Abstract
Kynurenic acid (KYNA), a major tryptophan metabolite, is a glutamate receptor antagonist, which is also reported to inhibit α7 nicotinic acetylcholine receptors (α7nAChRs). Due to variations in experimental approaches, controversy has arisen regarding the ability of KYNA to directly influence α7nAChR function. Here we summarize current concepts of KYNA neurobiology and review evidence pertaining to the proposed role of KYNA as an endogenous modulator of α7nAChRs and synaptic transmission. As dysfunction of α7nAChRs plays a major role in the pathophysiology of central nervous system disorders, elucidation of KYNA's action on this receptor subtype has significant therapeutic implications.
Kynurenic acid (KYNA), a neuroactive metabolite of the kynurenine pathway (KP) of tryptophan degradation, has received considerable attention from neurobiologists. Thus, as a non-selective antagonist of ionotropic glutamate receptors at high micromolar to millimolar concentrations, KYNA is widely used as a pharmacological tool to experimentally eliminate activation of all these receptors in vivo or in vitro. Moreover, endogenous KYNA has remarkable neuromodulatory properties, which do not appear to be initiated solely by KYNA’s ability to block glutamate receptors directly. Finally, the pharmacological manipulation of KYNA formation and disposition in the brain offers exciting new venues for the treatment of neurological and psychiatric diseases. Here we will briefly describe current knowledge regarding the main features of KYNA neurobiology and then discuss in greater depth the proposed role of α7 nicotinic acetylcholine receptors (α7nAChRs) as functionally important physiological targets of KYNA in the brain.
Synthesis and elimination of brain KYNA
Measured in crude tissue homogenates, KYNA concentrations in the mammalian brain are normally in the nanomolar range [1]. Remarkably, and of possible biological significance, KYNA levels are at least one order of magnitude higher in the human brain compared to other species [2]. As KYNA is not actively transported through the blood-brain barrier [3], its levels in the brain are normally determined by local neosynthesis from its brain-penetrable bioprecursor, the pivotal KP metabolite kynurenine. The irreversible transamination of kynurenine is catalyzed by several kynurenine aminotransferases (KATs), of which KAT II is primarily responsible for the rapid mobilization of neuroactive KYNA [4]. Cerebral KAT II is localized almost exclusively in astrocytes [5], which avidly accumulate kynurenine using the large neutral amino acid transporter [6], so that these glial cells are the major source of KYNA in the brain. Notably, KYNA synthesis in astrocytes is not only driven by the concentration of kynurenine, though the high Km values of all KATs assure that rising levels of endogenous kynurenine (normal concentration in the mammalian brain: ~2 µM; [7]) will result in proportional increases in KYNA production [8]. KYNA formation is also regulated by the availability of glucose and by the intracellular concentration of 2-oxoacids such as pyruvate, 2-oxoglutarate and oxaloacetate, which act as co-substrates in the enzymatic transamination process [9]. Furthermore, astrocytic KYNA formation is reduced by neuronal signals such as glutamate and other depolarizing stimuli [10]. Jointly, these mechanisms, which still need to be elaborated in detail, account for the production of KYNA within the glial cells and for its subsequent prompt liberation into the extracellular compartment. Efforts to characterize the cellular or molecular machinery underlying KYNA release from astrocytes have so far been unsuccessful. Similarly, no re-uptake processes for KYNA have been identified in the brain [2].
KYNA serves as a substrate for the probenecid-sensitive organic anion transporters OAT1 and OAT3, which are present on brain capillary endothelial cells, and it is likely that these proteins play an important role in the active removal of KYNA from the brain’s extracellular compartment [1,11]. In other words, it appears that the ambient extracellular KYNA concentration in the brain, under physiological conditions in vivo, is jointly determined by precursor availability, regulation of biosynthesis within astrocytes, and efflux mediated by non-specific, endothelial anion transporters.
KYNA function in brain physiology and pathology
Based on assays of human cerebrospinal fluid [12] and on microdialysis studies in rats and mice [13,14], the steady-state extracellular concentration of KYNA in the brain in vivo is commonly reported to be in the low nanomolar range. However, considering the tight apposition of astrocytic processes and pre- and postsynaptic elements (referred to as the “tripartite synapse”; [15]), the local concentration of newly formed, astrocyte-derived KYNA at neuronal receptor sites can be reasonably expected to be substantially higher, though it cannot be accurately determined with currently available methods. This issue is naturally important, and may in fact be critical, when debating the nature of KYNA’s functional receptor targets (see below). From a neurobiological perspective, it is noteworthy that even relatively minor fluctuations in brain KYNA levels have pronounced consequences, influencing prominent neurotransmitters such as dopamine and glutamate [16–20] and affecting cognitive and motor behaviors [21–24] in experimental animals. Awareness of these bi-directional effects has led to the hypothesis that endogenous KYNA functions as a significant neuromodulator in the mammalian brain [25]. Notably, this role likely extends to pathological situations, which frequently present with significant changes in brain KYNA levels. Examples include catastrophic neurodegenerative disorders (e.g. Huntington’s disease and Alzheimer’s disease [26,27]) as well as psychiatric diseases (e.g. schizophrenia; [12,28]) and diseases caused by viruses or parasites [29,30]). Therefore, understanding the nature and characteristics of the molecular target(s) mediating the effects of KYNA in the brain has implications for brain physiology and pathology alike.
