Abstract
Objective
Smokers often smoke during stressful events, which leads to large increases in cardiovascular measures such as blood pressure (BP) and heart rate (HR). Since exaggerated cardiovascular response to stress is associated with cardiovascular disease risk, this study examined paroxetine’s effect on the physiological response to combining stress and smoking.
Methods
Sixty-two participants completed this randomized, double blind, cross-over study in which BP, HR, plasma epinephrine, norepinephrine (NE) and cortisol concentrations were measured at rest, while smoking and during a speech and math task. Laboratory sessions occurred after one month of paroxetine and after one month of placebo.
Results
Significant increases occurred for all measures (except cortisol) during smoking with further increases occurring during the speech task (time effect p values <0.001). After one month of paroxetine, NE and HR values were lower and cortisol values higher (vs. placebo) throughout the lab session (treatment effect p values < 0.001). Treatment × time effects were observed for blood pressure and heart rate (all p<0.01). For systolic and diastolic BP, a smaller increase (from baseline to measures during speech) was observed after paroxetine compared to placebo (both p <0.006). In both measures, the increase in response to smoking was similar for both treatments, however the further increase during the speech was smaller when taking paroxetine (vs. placebo).
Conclusions
This study suggests that paroxetine affects physiological response to stress in smokers. Further research is needed to determine the impact of these results on cardiovascular health.
Keywords: mental stress, smoking, paroxetine, heart rate, blood pressure, catecholamines
Introduction
When individuals are asked to do stressful activities in a laboratory setting (such as giving a speech or performing mental arithmetic problems), an increase in sympathetic output as well as activation of the hypothalamic-pituitary-adrenal axis occurs (1). These changes are associated with physiological responses including increases in heart rate, blood pressure, plasma catecholamine concentrations and plasma cortisol concentrations. Although there is much variability between individuals in the magnitude of the stress response, a given individual’s response to stress is relatively stable over time (2, 3). Both naturalistic and laboratory studies suggest that large responses to mental stress are associated with cardiovascular morbidity and mortality. For example, naturalistic studies have found that the rate of myocardial infarctions and cardiac death is increased following natural disasters, is increased in conjunction with work related stressors and is increased following an anger episode (4-7). Additionally, studies that have evaluated the magnitude of the physiological response (e.g. blood pressure, heart rate, plasma catecholamine concentrations) in the laboratory and then followed individuals for a prolonged period of time have found that greater cardiovascular reactivity is associated with poor cardiovascular risk status at follow-up (8). In fact, a study in which patients with stable coronary artery disease underwent both mental stress and exercise stress testing found that myocardial ischemia (assessed by radionuclide ventriculography) during mental stress was more predictive of future cardiac events than when observed during exercise stress (9). The association between larger cardiovascular responses to stress and poor cardiovascular outcome suggests that interventions that decrease the physiological response to stress could potentially have cardiovascular benefit.
Cigarette smokers are a particularly relevant population in which to assess interventions that reduce the physiological response to stress. Similar to stress, smoking a cigarette has also been found to increase heart rate, blood pressure and in many studies plasma catecholamine concentrations (10, 11). The cardiovascular risks associated with cigarette smoking have been well described with an estimated 128,000 annual cardiovascular disease deaths in the United States attributable to smoking (12). The potential mechanisms by which smoking contributes to cardiovascular disease are numerous and not completely understood but alterations in blood pressure and catecholamine concentrations may contribute to the development of atherosclerosis and vascular dysfunction associated with smoking (13). Since stress is among the two most commonly cited smoking triggers (14, 15) and has been found in laboratory studies to potentiate smoking (16), the co-occurrence of smoking and stress are likely to occur frequently. Studies have demonstrated that when stress and smoking are combined, the cardiovascular responses are additive (17, 18). This exaggerated sympathetic response that occurs in smokers during stressful stimuli may contribute to the well-established cardiovascular risks that smoking imparts (19).
One category of medications that may decrease the physiological response to stress is the selective serotonin reuptake inhibitors (SSRIs). These agents are known to be effective in the treatment of psychiatric diseases that are characterized by increases in autonomic activity (e.g. panic disorder, post-traumatic stress disorder) (20-23). Several studies suggest that in the treatment of these disorders, physiological response to stressful situations is attenuated (24, 25), as demonstrated by smaller heart rate and blood pressure increases after fluvoxamine therapy in patients with post-traumatic stress disorder who are asked to describe their traumatic event (24) and smaller blood pressure response in patients treated with paroxetine than in healthy controls (26). The effects of SSRIs on stress response in psychiatrically healthy individuals are unclear. Although several small studies suggest that SSRIs may have a similar effect in those without psychiatric diagnoses (27-29), further research is needed. Among the SSRIs, paroxetine is commonly described as being the most calming and is the only one currently approved by the United States Food and Drug Administration for the treatment of generalized anxiety disorder, panic disorder, post-traumatic stress disorder and social anxiety disorder (all of which are associated with autonomic responses during certain stressful situations). Additionally, small studies suggest that paroxetine may have effects on the physiological response to stress and it was therefore the agent chosen for this study (28, 29).
In summary, there is substantial evidence that larger cardiovascular responses to stress are associated with poor cardiovascular outcomes and that smoking and stress individually lead to cardiovascular responses that are additive when smoking and stress occur concurrently. The mechanisms by which smoking leads to cardiovascular morbidity and mortality are not completely understood but may be due (at least in part) to the cardiovascular response that occurs in response to stress and smoking. Smokers would therefore be an important population in which to test if an intervention could potentially decrease the physiological response to stress so that future studies can determine if such an effect results in cardiovascular benefit. There is some limited data suggesting that paroxetine may be an agent that decreases the physiological response to stress, however more data is needed to demonstrate if that is the case. The purpose of the current study was therefore to determine the effect of paroxetine on the physiological response that occurs after the combination of smoking and stress. Our hypothesis was that the physiological response to stress observed after taking paroxetine would be less than that observed after taking placebo.
