Abstract
Objective
We sought to determine whether midtrimester amniotic fluid levels of matrix metalloproteinase-8 were associated with subsequent preterm premature rupture of membranes.
Study design
We conducted a case-control study examining 57 asymptomatic women who underwent genetic amniocentesis from 14 to 21 weeks’ gestation and subsequently had preterm premature rupture of membranes (<35 wk) and 58 women with subsequent term delivery. Measurement of total matrix metalloproteinase-8 level in amniotic fluid was conducted using a commercially available enzyme-linked immunosorbent assay and association with preterm birth due to preterm premature rupture of membranes was assessed.
Results
The overall distribution of matrix metalloproteinase-8 concentrations was similar in women who had preterm premature rupture of membranes and term controls (median 2.39 ng/mL, 25th to 75th percentile 1.1-10.1 vs 2.37 ng/mL, 25th to 75th percentile 1.5-4.7, P = .94). However, 26% of women who had preterm premature rupture of membranes had a matrix metalloproteinase-8 concentration above the 90th percentile (8.7 ng/mL), compared with only 10% of term controls (odds ratio 3.1, 95% CI 1.1-8.7; P = .03). Elevated matrix metalloproteinase-8 remained associated with preterm premature rupture of membranes after adjustment for maternal age, race, parity, gestational age, and year of amniocentesis (odds ratio 3.4, 95% CI 1.2-9.9; P = .03).
Conclusions
The overall distribution of midtrimester amniotic fluid matrix metalloproteinase-8 levels did not differ between women who had preterm premature rupture of membranes and those delivered at term. However, marked elevations of midtrimester amniotic fluid matrix metalloproteinase-8 were highly associated with subsequent preterm premature rupture of membranes, suggesting that the pathophysiologic processes that contribute to preterm premature rupture of membranes may begin in early pregnancy.
Keywords: Matrix metalloproteinase-8, Premature rupture of membranes, Intrauterine inflammation, Preterm birth
Collagens are a family of proteins responsible for the maintenance of tissue tensile strength. Each collagen molecule is composed of 3 polypeptide chains in a triple helical configuration with the specific composition of the monomeric chains defining the collagen subtype. The predominant collagens of the chorioamnion extracellular matrix are types I and III, but types IV, V, and VI have been localized to the amnion as well.1,2 Matrix metalloproteinases (MMP), a family of potent enzymes capable of digesting the extracellular matrix, can be classified according to substrate-specific activity. Three distinct collagenases have been described: MMP-1, interstitial collagenase; MMP-8, neutrophil collagenase; and MMP-13, collagenase-3. These enzymes cleave all three α chains of types I, II, and III collagens, resulting in instability of the triple helix configuration and subsequent degradation by other proteases.3
MMP-8 is synthesized by neutrophils and stored in the specific granules until neutrophil activation, whereupon it is released locally into the extracellular matrix. Intra-amniotic inflammation has been repeatedly associated with spontaneous preterm labor and preterm premature rupture of membranes (PROM).4-6 The migration of fetal neutrophils into the amniotic cavity and their subsequent activation and release of proteolytic enzymes, including MMP-8, characterizes the intraamniotic inflammatory response. Previously Maymon et al7-9 reported an increase in amniotic fluid MMP-8 levels in the setting of both preterm labor with intact membranes and preterm PROM. Because markers of intrauterine inflammation at the time of midtrimester genetic amniocentesis have been associated with preterm delivery and pregnancy loss,4,7 we hypothesized that MMP-8 levels measured in amniotic fluid obtained from asymptomatic women at the time of midtrimester genetic amniocentesis would be associated with subsequent preterm PROM because of its effect on weakening the extracellular matrix of the fetal membranes.
Material and methods
With institutional review board approval, we utilized our computerized obstetric outcomes database to identify a cohort of patients with nonanomalous fetuses that underwent midtrimester genetic amniocentesis and subsequently had preterm PROM prior to 35 weeks’ gestation (n = 58). Each subject was matched for maternal age and length of specimen storage with a control (n = 58) who underwent midtrimester genetic amniocentesis and was delivered at or beyond 37 weeks’ gestation. Initially 58 subjects were identified, but on review of pregnancy outcomes, it was discovered that a gestation complicated by fetal omphalocele was inadvertently included in the case cohort. This subject was excluded from further analysis, reducing the number of subjects to 57. Gestational age was determined by last menstrual period and confirmatory ultrasound at the time of amniocentesis; ultrasound dating criteria were used preferentially if they differed from the last menstrual period by more than 9 days.