Receptor targets for KYNA: an assortment of riches, and the case for α7nAChRs
KYNA’s neuroinhibitory qualities [31,32] and its neuroprotective and anticonvulsant effects [33] were discovered using concentrations of the compound in the millimolar range. This, as well as the low affinity of KYNA at each of the three ionotropic glutamate receptors responsible for these effects [NMDA, alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and kainate], together with the realization that KYNA concentrations in the mammalian brain are in the sub-micromolar range (see above), suggested that other receptors might serve as targets of endogenous KYNA (see Table 1). Indeed, KYNA was soon found to inhibit the obligatory glycine co-agonist site of the NMDA receptor (the “glycineB” receptor) competitively at a much lower concentration, with an IC50 of approximately 10 µM [34]. A decade later, we reported that KYNA, with a shallower inhibition curve and non-competitively, antagonizes α7nAChRs on cultured hippocampal neurons with an IC50 in the low micromolar range and proposed that these receptors might be preferred targets of endogenous KYNA [35]. Several follow-up studies, by our laboratories and others, and using neurochemical and electrophysiological outcome measures in vitro and in vivo, were consistent with the idea that α7nAChRs could be a biologically significant target of KYNA in the mammalian brain [17,36–40]. α7nAChRs are certainly critically involved in the actions of endogenous KYNA, as galantamine, an agent acting competitively as an agonist at an allosteric receptor site similar to that targeted by KYNA [41], readily neutralizes the neurochemical and behavioral effects of nanomolar concentrations of KYNA in vivo [20,42]. However, these studies do not provide unequivocal proof that endogenous KYNA targets α7nAChRs directly and selectively. In fact, low concentrations of KYNA have also been proposed to positively modulate AMPA receptors [43] and, more recently, were found to stimulate the G-protein-coupled receptor GPR35 [44], the arylhydrocarbon receptor [45]. Moreover, KYNA can also affect another as yet undefined target, as shown by dissecting KYNA-induced inhibition of glutamatergic activity in hippocampal CA1 pyramidal neurons [46,47]. Alone or together, additional receptors may therefore also contribute to the role of KYNA in brain physiology and/or pathology, though GPR35 and the arylhydrocarbon receptor may be especially important as KYNA targets in peripheral organs [48].
Table 1.
Sensitivity of various biological receptors/targets for the actions of KYNA
| Receptors | Locations | Effect | KYNA’s IC50/EC50 (µM) |
References |
|---|---|---|---|---|
| AMPA/ kainate |
CA1 region CA1 region |
Inhibition of DC potential Inhibition of synaptic currents |
500–1000 µM ~500 µM |
Ganong & Cotman, 1986 [61] see review Stone, 1993 [62] |
| Heterologous GluR2 subunits |
Inhibition of currents | 1000 –1900 µM | Prescott et al., 2006 [43] | |
| CA1 region | Enhancement of fEPSPs | ~10 µM | Prescott et al., 2006 [43] | |
| Inhibition of fEPSPs | 1000 µM | Prescott et al., 2006 [43] | ||
| NMDA | Cerebral cortex | Inhibition of spike activity induced by quisqualate and NMDA |
<50 mM | Perkins and Stone, 1982 [63] |
| Dentate gyrus | Inhibition of DC potential | ~100 µM | Ganong et al., 1983 [64] | |
| CA1 region | Inhibition of depolarization | ~100 µM | Ganong & Cotman, 1986 [61] | |
| Cortical membrane GlycineB site |
Inhibition of binding of [3H]glycine [3H]MDL 105519 |
~15 µM ~8 µM |
Kessler et al., 1989 [65] Parsons et al., 1997 [66] |
|
| Hippocampal neuron in culture |
Inhibition of currents | 15 µM (no glycine) 235 µM (10 µM glycine) |
Hilmas et al., 2001 [35] | |
| Heterologous NR1a and NR2A |
Inhibition of currents | 24.4 µM (1 µM glycine) 158 µM (30 µM glycine) |
Mok et al., 2009 [49] | |
| GABAA | Hippocampal neuron in culture |
Inhibition of currents | 2900 µM | Mok et al., 2009 [49] |
| α7 nAChR | Hippocampal neuron in culture |
Inhibition of currents | 7 – 14 µM | Hilmas et al., 2001 [35] Lopes et al., 2007 [41] |
| Interneuron in hippocampal slice |
Inhibition of currents | 100 – 136 µM | Stone, 2007 [38] Alkondon et al., 2011 [40] see review Stone et al, 2012 [67] |
|
| GPR35 | Transfected CHO cells |
Activation in aequorin assay |
7.4 µM (rat) 39.2 µM (human) |
Wang et al., 2006 [44] |
| U2OS cells | Trafficking of βarr2-GFP | 217 µM (human) | Zhao et al., 2010 [68] | |
| AHR* | HepG2 40/6 cell line | Induce transcriptional activity | ~300 nM (human) | DiNatale et al., 2010 [69] |
| Synaptic transmission |
CA1 pyramidal neuron in hippocampal slice |
Inhibition of MLA-sensitive spontaneous GABA transmission |
20 –200 µM | Banerjee et al., 2012 [52] |
| Synaptic transmission |
CA1 pyramidal neuron in hippocampal slice |
Inhibition of MLA-sensitive spontaneous glutamate transmission |
~ 1 µM | Banerjee et al., 2012 [47] |
| Excitability | CA1 stratum radiatum Interneurons |
Inhibition of spontaneous action potentials |
< 2 µM | Alkondon et al., 2011 [46] |
AHR = Aryl Hydrocarbon receptor
Whereas the effects of KYNA on other receptors are not controversial, some investigators have failed to observe an inhibition of α7nAChR currents even by high (millimolar) concentrations of KYNA [49,50]. Because of the conceptual significance of these negative data for a spectrum of (patho)physiological events involving brain KYNA, and in an attempt to reconcile seemingly disparate experimental results, we decided to re-evaluate all relevant published studies in depth.