Methods
Design
In this randomized, double blind, cross-over study, physiological response to mental stress was assessed after smoking a cigarette during two laboratory sessions: following a month of taking paroxetine and following a month of taking a matching placebo. After an initial screening visit at which eligibility was confirmed, all eligible and interested participants were randomized to receive either paroxetine or placebo. Paroxetine was started at 10 mg taken once daily for a week and then increased to 20 mg taken once daily for the remainder of the month (consistent with dosing when paroxetine is used clinically). One month of treatment was selected since this is typically the length of time required for paroxetine to begin exerting its effects when used in the treatment of psychiatric disorders (30). A washout period was not used since paroxetine’s half-life is approximately 24 hours and steady state concentrations are achieved within 1 to 2 weeks (31). One month should therefore be sufficient time for any effects of paroxetine to wear off and therefore no carry over effects should exist. After the screening visit, participants returned to the research clinic for a total of 5 additional visits. Three of the visits were short visits at which participants received study medication and two were longer laboratory sessions at which the physiological response to smoking combined with speech and math tasks was assessed. At each of these visits participants were asked to list any side effects they experienced since the previous visit regardless of whether they thought the side effects were related to the treatment. Each of the two laboratory sessions occurred after the participant took the study medication (i.e. paroxetine or placebo) for a 1 month period. Since sex and nicotine dependence could potentially have effects on stress response, randomization was stratified such that four approximately equally sized groups (i.e. male high dependence smokers, male low dependence smokers, female high dependence smokers, female low dependence smokers) were formed with order of treatment randomized within each group. Nicotine dependence was determined using the Fagerstrom Test for Nicotine Dependence (FTND) with a score of 6 or greater indicating high nicotine dependence (32). This cut-off is consistent with previous studies utilizing the FTND (32, 33).
Participants
Volunteers for this study were recruited through flyers and newspaper advertisement. To be eligible for the study, individuals had to be between the ages of 18 and 65 and smoke on average 10 or more cigarettes per day for the past year and have an expired carbon monoxide concentration of > 10 parts per million (ppm) at the time of screening. In explaining the rationale for using paroxetine in the absence of psychiatric disorders, volunteers were told that since smokers often report smoking in response to stress, the purpose of the study was to examine how a medication commonly used to treat anxiety disorders affects response to a stressful task in smokers. Individuals were excluded if they had any unstable or serious medical conditions at the time of screening, had abused substances other than nicotine within one year of beginning the study, used any medications that could have interfered with measures to be studied (e.g. psychoactive medications, antihypertensives) or that could interact with paroxetine (e.g. CYP2D6 substrates), had any psychiatric diagnosis as assessed by the PRIME-MD (34) or regularly used any form of tobacco other than cigarettes. Additionally, since this was not a smoking cessation study, anyone interested in quitting smoking in the 3 months subsequent to the screening visit was excluded from the study. Participants were recruited between November 2005 and August 2010. Written informed consent was obtained from all subjects and this study was approved by the University of Minnesota Institutional Review board.
Laboratory Sessions
Participants were asked to smoke normally until the evening prior to the day of each laboratory session but to abstain from smoking overnight prior to the session and the morning of the session. In order to facilitate blood draws an indwelling catheter was inserted into an arm vein and an automated sphygmomanometer was attached to the other arm. Participants then relaxed in a quiet room for a 30 minute period after which they smoked a single cigarette of their usual brand. They were given 5 minutes to smoke the cigarette and were then presented with a scenario involving an interpersonal conflict about which they would need to give a short speech. They were given 3 minutes to plan their speech and 3 minutes to deliver it. The speech was then played back to the participant. The speech task used is based on previously published methods and has been found to increase physiological measures of stress (such as heart rate, blood pressure and plasma catecholamine concentrations) in other studies (35-37). Immediately after the speech, the mental arithmetic task which consisted of performing a series of additions for a 3 minute period was explained to participants. This arithmetic task has been used previously and shown to increase physiological measures of stress (36, 38). A thirty minute relaxation period followed the conclusion of the mental arithmetic task. All laboratory sessions were started at the same time of day (i.e. between 8 and 10 AM in the morning) in order to minimize the effect of circadian differences on stress response.
Blood, from which plasma epinephrine, norepinephrine and cortisol concentrations were assayed, was drawn at the conclusion of the initial rest period, at the conclusion of the 5 minute smoking period, half-way through the speech task (when catecholamine response would be expected to be greatest), half-way through the math task, 15 minutes after the conclusion of the math task and 30 minutes after the conclusion of the math task (by which time cortisol concentrations could be expected to have increased). Blood pressure was recorded at 2 minute intervals during the relaxation periods and at 1 minute intervals during the stress tasks. Commercially available kits were used to determine plasma catecholamine and cortisol concentrations. Catecholamine concentrations were determined using extraction kits acquired though ESA Inc. (Chelmsford, MA) with concentrations quantified with electrochemical detection. The lower limit of quantitation for both epinephrine and norepinephrine concentrations was 10 pg/ml. For samples in which epinephrine concentrations were below the lower limit of quantitation, a value of 5 pg/ml was used for purposes of analysis. Cortisol concentrations were determined using enzyme immunoassay kits acquired from Calbiotech (Spring Valley, CA) the lower limit of quantitation for which was 0.5 μg/dL.
Statistical analysis
Given the completely nested design of this study, a hierarchical linear model was used to test for change across time within laboratory sessions and to examine treatment by time effects. We used SAS v. 9.2 PROC MIXED to conduct the analyses with period or draw, lab, and treatment condition as level 2 fixed effects and the intercept as a random effect. All tests of significance were two-tailed with threshold for significance at p<0.05.
Results
A total of 75 participants completed the first laboratory session and 62 participants completed both laboratory sessions. One additional participant completed both laboratory sessions but did not have usable cardiovascular measures and was not included in the analysis. In those that completed both laboratory sessions, 29 received paroxetine for the month prior to the first laboratory session (and therefore placebo for the month prior to the second laboratory session) and 33 received placebo for the month prior to the first laboratory session (and therefore paroxetine for the month prior to the second laboratory session). A summary of the demographic characteristics of participants at baseline is provided in Table 1. Comparing the demographics of participants who completed both laboratory sessions with those that dropped out after completing the first laboratory session did not find any significant differences between groups.