All amniotic fluid was obtained by a maternal-fetal medicine attending physician or fellow using a 22-gauge spinal needle with ultrasound guidance. After processing, aliquots were obtained for karyotype and α-fetoprotein measurement, and excess fluid was stored at −70°C within 6 hours of the procedure. Levels of MMP-8 were measured, in duplicate, utilizing a commercially available enzyme-linked immunosorbent assay (Quantikine human MMP-8 [total], R&D Systems, Minneapolis, Minn). The intra-assay coeffcient of variation was 3.1%; the interassay coeffcient of variation was 2.7%. A 96-well micro-plate reader (Bio-Tek Instruments, Winooski, Vt) was used to determine the optical density of each specimen at 450 nm and 540 nm (to correct for optical imperfections in the plate). We plotted the optical density for standards versus the known concentrations and log transformed the data to generate a standard curve that could be used to determine sample MMP-8 concentrations based on the absorbance value. All samples were diluted with manufacturer-provided diluent (assay diluent RD1-52, R&D Systems) to maintain values within the maximal sensitivity of the assay; the dilution factor was incorporated into final concentration calculations.
The results of the MMP-8 assays were analyzed with the Shapiro-Wilk test to determine whether the results were normally distributed.10 The overall distribution of amniotic fluid MMP-8 levels in subjects and controls were compared using the Wilcoxon rank sum test. Differences in proportions were assessed using the uncorrected Pearson χ2 test. The data were subjected to receiver-operator curve analysis. In addition, outcome data were stratified and analyzed by race using the Cochran-Mantel-Haenszel test. Unadjusted odds ratios and 95% CIs were calculated. Multivariable logistic regression was performed to control for other possible confounding variables and to calculate an adjusted odds ratio. Amniotic fluid levels of markers of collagen synthesis (C-terminal propeptide of procollagen [CICP]) and degradation (C-terminal telopeptide of type I collagen, ICTP) had been previously measured in the same specimens and reported by our group.11,12 Correlation between amniotic fluid levels of MMP-8 and these markers of collagen turnover was examined using the Pearson correlation coeffcient. Statistical significance was defined as P ≤ .05, and all tests of significance were two tailed.
Results
Subjects and controls were similar with regard to parity, tobacco use, gestational age at amniocentesis, maternal age and weight, length of specimen storage, and indication for amniocentesis. There was a greater proportion of African-American subjects than controls, and, as expected, the mean gestational age at delivery for subjects was earlier than that for controls (Table I).
Table I.
Characteristics of cohort: demographics and procedure indications
| Preterm PROM cases (n = 57) | Term controls (n = 58) | P value | |
|---|---|---|---|
| Maternal age (y) | 34.0 ± 5.9 | 34.9 ± 5.8 | .44 |
| Gestational age at amniocentesis (wk) | 16.2 ± 2.0 | 16.1 ± 1.8 | .67 |
| Gestational age at delivery (wk) | 28.9 ± 5.6 | 39.0 ± 1.2 | < .001 |
| Indication for amniocentesis | .2 | ||
| Maternal age ≥35 y | 61% | 65% | |
| Abnormal serum screen | 21% | 28% | |
| Other | 18% | 7% | |
| Maternal weight (pounds) | 166 ± 36 | 163 ± 40 | .73 |
| Race | .03 | ||
| African-American | 32% | 14% | |
| Caucasian | 68% | 84% | |
| Nulliparous | 35% | 28% | .39 |
| Smoker | 20% | 31% | .16 |
| Length of specimen storage (y) | 4.9 ± 1.7 | 5.2 ± 1.6 | .35 |
Data presented as mean ± SD or as a proportion of n.
The mean interval from amniocentesis to delivery was 13 ± 6 weeks (mean ± SD) in the women who had preterm PROM. The levels of MMP-8 in the amniotic fluid were not normally distributed because of a marked skewing of values to the left (P < .001); we therefore used nonparametric statistics to analyze the data. The overall distribution of amniotic fluid MMP-8 levels and the medians of subjects and controls were similar when examined with the Wilcoxon rank sum test (Table II).