Methodological considerations: effects of KYNA on α7nAChRs in vitro
The present re-assessment of the action of KYNA at α7nAChRs focuses primarily on methodological issues, which we believe to hold the key to the contrasting results obtained in different laboratories. In particular, we concentrate our attention on the following experimental variables and approaches, presented in the order of their importance: (i) method of agonist application to study α7nAChR currents; (ii) cell culture versus brain slices; (iii) the presence or absence of cell dialysis in the various experimental protocols; (iv) KYNA’s effects on GABAergic vs. glutamatergic transmission; (v) native vs. heterologously expressed α7nAChRs; (vi) age differences in animals or neuronal cultures; and (vii) the vehicle used to dissolve KYNA for experimental use.
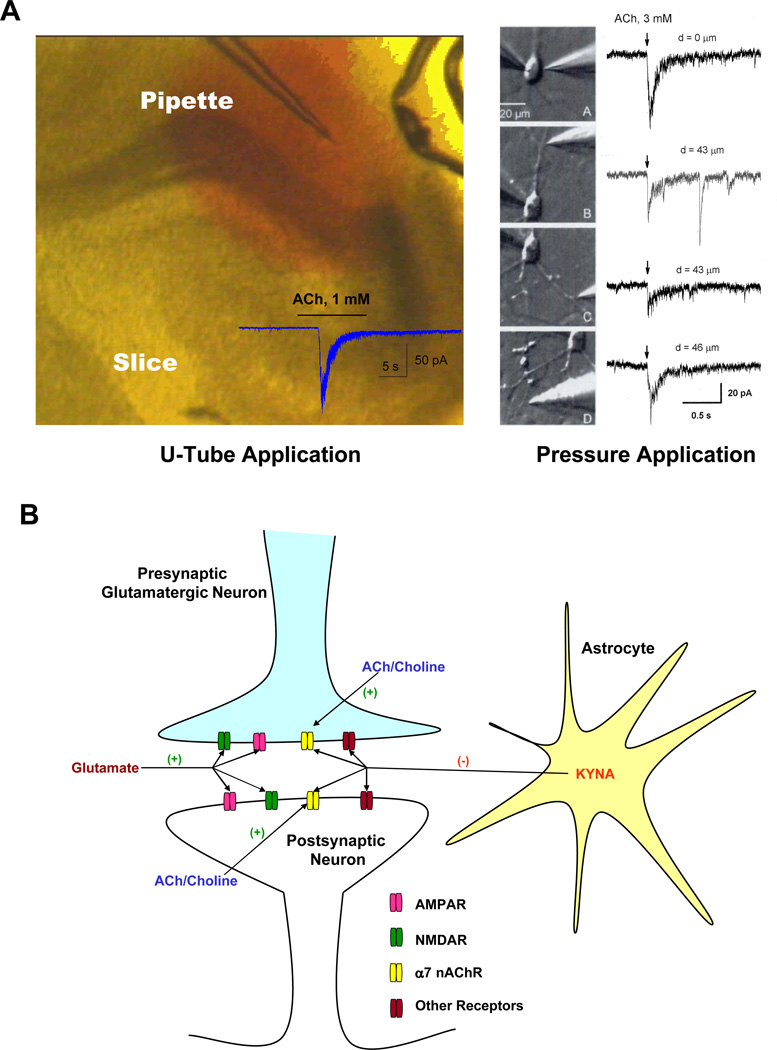
Method of agonist application
Two methods are routinely used in many laboratories to apply cholinergic agonists to neurons to study α7nAChR currents. In the pressure application method, a patch pipette with a tip diameter of <1 µm is typically filled with the agonist, positioned near the soma of the targeted neuron, and the test agent is ejected for periods of 10–100 msec at 10–15 pSi. This method prevents agonist leak and possible desensitization of α7nAChRs, and allows one to reliably study receptors located in highly localized regions of the neuron. Thus, if the pipette releasing the agonist is located close to the cell soma, only somatic α7nAChRs will be activated. In an alternative method, the use of a U-tube also prevents leak of the agonist and possible desensitization of receptors, but allows application of the agonist over a wide region of the neuron, activating both somatic and dendritic α7nAChRs [51]. This U-tube method was used by Hilmas et al. [35] and Alkondon et al. [40] to identify and study the inhibitory effect of KYNA on α7nAChRs. In contrast, only somatic α7nAChRs may have been activated in the studies of Mok et al. [49] and Dobelis et al. [50], who used the pressure ejection system in their failed attempts to observe KYNA inhibition of α7nAChRs. The divergent results might therefore indicate that α7nAChRs located in cell somata and dendrites have different properties, including different sensitivities to KYNA, by virtue of having different composition or association with different sets of auxiliary proteins (see below). In fact, we very recently showed that the effect of MLA on GABAergic transmission is larger than that of α-BGT [52], possibly due to the ability of MLA to inhibit heteromeric α7nAChRs in addition to blocking homomeric α7nAChRs. Yu and Role [59], in agreement with our previous findings [60], also suggested the presence of both homomeric and heteromeric α7nAChRs based on the sensitivity of the low conductance channel to α-BGT and the sensitivity of the high conductance channel to MLA.. The wavering effects of KYNA on α7nAChRs may therefore be caused by the differential sensitivity of homomeric and heteromeric α7nAChRs to KYNA. The location of homomeric and heteromeric α7nAChRs on the somatic and/or dendritic region could add further to the complexities of mechanism of action of KYNA clearly requires additional investigation.