Table 1.
Baseline demographics for those assigned to paroxetine or placebo for the first month
| Paroxetine (n= 29) | Placebo (n= 33) | |
|---|---|---|
| Age: mean (SD) | 40.0 (11.0) | 40.4 (14.1) |
| Women: n (%) | 14 (48.3%) | 16 (48.5%) |
| Race | ||
| White: n (%) | 17 (58.6%) | 26 (78.8%) |
| African American: n (%) | 4 (13.8%) | 4 (12.1%) |
| Number of cigarettes per day: mean (SD) | 19.4 (8.7) | 18.2 (5.2) |
| Fagerstrom Test for Nicotine Dependence: mean (SD) | 4.8 (2.0) | 5.1 (1.8) |
| BMI: mean (SD) | 28.3 (5.9) | 26.8 (4.8) |
Of the 62 participants who completed the entire study, 56% self-reported at least one side effect while taking paroxetine and 55% reported at least one side effect while taking placebo (31% reported at least one side effect while taking both paroxetine and placebo). The most common side effects reported while taking paroxetine were tiredness / drowsiness (27%) and gastrointestinal symptoms (such as diarrhea, nausea, upset stomach, constipation) (21%). The most common side effects reported while taking placebo were tiredness / drowsiness (16%), gastrointestinal symptoms (11%) and feelings of anxiousness, irritability or frustration (13%).
Effect of treatment on blood pressure, heart rate and catecholamine concentrations among completers
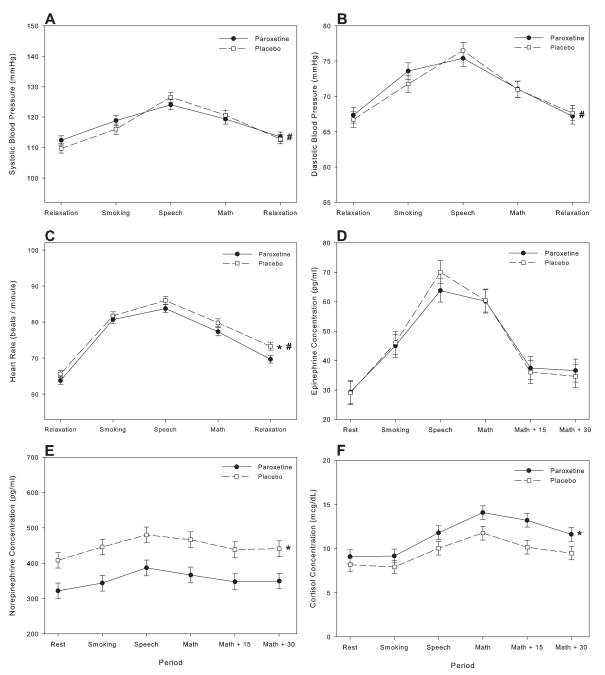
Among those who completed the study, a significant time effect (all p<0.001) was found for all of the physiological measures which would be expected to change rapidly in response to stress (i.e. systolic blood pressures (SBP), diastolic blood pressure (DBP), heart rate, plasma epinephrine concentration, plasma norepinephrine concentration). For all of these measures, concentrations increased between the initial baseline period and the post smoking period with an additional significant increase occurring from the end of the smoking period to the measures obtained during the speech task (Figure). There was then a significant decrease in all measures occurring between the speech and math task with an additional significant decrease occurring between the math task and the subsequent relaxation period.
Figure.
Systolic blood pressure (panel A), diastolic blood pressure (panel B), heart rate (panel C), plasma epinephrine concentrations (panel D), plasma norepinephrine concentrations (panel E) and plasma cortisol concentrations (panel F) (mean±SEM) after 1 month of paroxetine and placebo in participants who completed both laboratory sessions; M+15=15 min after math task, M + 30 = 30 min after math task
* p<0.001 for treatment effect
# p<0.01 for treatment × time effect
Significant treatment effects were found for plasma norepinephrine concentration and heart rate (p<0.001). Subjects had significantly lower values of these measures throughout the laboratory session after a month of paroxetine compared to measures observed after a month of placebo. No significant treatment effects were seen for any of the other measures. Significant treatment × time effects were observed for blood pressure and heart rate (all p<0.01) but not for plasma epinephrine or norepinephrine concentrations. For SBP and DBP, a smaller increase (when comparing baseline values to those obtained during the speech) was observed in participants when treated with paroxetine compared to treatment with placebo (F(1,60)=27.4, p<0.001; F(1,60)=8.04, p<0.006; respectively). In both of these measures, the increase observed in response to smoking was similar for both treatments, however the further increase that occurred during the speech was smaller when taking paroxetine than when taking placebo (table 2).
Table 2.