Table II.
Midtrimester amniotic fluid levels of MMP-8 and markers of collagen of collagen metabolism
| Preterm PROM cases (n = 57) | Term controls (n = 58) | P value | |
|---|---|---|---|
| MMP-8 (ng/ml) | |||
| Median (25th-75th percentile) | 2.39 (1.1-10.1) | 2.37 (1.5-4.7) | .94 |
| Range | 0.08-262.1 | 0.08-64.4 |
We next sought to dichotomize MMP-8 levels to evaluate the association of elevated levels with subsequent preterm PROM. Receiver-operator curve analysis identified a value at approximately the 91st percentile as the optimal cutoff value in this analysis, although at no value were sensitivity and specificity ideal (Figure 1). Therefore, based on the typically expected distribution of biologic markers, we utilized the 90th percentile of the control group as the upper limit of normal. Of women who subsequently had preterm PROM, 26.3% had amniotic fluid MMP-8 levels greater than the 90th percentile (8.7 ng/ml), compared with only 10.3% of term controls (crude odds ratio [OR] 3.1, 95% confidence interval [CI] 1.1-8.7; P = .03) (Figure 2). The 90th percentile cutoff resulted, therefore, in a sensitivity of 26.3% and a specificity of 89.7%. MMP-8 levels greater than the 90th percentile remained associated with subsequent preterm PROM after controlling for maternal age, race, parity, gestational age at amniocentesis, and year of amniocentesis in a multiple logistic regression model (adjusted OR 3.4, 95% CI 1.2-9.9; P = 0.03). There was no relationship between the degree of elevation of MMP-8 and timing of preterm PROM in subjects (P = .29). Because of the different racial distribution between subjects and controls, outcomes were stratified and analyzed by race. After controlling for race, an elevated MMP-8 concentration was even more strongly associated with subsequent preterm PROM (OR 3.8, 95% CI 1.3-11.0; P = .01).
Figure 1.
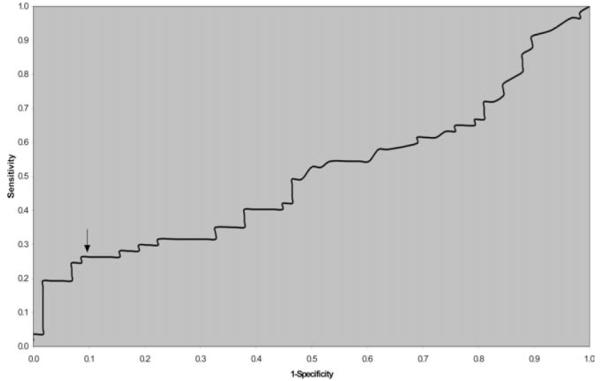
Receiver operator curve analysis for midtrimester amniotic fluid MMP-8 levels and the prediction of subsequent preterm PROM (<35 weeks). Analysis of the curve showed no ideal cutoff; however, sensitivity and specificity were optimized at an MMP-8 level that represented approximately the 91st percentile. The 90th percentile (8.7 ng/mL) was therefore used as the cutoff value of normal; all further analysis was based on this level.
Figure 2.

Amniotic fluid levels of MMP-8 in asymptomatic women at the time of midtrimester genetic amniocentesis. More women with subsequent preterm PROM had MMP-8 levels greater than the 90th percentile (8.7 ng/mL) than did term controls (26% vs 10%, P = .03).
Amniotic fluid levels of markers of collagen synthesis (CICP) and degradation (ICTP) had been previously measured in the same specimens and reported by our group.11,12 Amniotic fluid levels of MMP-8 and ICTP were correlated in women who had preterm PROM (r = 0.32, P = .02) but not in term controls (r = 0.15, P = .27). There was no correlation between amniotic fluid levels of MMP-8 and CICP in either subjects or controls (P > .05).