Cell culture versus brain slices
In the original study reporting inhibition of α7nAChR currents by KYNA, we noted that the potency of KYNA on cultured hippocampal neurons (IC50: 7 µM) exceeded the efficacy of the inhibitor at interneurons in hippocampal slices (IC50: ~100 µM) [35]. Subsequent experiments using hippocampal slices and various experimental modalities such as whole-cell recording of α7nAChR currents, recording of α7nAChR-dependent spontaneous and evoked GABAergic IPSCs, and cholinergic fiber-stimulated α7nAChR synaptic currents, confirmed that α7nAChRs on interneurons have a comparatively low sensitivity to KYNA (tested in the range of 10–200 µM; [38,40,52]. This difference was not due to slow diffusion of the test compound in the slices, because KYNA efficiently antagonized glutamate- and NMDA-evoked EPSCs under the same experimental conditions [38,40]. Re-examination of the results of the original study [35] revealed that the majority of neurons examined in hippocampal neuron cultures were excitatory pyramidal neurons rather than inhibitory interneurons. This suggests that α7nAChRs on pyramidal neurons may have different properties and, in particular, may be preferentially sensitive to KYNA compared to α7nAChRs on GABAergic interneurons (see below). In fact, our recent experiments in hippocampal slices confirmed that α7nAChR-dependent glutamatergic transmission is more susceptible to inhibition by KYNA than α7nAChR-dependent GABAergic transmission [47,52].
Role of cell dialysis
Cell dialysis during whole-cell patch-clamp recordings may interfere with the inhibitory action of KYNA on α7nAChRs. When α7nAChR-containing cells are intact, as in experiments in which choline-induced GABAergic PSCs are recorded, reversible inhibition by KYNA can be reliably elicited [41]. Moreover, though these effects are indirect, a robust reduction in choline-induced GABAergic PSCs can be readily demonstrated in stratum radiatum interneurons of post-weaned rats when slices are incubated with either kynurenine or KYNA [40]. During whole-cell patch-clamp experiments, cell dialysis is complete in cell soma, whereas it is incomplete in dendritic compartments. Compared to dendritic α7nAChRs, dialysis therefore preferentially affects α7nAChRs on cell somata, removing functionally relevant components from the intracellular milieu and resulting in “run down” of these receptors [53]. If the binding of KYNA to α7nAChRs depends on such intracellular factors, KYNA’s inhibitory action would be lost under these circumstances. It is likely that this situation applied to the studies of Mok et al. [49] and Dobelis et al. [50], as both studies investigated predominantly somatic α7nAChRs. In contrast, studies where an inhibitory effect of KYNA was observed [35,40,41] involved dendritic α7nAChRs that were spared the actions of dialysis.
KYNA’s effects on GABAergic vs. glutamatergic transmission
In recent experiments using hippocampal slices, whole cell recordings of spontaneous GABAergic IPSCs in CA1 pyramidal neurons revealed a decrease in frequency in the presence of the α7nAChR antagonists MLA or α-bungarotoxin (α-BGT) [47,52]. This indicates that basal levels of choline or ACh activate α7nAChRs, which in turn contribute to GABAergic synaptic transmission. Exposure of the slices to kynurenine (20 to 200 µM) also caused a significant reduction in the frequency of GABAergic events. This effect implied a role of endogenously synthesized KYNA and was, in fact, duplicated by the direct application of KYNA (20 to 200 µM) [52]. Notably, while the effect of 20 µM kynurenine was occluded by MLA or α-BGT, 200 µM kynurenine suppressed IPSCs even in the presence of the α7nAChR antagonists [52], suggesting a contribution of mechanisms independent of α7nAChR function in the action of kynurenine. Interestingly, the frequency of spontaneous glutamatergic EPSCs recorded from CA1 pyramidal neurons is attenuated by KYNA concentrations as low as 1 µM, and this effect is also occluded in the presence of MLA or α-BGT [47]. In other words, α7nAChR-dependent glutamatergic transmission is more sensitive to KYNA than α7nAChR-dependent-GABAergic transmission. At higher concentrations (>5 µM), KYNA was effective even in the presence of α7nAChR antagonists [47]. These findings provide further evidence that KYNA inhibits α7nAChR-dependent synaptic activity, but the contribution of additional mechanisms independent of α7nAChR function to the action of KYNA cannot be ruled out (see Figure 1).
Figure.
Astrocyte-derived KYNA inhibits glutamatergic synaptic activity in CA1 area of the hippocampus. This simplified scheme illustrates the mechanism of action of low micromolar concentration of KYNA in modulating synaptic transmission between a presynaptic glutamate neurons and a postsynaptic CA1 pyramidal neuron. For ease of understanding, receptors are shown on the terminals of the pre-synaptic neuron, but they may also be present in the somato-dendritic region, on axons or on any other pre-terminal region; and all these receptors can be affected by astrocyte-derived KYNA. KYNA inhibits glutamatergic transmission to the CA1 pyramidal neuron via α7nAChR-dependent and -independent mechanisms [47]. Thus, it is plausible that receptors other than α7nAChRs on the glutamate neuron might also be inhibited by endogenous KYNA, thereby affecting glutamatergic transmission [46, 47]. However, low concentrations of KYNA (1–2 µM) are unlikely to inhibit NMDA or AMPA receptors because of its lower potency on these receptors (see Table 1). This indicates that the effect of KYNA on synaptic transmission is a complex phenomenon involving multiple receptor functions.