Physiological response among participants who completed both laboratory sessions (n=62)
| Resting value on paroxetine |
Resting value on placebo |
Increase from rest to smoking on paroxetine |
Increase from rest to smoking on placebo |
P value |
Increase from smoking to speech on paroxetine |
Increase from smoking to speech on placebo |
P value | Total increase on paroxetine* |
Total increase on placebo* |
P value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SBP (mmHG) |
112.26 (1.64) | 109.84 (1.64) | 6.54 (0.80) | 6.35 (0.81) | 0.16 | 5.17 (1.03) | 10.5 (1.03) | <0 .001 | 11.71 (0.78) | 16.85 (0.78) | <0.001 |
| DBP (mmHG) |
67.28 (1.17) | 66.94 (1.17) | 6.24 (0.51) | 5.03 (0.51) | 0.01 | 1.83 (0.65) | 4.75 (0.65) | 0.002 | 8.07 (0.50) | 9.78 (0.49) | 0.006 |
| HR (bpm) |
63.78 (1.00) | 65.62 (1.01) | 16.93 (0.58) | 16.14 (0.58) | 0.09 | 3.09 (0.74) | 4.25 (0.74) | 0.40 | 20.02 (0.56) | 20.39 (0.56) | 0.56 |
| Epi Conc (pg/ml) |
29.26 (3.92) | 29.00 (3.92) | 16.99 (3.42) | 17.98 (3.40) | 0.81 | 17.95 (3.49) | 23.0 (3.42) | 0.47 | 34.94 (3.48) | 40.98 (3.42) | 0.33 |
| NE Conc (pg/ml) |
321.83 (22.18) |
408.28 (22.18) |
17.47 (18.32) |
34.20 (18.32) |
0.52 | 45.90 (18.32) |
38.11 (18.32) |
0.71 | 63.37 (18.32) | 72.31 (18.32) |
0.82 |
SBP = Systolic Blood Pressure; DBP = Diastolic Blood Pressure; HR = Heart Rate; Epi Conc = Plasma epinephrine concentration; NE Conc = Plasma norepinephrine concentration
Total increase is from baseline to speech
Significant session × time effects were seen in heart rate, systolic and diastolic blood pressure with the maximal increase in these measures lower in the second laboratory session compared to the first (all p<0.001). This suggests that there was some attenuation of response (i.e. habituation) that occurred between the first and second laboratory session. We examined the effect of treatment order on differences in response between sessions. For those on paroxetine first, there was little difference between laboratory sessions. For those on placebo first, the second session appears to be more attenuated compared to the first. This was observed for heart rate (F(9,232)=20.76, p<0.001), SBP (F(9,232)=11.62, p<0.001) and DBP (F(9,232)=3.07, p <0.002).
Effect of treatment on cortisol among completers
For participants who completed the study, there were significant differences in cortisol levels across the measurement points [F(3,183)=37.5, p<0.001] with significant increases occurring from the measure obtained at the end of smoking to the measure obtained during speech (t(183)=4.92, p<0.001) and an additional increase occurring for the measure obtained during the math task (t(183)=9.07, p<0.001). Overall, when participants were taking paroxetine their cortisol levels were higher (11.00 mcg/dL) than when they were on placebo (9.47 mcg/dL) [F(1,60)=20.09, p<0.001]. However, no significant difference in change across time was found between the two conditions [F(3,183)=.84, p=0.48]. Nor were significant session × time effects found, with changes across the second laboratory session not significantly different than changes across the first session [F(3,180)=.22, p=0.88].
Effect of gender and FTND on physiologic measures
All the above analyses were repeated with sex and FTND included as covariates in the analyses. FTND was not a significant predictor of any of the physiologic measures. Women had significantly lower values on systolic blood pressure (t(59)=3.36, p=0.002), diastolic blood pressure (t(59)=2.26, p=0.02), plasma epinephrine (t(59)=3.96, p<0.001) and plasma cortisol concentrations (t(59)=3.39, p=0.002). The inclusion of gender and FTND however did not alter the results found in earlier analyses of treatment and treatment × time effects.
Effect of treatment on physiological measures among all participants who completed the first laboratory session
Among the 75 participants who completed the first laboratory session, 13 dropped out prior to the second laboratory session. Of these participants, 5 received paroxetine and 8 received placebo. Since cross-over and learning effects would not be a concern in an analysis only looking at the first laboratory session data, we report these results as additional confirmatory data regarding the effects of paroxetine on physiological response to stress.
A significant time effect was seen for all acutely reactive physiological measures with increases in heart rate, catecholamine, and blood pressure measures occurring during the smoking period and further during the stress period. Significant treatment × time effects were seen for systolic and diastolic blood pressure (F(1,71)=17.08, p<0.001; F(1,71)=9.20, p<0.004). The increase from smoking to speech in systolic and diastolic blood pressure is smaller in those receiving paroxetine than in those receiving placebo (consistent with the results of the cross-over analysis) (table 3). For cortisol, a significant time effect was seen (F(5,361)=20.97, p<0.001) but no significant treatment effect was observed. A significant treatment × time effect was found (F(5,361)=2.64, p=0.02) with greater cortisol increases observed in the paroxetine than in the placebo treated group.
Table 3.
Physiological response among all participants who completed the first lab session (n=34 for paroxetine; n=41 for placebo)
| Resting value on paroxetine |
Resting value on placebo |
Increase from rest to smoking on paroxetine |
Increase from rest to smoking on placebo |
P value | Increase from smoking to speech on paroxetine |
Increase from smoking to speech on placebo |
P value |
Total increase on paroxetine* |
Total increase on placebo* |
P value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SBP (mmHG) |
110.96 (2.37) |
109.09 (2.16) |
7.85 (0.87) | 4.13 (0.79) | <0.001 | 3.94 (1.10) | 15.46 (1.01) | <0.001 | 11.79 (0.83) | 19.59 (0.76) | <0.001 |
| DBP (mmHG) |
68.21 (1.60) | 65.83 (1.45) | 6.17 (0.56) | 4.08 (0.51) | 0.003 | 2.60 (0.71) | 7.05 (0.65) | <0.001 | 8.77 (0.54) | 11.13 (0.49) | <0.001 |
| HR (bpm) |
62.19 (1.63) | 64.65 (1.49) | 17.01 (0.59) | 16.78 (0.54) | 0.26 | 3.59 (0.92) | 4.83 (0.84) | 0.22 | 20.6 (0.69) | 21.61 (0.63) | 0.28 |
| Epi Conc (pg/ml) |
29.50 (5.63) | 29.49 (5.19) | 17.81 (4.66) | 17.3 (4.34) | 0.92 | 17.71 (4.83) | 23.98 (4.28) | 0.42 | 35.52 (4.82) | 41.28 (4.28) | 0.54 |
| NE Conc (pg/ml) |
349.72 (28.14) |
381.13 (25.95) |
11.45 (15.23) |
32.62 (14.04) |
0.29 | 56.22 (15.4) | 52.04 (14.04) |
0.93 | 67.67 (15.4) | 84.66 (14.04) |
0.54 |
SBP = Systolic Blood Pressure; DBP = Diastolic Blood Pressure; HR = Heart Rate; Epi Conc = Plasma epinephrine concentration; NE Conc = Plasma norepinephrine concentration
Total increase is from baseline to speech
Discussion
This study demonstrated that smoking a cigarette results in significant increases in blood pressure, heart rate and plasma catecholamine concentrations. Being subjected to a stressful situation immediately after smoking further increases these physiological parameters. Administration of paroxetine for one month did not attenuate the initial increase that occurred in physiological measures as a result of smoking but did attenuate the additional increase in blood pressure that occurred during the mental stress task. Overall, the increase in blood pressure that occurred when smoking is combined with stress was lower when smokers were receiving paroxetine than when receiving placebo. Additionally, paroxetine resulted in lower plasma norepineprhine concentrations and heart rate throughout the laboratory session. Since greater cardiovascular responses to stress, high plasma norepinephrine concentrations and elevated heart rate have been associated with poor cardiovascular outcomes (8, 39, 40), additional research is needed to determine if paroxetine may have cardiovascular benefits when used in smokers.
