A. Ku70 can translocate to the nucleus independent of Ku80 translocation and DNA-PK autophosphorylation. Biochem Biophys Res Commun. 2000;276:1105–1111. doi: 10.1006/bbrc.2000.3567. [DOI] [PubMed] [Google Scholar]
- 59.Lim JW, Kim KH, Kim H. NF-kappaB p65 regulates nuclear translocation of Ku70 via degradation of heat shock cognate protein 70 in pancreatic acinar AR42J cells. Int J Biochem Cell Biol. 2008;40:2065–2077. doi: 10.1016/j.biocel.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 60.Muller C, Dusseau C, Calsou P, Salles B. Human normal peripheral blood B-lymphocytes are deficient in DNA-dependent protein kinase activity due to the expression of a variant form of the Ku86 protein. Oncogene. 1998;16:1553–1560. doi: 10.1038/sj.onc.1201676. [DOI] [PubMed] [Google Scholar]
- 61.Morimatsu M, Donoho G, Hasty P. Cells deleted for Brca2 COOH terminus exhibit hypersensitivity to gamma- radiation and premature senescence. Cancer Res. 1998;58:3441–3447. [PubMed] [Google Scholar]