Native vs. heterologously expressed α7nAChRs
KYNA-induced inhibition of α7nAChRs may depend on (changes in) the expression of functional surface receptors, which is controlled by proteins such as Ric-3 [54]. Lack of, or abnormalities in, such regulatory mechanisms in heterologous-expression systems, like the Xenopus oocytes used by Mok et al. [49], could cause α7nAChRs to become insensitive to KYNA. Moreover, the composition and association with auxiliary proteins of α7nAChRs may be different between somatic and dendritic sites, and therefore their sensitivity to pharmacological agents could be different. For example, AMPA receptors, when associated with TARP proteins, change pharmacological sensitivity to CNQX. CNQX becomes an agonist under these conditions [55].
Age differences
Both in cell culture and in intact animals, neuronal maturation appears to play an important role in determining the inhibitory potency of KYNA at α7nAChRs. Hilmas et al. [35] used hippocampal cultures at 7–42 days after plating, but older cultures, which present with larger α7nAChR currents [56](Alkondon and Albuquerque, 1991), were preferentially used to reveal the inhibitory effect of KYNA. In contrast, Mok et al. [49] used hippocampal cultures between 7 and 18 days after plating. Although possible changes in sensitivity to KYNA of α7nAChRs with the number of days after plating have so far not been investigated systematically, recent experiments provide support for an age-dependency of KYNA’s inhibitory effect in rats. Thus, in CA1 stratum radiatum interneurons, sensitivity of α7nAChR currents to inhibition by KYNA was found to increase with age, as KYNA significantly inhibited currents in from postweaned [postnatal day (P)23–P35] rats but was far less effective in neurons from preweaned (P10-P18 days) animals [40]. This age-related change in sensitivity could contribute to the failure of Mok et al. [49] to observe inhibition of α7nAChR currents by KYNA in rat stratum radiatum interneurons on P12-P24.
Vehicle used to dissolve KYNA
As KYNA is not readily soluble in water, the compound must be dissolved in an appropriate vehicle before use. In the study of Hilmas et al. [35], KYNA was dissolved in DMSO, prompting Mok et al. [49] to suggest that α7nAChR inhibition by KYNA might be a DMSO-induced artifact. However, corresponding concentrations of DMSO were used in all control experiments [35), and DMSO (10 µM) has no effect on the acetylcholine-evoked α7nAChR currents in Xenopus oocytes [57]. To further rule out the possibility of any influence of DMSO, we recently studied the effect of KYNA dissolved in NaOH (at physiological final pH). Under these conditions, too, KYNA reduced agonistevoked α7nAChR currents in hippocampal interneurons [40] and GABAergic [52] as well as glutamatergic [47] transmission in CA1 pyramidal neurons.
Concluding remarks
Careful evaluation of the effects of KYNA on α7nAChRs in the rodent hippocampus leave no doubt that the metabolite can indeed antagonize receptor function and should therefore be considered a bona fide endogenous receptor modulator. In part due to the studies of Mok et al. [49] and Dobelis et al. [50], it is becoming increasingly clear, however, that KYNA inhibition of the α7nAChR is a complex phenomenon, which depends on cell maturation, receptor expression on interneurons versus pyramidal neurons, compartmentalization of receptors to dendritic versus somatic locations, and additional variables such as modification of subunits and association with other proteins and intracellular regulatory factors. Moreover, it is certainly possible that KYNA may also act on (a) target(s) downstream to α7nAChR function. Elucidation of the intricacies of α7nAChRs inhibition by KYNA in the hippocampus as well as elsewhere in the central nervous system, and possibly also in the periphery [58], presents formidable methodological challenges but promises important new insights into the regulation and function of this interesting receptor subtype and its diverse roles in physiology and pathology [51].
Acknowledgements
We are especially grateful for the many critical contributions of Drs. M. Alkondon, J. Banerjee, P. Guidetti and H.-Q. Wu, as well as the important roles of several other members of our laboratories. We would like to thank Dr. J. Banerjee for his significant contribution to the preparation of the figure as well as the text. Our collaborative work is supported by USPHS grant NS25296.