Our finding that smoking results in rapid blood pressure and heart rate increases is consistent with previously published data (10). Similarly, our findings that these measures increase further when stress is combined with smoking is consistent with previously published data demonstrating that stress and smoking produce greater effects on cardiovascular parameters than does smoking alone (17, 18). The effect of paroxetine (or other SSRIs more generally) on cardiovascular measures during times of stress, particularly in psychiatrically healthy individuals, has not been studied extensively. Several small studies assessing the effect of SSRIs in patients with psychiatric disorders that are characterized by increased autonomic response to certain situations (e.g. panic disorder, post-traumatic stress disorder, depression) have generally found a decreased response to stressful situations (24, 26, 41, 42). Since these studies were conducted in individuals with psychiatric disorders, it is not known if the observed effects are independent effects of the medication or are secondary to improvement of the psychiatric disorder being treated. There is currently very little data assessing the effects of SSRIs in those without psychiatric disorders, although a small study has found decreased heart rate and blood pressure response to stress in psychiatrically healthy individuals with a history of coronary artery disease and another has found similar results in obese individuals (27, 28). Our study adds to the literature by demonstrating an attenuated blood pressure response to the combination of stress and smoking. Considering that larger blood pressure responses to stress are associated with cardiovascular morbidity and mortality, studies that examine the effects of paroxetine on cardiovascular outcomes in non-depressed smokers are needed.
Although there were no differences in the magnitude of norepinephrine and heart rate increase in response to smoking or the stress tasks, norepinephrine concentrations and heart rate were lower overall during paroxetine administration. There is limited data regarding the effect of serotonergic antidepressants on plasma catecholamine concentrations; however a small study of 12 healthy volunteers found that short term administration of sertraline (an SSRI) resulted in decreased plasma norepinephrine appearance rate (43). Our finding of lower heart rate but no difference in blood pressure after a month of paroxetine than after a month of placebo is generally consistent with findings that SSRI use in the treatment of psychiatric disorders is associated with no change or slight decreases in resting heart rate and little effect on blood pressure (44, 45). Small studies in psychiatrically healthy volunteers similarly found sertraline to be associated with decreased resting heart rate (46) and paroxetine to be associated with lower heart rate throughout a laboratory session during which participants were asked to complete a mental stress task (28). The observed slightly (but statistically significantly) larger increase in DBP in response to smoking after paroxetine (vs. placebo) administration was not found with the other measures assessed. Future studies are therefore needed to determine if this effect is replicated and if there are any clinical implications for instances when smoking does not occur in the context of a stressful situations.
This study found that cortisol concentrations were higher in smokers when they were taking paroxetine than when they were taking placebo. Neither the mechanism nor the significance of these findings is clear. Single doses of SSRIs have been demonstrated to increase plasma cortisol concentrations (47, 48); however, longer term studies have been less consistent with most studies finding plasma, salivary or urinary cortisol to either be unchanged or decreased after SSRI administration (42, 49-51). These longer term treatment studies have generally measured cortisol concentrations in the context of treating psychiatric disorders such as depression, generalized anxiety disorder or panic disorder, which are associated with increased cortisol concentrations (50-52). It is therefore unclear from those earlier studies if the decreases in cortisol concentrations were independent effects of the SSRIs or secondary to improvement in psychiatric symptoms. The increase in cortisol in the current study suggests that the effects seen in previous studies may have been secondary to the resolution of psychiatric symptoms.
There are a number of limitations to the current study. One limitation is that, in order to best compare the two treatment conditions, the procedure was standardized such that smoking and stress occurred in a fixed order. It is likely however that in the natural environment there are times when stress precedes smoking and other times when stress and smoking occur concurrently. Although stress and smoking occurred in very close proximity to each other, it is nonetheless not known if the results would have been affected had the order in which stress and smoking occurred been altered. An additional limitation is that some habituation occurred to the speech stressor which likely resulted in the observed order effects. Habituation effects combined with the observed treatment effects likely explain the finding that attenuation of stress response was only observed in those receiving placebo first since in those receiving paroxetine first the habituation and treatment effects would have occurred at different lab session. The consistent results observed for physiological measures in both the cross-over analysis and in the analysis in which treatment effects were compared in only the first laboratory session provide evidence that the overall effects observed are due to treatment rather than habituation.
Other limitations of this study are related to the generalizability of these results to other patient populations and to other SSRIs. Specifically, since paroxetine did not attenuate the increase in blood pressure that occurred in response to smoking but only the incremental increase that occurred in response to the stress task that occurred after smoking, it is not known if a similar (or greater) effect would be seen in non-smokers without psychiatric disorders. If so, that would suggest potential benefits of SSRIs would not be limited to smokers. Similarly, it is not known if these results would generalize to other SSRIs. Although paroxetine is a commonly used SSRI, it is inappropriate for some patients to due to its side effect profile or the possibility of cytochrome P450 mediated drug interactions. Therefore it is necessary to determine if other SSRIs have similar effects.