References
- 1.Moroni F, Russi P, Lombardi G, Beni M, Carlá V. Presence of kynurenic acid in the mammalian brain. J Neurochem. 1988;51:177–180. doi: 10.1111/j.1471-4159.1988.tb04852.x. [DOI] [PubMed] [Google Scholar]
- 2.Turski WA, Schwarcz R. On the disposition of intrahippocampally injected kynurenic acid in the rat. Exp Brain Res. 1988;71:563–567. doi: 10.1007/BF00248748. [DOI] [PubMed] [Google Scholar]
- 3.Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991;56:2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 4.Guidetti P, Amori L, Sapko MT, Okuno E, Schwarcz R. Mitochondrial aspartate aminotransferase: a third kynurenate-producing enzyme in the mammalian brain. J Neurochem. 2007;102:103–111. doi: 10.1111/j.1471-4159.2007.04556.x. [DOI] [PubMed] [Google Scholar]
- 5.Guidetti P, Hoffman GE, Melendez-Ferro M, Albuquerque EX, Schwarcz R. Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia. 2007;55:78–92. doi: 10.1002/glia.20432. [DOI] [PubMed] [Google Scholar]
- 6.Speciale C, Hares K, Schwarcz R, Brookes N. High-affinity uptake of L-kynurenine by a Na+-independent transporter of neutral amino acids in astrocytes. J Neurosci. 1989;9:2066–2072. doi: 10.1523/JNEUROSCI.09-06-02066.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joseph MH. Determination of kynurenine by a simple gas-liquid chromatographic method applicable to urine, plasma, brain and cerebrospinal fluid. J Chromatogr. 1978;146:33–41. doi: 10.1016/s0378-4347(00)81287-6. [DOI] [PubMed] [Google Scholar]
- 8.Han Q, Cai T, Tagle DA, Li J. Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell Mol Life Sci. 2010;67:353–368. doi: 10.1007/s00018-009-0166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodgkins PS, Wu HQ, Zielke HR, Schwarcz R. 2-Oxoacids regulate kynurenic acid production in the rat brain: studies in vitro and in vivo. J Neurochem. 1999;72:643–651. doi: 10.1046/j.1471-4159.1999.0720643.x. [DOI] [PubMed] [Google Scholar]
- 10.Gramsbergen JB, Hodgkins PS, Rassoulpour A, Turski WA, Guidetti P, Schwarcz R. Brain-specific modulation of kynurenic acid synthesis in the rat. J Neurochem. 1997;69:290–298. doi: 10.1046/j.1471-4159.1997.69010290.x. [DOI] [PubMed] [Google Scholar]
- 11.Uwai Y, Honjo H, Iwamoto K. Interaction and transport of kynurenic acid via human organic anion transporters hOAT1 and hOAT3. Pharmacol Res. 2012;65:254–260. doi: 10.1016/j.phrs.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001;313:96–98. doi: 10.1016/s0304-3940(01)02242-x. [DOI] [PubMed] [Google Scholar]
- 13.Swartz KJ, During MJ, Freese A, Beal MF. Cerebral synthesis and release of kynurenic acid: an endogenous antagonist of excitatory amino acid receptors. J Neurosci. 1990;10:2965–2973. doi: 10.1523/JNEUROSCI.10-09-02965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu HQ, Rassoulpour A, Schwarcz R. Kynurenic acid leads, dopamine follows: a new case of volume transmission in the brain? J Neural Transm. 2007;114:33–41. doi: 10.1007/s00702-006-0562-y. [DOI] [PubMed] [Google Scholar]
- 15.Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Carpenedo R, Pittaluga A, Cozzi A, Attucci S, Galli A, Raiteri M, Moroni F. Presynaptic kynurenate-sensitive receptors inhibit glutamate release. Eur J Neurosci. 2001;13:2141–2147. doi: 10.1046/j.0953-816x.2001.01592.x. [DOI] [PubMed] [Google Scholar]
- 17.Rassoulpour A, Wu H-Q, Ferré S, Schwarcz R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. J Neurochem. 2005;93:762–765. doi: 10.1111/j.1471-4159.2005.03134.x. [DOI] [PubMed] [Google Scholar]
- 18.Amori L, Wu HQ, Marinozzi M, Pellicciari R, Guidetti P, Schwarcz R. Specific inhibition of kynurenate synthesis enhances extracellular dopamine levels in the rodent striatum. Neuroscience. 2009;159:196–203. doi: 10.1016/j.neuroscience.2008.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zmarowski A, Wu HQ, Brooks JM, Potter MC, Pellicciari R, Schwarcz R, Bruno JP. Astrocyte-derived kynurenic acid modulates basal and evoked cortical acetylcholine release. Eur J Neurosci. 2009;29:529–538. doi: 10.1111/j.1460-9568.2008.06594.x. [DOI] [PubMed] [Google Scholar]
- 20.Wu HQ, Pereira EF, Bruno JP, Pellicciari R, Albuquerque EX, Schwarcz R. The astrocyte-derived alpha7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in the prefrontal cortex. J Mol Neurosci. 2010;40:204–210. doi: 10.1007/s12031-009-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erhardt S, Schwieler L, Emanuelsson C, Geyer M. Endogenous kynurenic acid disrupts prepulse inhibition. Biol Psychiatry. 2004;56:255–260. doi: 10.1016/j.biopsych.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Chess AC, Simoni MK, Alling TE, Bucci DJ. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr Bull. 2007;33:797–804. doi: 10.1093/schbul/sbl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregoire L, Rassoulpour A, Guidetti P, Samadi P, Bédard PJ, Izzo E, Schwarcz R, Di Paolo T. Prolonged kynurenine 3-hydroxylase inhibition reduces development of levodopa-induced dyskinesias in parkinsonian monkeys. Behav Brain Res. 2008;186:161–167. doi: 10.1016/j.bbr.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Pocivavsek A, Wu HQ, Potter MC, Elmer GI, Pellicciari R, Schwarcz R. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology. 2011;36:2357–2367. doi: 10.1038/npp.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beal MF, Matson WR, Swartz KJ, Gamache PH, Bird ED. Kynurenine pathway measurements in Huntington's disease striatum: evidence for reduced formation of kynurenic acid. J Neurochem. 1990;55:1327–1339. doi: 10.1111/j.1471-4159.1990.tb03143.x. [DOI] [PubMed] [Google Scholar]