Future studies are needed to determine the clinical relevance of these findings. Exaggerated responses to stress have been associated with the development and progression of coronary artery disease (8). Similarly, elevated concentrations of norepinephrine and increased heart rate have been associated with poor cardiovascular outcomes in patients with cardiovascular disease (39, 53). Further research is therefore needed to determine if paroxetine has cardiovascular benefit when used in smokers without psychiatric disorders. The clinical relevance of these findings as they relate to smoking cessation success also needs to be further evaluated. Studies have been inconsistent regarding the pattern of physiological stress response that is predictive of successful cessation (i.e. whether exaggerated or attenuated stress response is associated with successful smoking cessation) although differences in study design make it difficult to directly compare the various studies that have examined this association (54-57). Although SSRIs have not been found to be effective at generally increasing smoking cessation rates (58), research is needed to determine if paroxetine might be effective in the sub-population of smokers who are particularly responsive to stress.
In summary, this study found that one month of paroxetine administration (relative to placebo) resulted in an attenuated blood pressure response to a stress tasks when such a task is presented immediately after smoking a cigarette. Additionally, paroxetine administration resulted in lower heart rate and plasma norepinephrine concentrations throughout the laboratory procedure. Additional research is needed to determine the clinical implications of these results both as they apply to cardiovascular health and to smoking cessation efficacy.
Acknowledgements
This research was supported by Grant K23DA017307 to the first author from the National Institute on Drug Abuse and Grant M01-RR00400 from the General Clinical Research Centers program of the National Center for Research Resources. We would like to thank our research staff – Erin Syrjanen, Megan Mrozek, Lindsey Streamer and Elizabeth Amiot – for their work with participant recruitment and follow-up, as well as data collection, entry and management. We would also like to thank James Fisher and the Clinical Pharmacology Analytical Services and Laboratory for assistance with sample analysis.
Acronyms used in text
- DBP
Diastolic Blood Pressure
- FTND
Fagerstrom Test for Nicotine Dependence
- SBP
Systolic Blood Pressure
- SSRI
Selective Serotonin Reuptake Inhibitor
Footnotes
Trial Registration: www.clinicaltrials.gov # NCT00218439
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brotman DJ, Golden SH, Wittstein IS. The cardiovascular toll of stress. Lancet. 2007;370:1089–100. doi: 10.1016/S0140-6736(07)61305-1. [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Iwanaga K, Shimomura Y, Katsuura T. The reproducibility of cardiovascular response to a mental task. Journal of physiological anthropology. 2010;29:35–41. doi: 10.2114/jpa2.29.35. [DOI] [PubMed] [Google Scholar]
- 3.Jern S, Wall U, Bergbrant A. Long-term stability of blood pressure and pressor reactivity to mental stress in borderline hypertension. Am J Hypertens. 1995;8:20–8. doi: 10.1016/0895-7061(94)00157-7. [DOI] [PubMed] [Google Scholar]
- 4.Kivimaki M, Leino-Arjas P, Luukkonen R, Riihimaki H, Vahtera J, Kirjonen J. Work stress and risk of cardiovascular mortality: prospective cohort study of industrial employees. BMJ (Clinical research ed. 2002;325:857. doi: 10.1136/bmj.325.7369.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. N Engl J Med. 1996;334:413–9. doi: 10.1056/NEJM199602153340701. [DOI] [PubMed] [Google Scholar]
- 6.Strike PC, Perkins-Porras L, Whitehead DL, McEwan J, Steptoe A. Triggering of acute coronary syndromes by physical exertion and anger: clinical and sociodemographic characteristics. Heart (British Cardiac Society) 2006;92:1035–40. doi: 10.1136/hrt.2005.077362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mittleman MA, Maclure M, Sherwood JB, Mulry RP, Tofler GH, Jacobs SC, Friedman R, Benson H, Muller JE. Triggering of acute myocardial infarction onset by episodes of anger. Determinants of Myocardial Infarction Onset Study Investigators. Circulation. 1995;92:1720–5. doi: 10.1161/01.cir.92.7.1720. [DOI] [PubMed] [Google Scholar]
- 8.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55:1026–32. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- 9.Jiang W, Babyak M, Krantz DS, Waugh RA, Coleman RE, Hanson MM, Frid DJ, McNulty S, Morris JJ, O’Connor CM, Blumenthal JA. Mental stress-induced myocardial ischemia and cardiac events. JAMA. 1996;275:1651–6. doi: 10.1001/jama.275.21.1651. [DOI] [PubMed] [Google Scholar]
- 10.Benowitz NL, Porchet H, Sheiner L, Jacob P., 3rd Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clinical pharmacology and therapeutics. 1988;44:23–8. doi: 10.1038/clpt.1988.107. [DOI] [PubMed] [Google Scholar]
- 11.Grassi G, Seravalle G, Calhoun DA, Bolla GB, Giannattasio C, Marabini M, Del Bo A, Mancia G. Mechanisms responsible for sympathetic activation by cigarette smoking in humans. Circulation. 1994;90:248–53. doi: 10.1161/01.cir.90.1.248. [DOI] [PubMed] [Google Scholar]
- 12.CDC Annual Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivty Losses --- United States, 2000--2004. MMWR. 2008;57:1226–28. [PubMed] [Google Scholar]
- 13.Erhardt L. Cigarette smoking: an undertreated risk factor for cardiovascular disease. Atherosclerosis. 2009;205:23–32. doi: 10.1016/j.atherosclerosis.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: Within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64:366–79. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- 15.Borland R. Slip-ups and relapse in attempts to quit smoking. Addict Behav. 1990;15:235–45. doi: 10.1016/0306-4603(90)90066-7. [DOI] [PubMed] [Google Scholar]
- 16.McKee SA, Sinha R, Weinberger AH, Sofuoglu M, Harrison EL, Lavery M, Wanzer J. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. Journal of psychopharmacology (Oxford, England) 2011;25:490–502. doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacDougall JM, Dembroski TM, Slaats S, Herd JA, Eliot RS. Selective cardiovascular effects of stress and cigarette smoking. Journal of human stress. 1983;9:13–21. doi: 10.1080/0097840X.1983.9936125. [DOI] [PubMed] [Google Scholar]
- 18.Dembroski TM, MacDougall JM, Cardozo SR, Ireland SK, Krug-Fite J. Selective cardiovascular effects of stress and cigarette smoking in young women. Health Psychol. 1985;4:153–67. [PubMed] [Google Scholar]
- 19.Epstein LH, Perkins KA. Smoking, stress, and coronary heart disease. J Consult Clin Psychol. 1988;56:342–9. doi: 10.1037//0022-006x.56.3.342. [DOI] [PubMed] [Google Scholar]
- 20.Masand PS, Gupta S. Selective serotonin-reuptake inhibitors: an update. Harv Rev Psychiatry. 1999;7:69–84. [PubMed] [Google Scholar]
- 21.Martinez JM, Garakani A, Kaufmann H, Aaronson CJ, Gorman JM. Heart rate and blood pressure changes during autonomic nervous system challenge in panic disorder patients. Psychosom Med. 2010;72:442–9. doi: 10.1097/PSY.0b013e3181d972c2. [DOI] [PubMed] [Google Scholar]
- 22.Blechert J, Michael T, Grossman P, Lajtman M, Wilhelm FH. Autonomic and respiratory characteristics of posttraumatic stress disorder and panic disorder. Psychosom Med. 2007;69:935–43. doi: 10.1097/PSY.0b013e31815a8f6b. [DOI] [PubMed] [Google Scholar]
- 23.Esler M, Alvarenga M, Lambert G, Kaye D, Hastings J, Jennings G, Morris M, Schwarz R, Richards J. Cardiac sympathetic nerve biology and brain monoamine turnover in panic disorder. Annals of the New York Academy of Sciences. 2004;1018:505–14. doi: 10.1196/annals.1296.062. [DOI] [PubMed] [Google Scholar]
- 24.Tucker P, Smith KL, Marx B, Jones D, Miranda R, Lensgraf J. Fluvoxamine reduces physiologic reactivity to trauma scripts in posttraumatic stress disorder. J Clin Psychopharmacol. 2000;20:367–72. doi: 10.1097/00004714-200006000-00014. [DOI] [PubMed] [Google Scholar]
- 25.DeVane CL, Ware MR, Emmanuel NP, Brawman-Mintzer O, Morton WA, Villarreal G, Lydiard RB. Evaluation of the efficacy, safety and physiological effects of fluvoxamine in social phobia. Int Clin Psychopharmacol. 1999;14:345–51. doi: 10.1097/00004850-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Straneva-Meuse PA, Light KC, Allen MT, Golding M, Girdler SS. Bupropion and paroxetine differentially influence cardiovascular and neuroendocrine responses to stress in depressed patients. Journal of affective disorders. 2004;79:51–61. doi: 10.1016/S0165-0327(02)00352-X. [DOI] [PubMed] [Google Scholar]
- 27.Ljung T, Ahlberg AC, Holm G, Friberg P, Andersson B, Eriksson E, Bjorntorp P. Treatment of abdominally obese men with a serotonin reuptake inhibitor: a pilot study. J Intern Med. 2001;250:219–24. doi: 10.1046/j.1365-2796.2001.00881.x. [DOI] [PubMed] [Google Scholar]
- 28.Golding M, Kotlyar M, Carson SW, Hoyler S, Lazarus C, Davidson C, Guzzo J, Sontz E, Garbutt JC. Effects of paroxetine on cardiovascular response to mental stress in subjects with a history of coronary artery disease and no psychiatric diagnoses. Psychopharmacology (Berl) 2005;182:321–6. doi: 10.1007/s00213-005-0075-7. [DOI] [PubMed] [Google Scholar]
- 29.Golding M, Kotlyar M, Garbutt JC, Guzzo J, Sontz E, Hinderliter A, Carson SW. Paroxetine modulates psychological and sympathetic responses during public speaking. J Clin Psychopharmacol. 2002;22:98–9. doi: 10.1097/00004714-200202000-00020. [DOI] [PubMed] [Google Scholar]
- 30.Schatzberg AF. Pharmacological principles of antidepressant efficacy. Hum Psychopharmacol. 2002;17(Suppl 1):S17–22. doi: 10.1002/hup.399. [DOI] [PubMed] [Google Scholar]
- 31.Bourin M, Chue P, Guillon Y. Paroxetine: a review. CNS drug reviews. 2001;7:25–47. doi: 10.1111/j.1527-3458.2001.tb00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 33.Fagerstrom KO, Kunze M, Schoberberger R, Breslau N, Hughes JR, Hurt RD, Puska P, Ramstrom L, Zatonski W. Nicotine dependence versus smoking prevalence: comparisons among countries and categories of smokers. Tob Control. 1996;5:52–6. doi: 10.1136/tc.5.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spitzer RL, Williams JB, Kroenke K, Linzer M, deGruy FV, Hahn SR, Brody D, Johnson JG. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272:1749–56. [PubMed] [Google Scholar]
- 35.Koo-Loeb JH, Costello N, Light KC, Girdler SS. Women with eating disorder tendencies display altered cardiovascular, neuroendocrine, and psychosocial profiles. Psychosom Med. 2000;62:539–48. doi: 10.1097/00006842-200007000-00013. [DOI] [PubMed] [Google Scholar]
- 36.al’Absi M, Bongard S, Buchanan T, Pincomb GA, Licinio J, Lovallo WR. Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology. 1997;34:266–75. doi: 10.1111/j.1469-8986.1997.tb02397.x. [DOI] [PubMed] [Google Scholar]