- 27.Baran H, Jellinger K, Deecke L. Kynurenine metabolism in Alzheimer’s disease. J Neural Transm. 1999;106:165–181. doi: 10.1007/s007020050149. [DOI] [PubMed] [Google Scholar]
- 28.Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50:521–530. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- 29.Hansen AM, Ball HJ, Mitchell AJ, Miu J, Takikawa O, Hunt NH. Increased expression of indoleamine 2,3-dioxygenase in murine malaria infection is predominantly localised to the vascular endothelium. Int J Parasitol. 2004;34:1309–1319. doi: 10.1016/j.ijpara.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Holtze M, Asp L, Schwieler L, Engberg G, Karlsson H. Induction of the kynurenine pathway by neurotropic influenza A virus infection. J Neurosci Res. 2008;86:3674–3683. doi: 10.1002/jnr.21799. [DOI] [PubMed] [Google Scholar]
- 31.Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247:184–187. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- 32.Ganong AH, Lanthorn TH, Cotman CW. Kynurenic acid inhibits synaptic and acidic amino acid-induced responses in the rat hippocampus and spinal cord. Brain Res. 1983;273:170–174. doi: 10.1016/0006-8993(83)91108-3. [DOI] [PubMed] [Google Scholar]
- 33.Foster AC, Vezzani A, French ED, Schwarcz R. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci Lett. 1984;48:273–278. doi: 10.1016/0304-3940(84)90050-8. [DOI] [PubMed] [Google Scholar]
- 34.Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem. 1989;52:1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 35.Hilmas C, Pereira EFR, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21:7463–7473. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alkondon M, Pereira EFR, Yu P, Arruda EZ, Almeida LEF, Guidetti P, Fawcett WP, Sapko MT, Randall WR, Schwarcz R, Tagle DA, Albuquerque EX. Targeted deletion of the kynurenine aminotransferase II gene reveals a critical role of endogenous kynurenic acid in the regulation of synaptic transmission via α7 nicotinic receptors in the hippocampus. J Neurosci. 2004;24:4635–4648. doi: 10.1523/JNEUROSCI.5631-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grilli M, Raiteri L, Patti L, Parodi M, Robino F, Raiteri M, Marchi M. Modulation of the function of presynaptic α7 and non-α7 nicotinic receptors by the tryptophan metabolites, 5-hydroxyindole and kynurenate in mouse brain. Br J Pharmacol. 2006;149:724–732. doi: 10.1038/sj.bjp.0706914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stone TW. Kynurenic acid blocks nicotinic synaptic transmission to hippocampal interneurons in young rats. Eur J Neurosci. 2007;25:2656–2665. doi: 10.1111/j.1460-9568.2007.05540.x. [DOI] [PubMed] [Google Scholar]
- 39.Arnaiz-Cot JJ, González JC, Sobrado M, Baldelli P, Carbone E, Gandía L, García AG, Hernández-Guijo JM. Allosteric modulation of alpha 7 nicotinic receptors selectively depolarizes hippocampal interneurons, enhancing spontaneous GABAergic transmission. Eur J Neurosci. 2008;27:1097–1110. doi: 10.1111/j.1460-9568.2008.06077.x. [DOI] [PubMed] [Google Scholar]
- 40.Alkondon M, Pereira EFR, Eisenberg HM, Kajii Y, Schwarcz R, Albuquerque EX. Age dependency of inhibition of α7 nicotinic receptors and tonically active N-methyl-D-aspartate receptors by endogenously produced kynurenic acid in the brain. J Pharmacol Exp Ther. 2011;337:572–582. doi: 10.1124/jpet.110.177386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopes C, Pereira EFR, Wu HQ, Purushottamachar P, Njar V, Schwarcz R, Albuquerque EX. Competitive antagonism between the nicotinic allosteric potentiating ligand galantamine and kynurenic acid at α7* nicotinic receptors. J Pharmacol Exp Ther. 2007;322:48–58. doi: 10.1124/jpet.107.123109. [DOI] [PubMed] [Google Scholar]
- 42.Alexander KS, Wu HQ, Schwarcz R, Bruno JP. Acute elevations of brain kynurenic acid impair cognitive flexibility: normalization by the alpha7 positive modulator galantamine. Psychopharmacology (Berl) 2012;220:627–637. doi: 10.1007/s00213-011-2539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prescott C, Weeks AM, Staley KJ, Partin KM. Kynurenic acid has a dual action on AMPA receptor responses. Neurosci Lett. 2006;402:108–112. doi: 10.1016/j.neulet.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Simonavicius N, Wu X, Swaminath G, Reagan J, Tian H, Ling L. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J Biol Chem. 2006;281:22021–22028. doi: 10.1074/jbc.M603503200. [DOI] [PubMed] [Google Scholar]
- 45.DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM, Omiecinski CJ, Perdew GH. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010;115:89–97. doi: 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alkondon M, Pereira EF, Albuquerque EX. Endogenous activation of nAChRs and NMDA receptors contributes to the excitability of CA1 stratum radiatum interneurons in rat hippocampal slices: effects of kynurenic acid. Biochem Pharmacol. 2011;82:842–851. doi: 10.1016/j.bcp.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Banerjee J, Alkondon M, Albuquerque EX. Kynurenic acid inhibits glutamatergic transmission to CA1 pyramidal neurons via α7 nAChR-dependent and -independent mechanisms. Biochem Pharmacol. 2012;84:1078–1087. doi: 10.1016/j.bcp.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 48.Moroni F, Cozzi A, Sili M, Mannaioni G. Kynurenic acid: a metabolite with multiple actions and multiple targets in brain and periphery. J Neural Transm. 2012;119:133–139. doi: 10.1007/s00702-011-0763-x. [DOI] [PubMed] [Google Scholar]