- 37.Kotlyar M, Brauer LH, al’absi M, Adson DE, Robiner W, Thuras P, Harris J, Finocchi ME, Bronars CA, Candell S, Hatsukami DK. Effect of bupropion on physiological measures of stress in smokers during nicotine withdrawal. Pharmacol Biochem Behav. 2006;83:370–9. doi: 10.1016/j.pbb.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 38.al’Absi M, Lovallo WR, McKey B, Sung BH, Whitsett TL, Wilson MF. Hypothalamic-pituitary-adrenocortical responses to psychological stress and caffeine in men at high and low risk for hypertension. Psychosom Med. 1998;60:521–7. doi: 10.1097/00006842-199807000-00021. [DOI] [PubMed] [Google Scholar]
- 39.Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, Steg PG, Tardif JC, Tavazzi L, Tendera M. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50:823–30. doi: 10.1016/j.jacc.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 40.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–23. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 41.Scalco AZ, Rondon MU, Trombetta IC, Laterza MC, Azul JB, Pullenayegum EM, Scalco MZ, Kuniyoshi FH, Wajngarten M, Negrao CE, Lotufo-Neto F. Muscle sympathetic nervous activity in depressed patients before and after treatment with sertraline. J Hypertens. 2009;27:2429–36. doi: 10.1097/HJH.0b013e3283310ece. [DOI] [PubMed] [Google Scholar]
- 42.Vermetten E, Vythilingam M, Schmahl C, C DEK, Southwick SM, Charney DS, Bremner JD. Alterations in stress reactivity after long-term treatment with paroxetine in women with posttraumatic stress disorder. Annals of the New York Academy of Sciences. 2006;1071:184–202. doi: 10.1196/annals.1364.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shores MM, Pascualy M, Lewis NL, Flatness D, Veith RC. Short-term sertraline treatment suppresses sympathetic nervous system activity in healthy human subjects. Psychoneuroendocrinology. 2001;26:433–9. doi: 10.1016/s0306-4530(01)00002-6. [DOI] [PubMed] [Google Scholar]
- 44.Alvarez W, Jr., Pickworth KK. Safety of antidepressant drugs in the patient with cardiac disease: a review of the literature. Pharmacotherapy. 2003;23:754–71. doi: 10.1592/phco.23.6.754.32185. [DOI] [PubMed] [Google Scholar]
- 45.Licht CM, de Geus EJ, Seldenrijk A, van Hout HP, Zitman FG, van Dyck R, Penninx BW. Depression is associated with decreased blood pressure, but antidepressant use increases the risk for hypertension. Hypertension. 2009;53:631–8. doi: 10.1161/HYPERTENSIONAHA.108.126698. [DOI] [PubMed] [Google Scholar]
- 46.Siepmann M, Grossmann J, Muck-Weymann M, Kirch W. Effects of sertraline on autonomic and cognitive functions in healthy volunteers. Psychopharmacology (Berl) 2003;168:293–8. doi: 10.1007/s00213-003-1448-4. [DOI] [PubMed] [Google Scholar]
- 47.Hawken ER, Owen JA, Hudson RW, Delva NJ. Specific effects of escitalopram on neuroendocrine response. Psychopharmacology (Berl) 2009;207:27–34. doi: 10.1007/s00213-009-1633-1. [DOI] [PubMed] [Google Scholar]
- 48.Kojima H, Terao T, Iwakawa M, Soya A, Inoue N, Shiraishi Y, Son Y, Soeda S, Ueda N, Yoshimura R, Nakamura J. Paroxetine as a 5-HT neuroendocrine probe. Psychopharmacology (Berl) 2003;167:97–102. doi: 10.1007/s00213-003-1406-1. [DOI] [PubMed] [Google Scholar]
- 49.Deuschle M, Hamann B, Meichel C, Krumm B, Lederbogen F, Kniest A, Colla M, Heuser I. Antidepressive treatment with amitriptyline and paroxetine: effects on saliva cortisol concentrations. J Clin Psychopharmacol. 2003;23:201–5. doi: 10.1097/00004714-200304000-00014. [DOI] [PubMed] [Google Scholar]
- 50.Lenze EJ, Mantella RC, Shi P, Goate AM, Nowotny P, Butters MA, Andreescu C, Thompson PA, Rollman BL. Elevated cortisol in older adults with generalized anxiety disorder is reduced by treatment: a placebo-controlled evaluation of escitalopram. Am J Geriatr Psychiatry. 2011;19:482–90. doi: 10.1097/JGP.0b013e3181ec806c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muck-Seler D, Pivac N, Sagud M, Jakovljevic M, Mihaljevic-Peles A. The effects of paroxetine and tianeptine on peripheral biochemical markers in major depression. Progress in neuro-psychopharmacology & biological psychiatry. 2002;26:1235–43. doi: 10.1016/s0278-5846(02)00259-2. [DOI] [PubMed] [Google Scholar]
- 52.Mantella RC, Butters MA, Amico JA, Mazumdar S, Rollman BL, Begley AE, Reynolds CF, Lenze EJ. Salivary cortisol is associated with diagnosis and severity of late-life generalized anxiety disorder. Psychoneuroendocrinology. 2008;33:773–81. doi: 10.1016/j.psyneuen.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benedict CR, Shelton B, Johnstone DE, Francis G, Greenberg B, Konstam M, Probstfield JL, Yusuf S. Prognostic significance of plasma norepinephrine in patients with asymptomatic left ventricular dysfunction. SOLVD Investigators. Circulation. 1996;94:690–7. doi: 10.1161/01.cir.94.4.690. [DOI] [PubMed] [Google Scholar]
- 54.al’Absi M, Hatsukami D, Davis GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology (Berl) 2005;181:107–17. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- 55.Emmons KM, Weidner G, Collins RL. Smoking cessation and cardiovascular reactivity to stress. Journal of behavioral medicine. 1989;12:587–98. doi: 10.1007/BF00844827. [DOI] [PubMed] [Google Scholar]
- 56.Niaura R, Abrams DB, Monti PM, Pedraza M. Reactivity to high risk situations and smoking cessation outcome. J Subst Abuse. 1989;1:393–405. [PubMed] [Google Scholar]
- 57.Swan GE, Ward MM, Jack LM, Javitz HS. Cardiovascular reactivity as a predictor of relapse in male and female smokers. Health Psychol. 1993;12:451–8. doi: 10.1037//0278-6133.12.6.451. [DOI] [PubMed] [Google Scholar]
- 58.Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane database of systematic reviews (Online) 2007:CD000031. doi: 10.1002/14651858.CD000031.pub3. [DOI] [PubMed] [Google Scholar]