- 49.Mok MH, Fricker AC, Weil A, Kew JN. Electrophysiological characterisation of the actions of kynurenic acid at ligand-gated ion channels. Neuropharmacology. 2009;57:242–249. doi: 10.1016/j.neuropharm.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 50.Dobelis P, Staley K, Cooper D. Lack of modulation of nicotinic acetylcholine alpha-7 receptor currents by kynurenic acid in adult hippocampal interneurons. PLoS ONE. 2012;7:e41108. doi: 10.1371/journal.pone.0041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Albuquerque EX, Pereira EFR, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banerjee J, Alkondon M, Pereira EF, Albuquerque EX. Regulation of GABAergic inputs to CA1 pyramidal neurons by nicotinic receptors and kynurenic acid. J Pharmacol Exp Ther. 2012;341:500–509. doi: 10.1124/jpet.111.189860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alkondon M, Reinhardt S, Lobron C, Hermsen B, Maelicke A, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. II. The rundown and inward rectification of agonist-elicited whole-cell currents and identification of receptor subunits by in situ hybridization. J Pharmacol Exp Ther. 1994;271:494–506. [PubMed] [Google Scholar]
- 54.Alexander JK, Sagher D, Krivoshein AV, Criado M, Jefford G, Green WN. Ric-3 promotes α7 nicotinic receptor assembly and trafficking through the ER subcompartment of dendrites. J Neurosci. 2010;30:10112–10126. doi: 10.1523/JNEUROSCI.6344-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milstein AD, Nicoll RA. Regulation of AMPA receptor gating and pharmacology by TARP auxiliary subunits. Trends Pharmacol Sci. 2008;29:333–339. doi: 10.1016/j.tips.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alkondon M, Albuquerque EX. Initial characterization of the nicotinic acetylcholine receptors in rat hippocampal neurons. J Receptor Res. 1991;11:1001–1021. doi: 10.3109/10799899109064693. [DOI] [PubMed] [Google Scholar]
- 57.Oz M, Spivak CE, Lupica CR. The solubilizing detergents, Tween 80 and Triton X-100 non-competitively inhibit alpha 7-nicotinic acetylcholine receptor function in Xenopus oocytes. J Neurosci Meth. 2004;137:167–173. doi: 10.1016/j.jneumeth.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 58.Filippini P, Cesario A, Fini M, Locatelli F, Rutella S. The Yin and Yang of non-neuronal α7-nicotinic receptors in inflammation and autoimmunity. Curr Drug Targ. 2012;13:644–655. doi: 10.2174/138945012800399008. [DOI] [PubMed] [Google Scholar]
- 59.Yu CR, Role LW. Functional contribution of the α7 subunit to multiple subtypes of nicotinic receptors in embryonic chick sympathetic neurons. J Physiol. 1998;509:651–665. doi: 10.1111/j.1469-7793.1998.651bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mike A, Castro NG, Albuquerque EX. Choline and acetylcholine have similar kinetic properties of activation and desensitization on the α7 nicotinic receptors in rat hippocampal neurons. Brain Res. 2000;882:155–168. doi: 10.1016/s0006-8993(00)02863-8. [DOI] [PubMed] [Google Scholar]
- 61.Ganong AH, Cotman CW. Kynurenic acid and quinolinic acid act at N-methyl-D-aspartate receptors in the rat hippocampus. J Pharmcol Exp Ther. 1986;236:293–299. [PubMed] [Google Scholar]
- 62.Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45:309–379. [PubMed] [Google Scholar]
- 63.Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247:184–187. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- 64.Ganong AH, Lanthorn TH, Cotman CW. Kynurenic acid inhibits synaptic and acidic amino acid-induced responses in the rat hippocampus and spinal cord. Brain Res. 1983;273:170–174. doi: 10.1016/0006-8993(83)91108-3. [DOI] [PubMed] [Google Scholar]
- 65.Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem. 1989;52:1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 66.Parsons CG, Danysz W, Quack G, Hartmann S, Lorenz B, Wollenburg C, Baran L, Przegalinski E, Kostowski W, Krzascik P, Chizh B, Headley PM. Novel systemically active antagonists of the glycine site of the N-methyl-D-aspartate receptor: electrophysiological, biochemical and behavioral characterization. J Pharmacol Exp Ther. 1997;283:1264–1275. [PubMed] [Google Scholar]
- 67.Stone TW, Stoy N, Darlington LG. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol Sci. 2012 doi: 10.1016/j.tips.2012.09.006. http://dx.doi.org/10.1016/j.tips.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 68.Zhao P, Sharir H, Kapur A, Cowan A, Geller EB, Adler MW, Seltzman HH, Reggio PH, Heynen-Genel S, Sauer M, Chung TDY, Bai Y, Chen W, Caron MG, Barak LS, Abood ME. Targeting of the orphan receptor GPR35 by pamoic acid: a potent activator of extracellular signal-regulated kinase and β-arrestin2 with antinociceptive activity. Mol Pharmacol. 2010;78:560–568. doi: 10.1124/mol.110.066746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM, Omiecinski CJ, Perdew GH. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010;15:89–97. doi: 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]