Comment
High levels of MMP-8 may weaken the tensile strength of the membranes leading to the observed increase in the risk of preterm PROM. Although the median and overall distribution of midtrimester amniotic fluid MMP-8 levels were similar between women who had preterm PROM and those delivered at term, we found that even at 16 weeks’ gestational age, women with midtrimester MMP-8 levels greater than the 90th percentile were more than 3 times more likely to experience preterm PROM than women with lower levels. In addition, we also found that MMP-8 levels correlated with actual collagen breakdown as reflected by increased levels of ICTP.
The levels of midtrimester amniotic fluid MMP-8 were not normally distributed but instead were markedly skewed to the left, reflecting that most patients typically have low values of this substance detectable in amniotic fluid. This was further supported by the similarity between the measures of central tendency and overall distribution of levels in both subjects and controls. Despite this similarity, it is, however, clear that patients with extreme elevations of MMP-8 levels are at increased risk for subsequent preterm PROM, reflecting a state of neutrophil activation as early as 16 weeks’ gestation. The lack of elevation in MMP-8 levels in some subjects may reflect the multitude of pathophysiologic pathways, proteases, and collagenases that can be involved in preterm PROM or may simply reflect timing of sample collection.
Neutrophils involved in intrauterine inflammation are thought to be predominantly of fetal lineage, and Romero et al5,13,14 have shown that the fetus may therefore play a role in preterm PROM. Amniotic fluid levels of MMP-8 obtained at the time of diagnosis of preterm PROM are elevated and are an indicator of intra-amniotic infection.8 In a cohort of 19 women who had either spontaneous preterm birth or preterm PROM, Yoon et al15 reported elevated MMP-8 levels. In their report, although the median MMP-8 level in subjects was similar to our case median (3.1 ng/mL versus 2.39 ng/mL), there was a wide range of MMP-8 levels, with one subject having a level of 1954.9 ng/mL. However, whether the highest levels occurred in women with preterm PROM or spontaneous preterm labor is unclear. We limited our study to women with preterm PROM to obtain a more uniform cohort because the pathophysiology leading to preterm PROM and spontaneous preterm labor may differ.
The dynamics of collagen metabolism play a role in the maintenance of the integrity of the fetal membranes. Our finding that an increase in collagenase activity, especially with an affnity for the collagens most commonly found in the fetal membranes (types I and III), is associated with preterm PROM is further substantiated by the correlation between MMP-8 and a marker of collagen degradation (ICTP) in subjects but not in controls. Although the production of MMP-8 is one of the critical early steps in collagen catabolism ultimately responsible for disruption of the triple helical structure, numerous other proteases play a role in total collagen metabolism.3 It is critical, therefore, not to consider MMP-8 in isolation but as part of a catabolic pathway. The lack of MMP-8 correlation with ICTP in controls most likely reflects the fact that in normal pregnancy there is a process of collagen turnover and degradation with resynthesis. However, MMP-8 (neutrophil collagenase) may not be a major contributor to degradation in the absence of inflammation.
Although measurement of amniotic fluid MMP-8 levels at the time of midtrimester amniocentesis could provide a risk assessment for the subsequent development of preterm PROM, and perhaps spontaneous preterm labor, several issues need to be addressed prior to implementation of such a strategy. First, both our study and Yoon’s were retrospective and used stored samples with small study groups. In both studies the sensitivity was fair, 26% and 42%, and the specificities were acceptable, 90% and 99%.15 However, the case-control design precludes calculation of positive and negative predictive values. A larger prospective study would need to be performed to better understand the test parameters before any screening and risk stratification is advocated. Second, if an elevated MMP-8 level were identified, there is currently no effective intervention that has been shown to reduce the risk of preterm PROM or preterm birth in the setting of intrauterine inflammation. Without an intervention the value of screening is questionable.
Acknowledgments
Supported by the National Institutes of Health/National Institute of Child Health and Human Development grant No. K12-HD01402 and the American College of Obstetrics and Gynecology/3M Pharmaceutical Research in Lower Genital Tract Infection Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 23rd Annual Meeting of the Society for Maternal-Fetal Medicine, San Francisco, Calif, Feb 3-8, 2003. Reprints not available from the authors.
References
- 1.Bogusiewicz M, Rechberger T, Skorupski P, Postawski K, Jakowicki JA. Local collagen turnover in human foetal membranes during full term vaginal delivery. Eur J Obstet Gynecol Reprod Biol. 1998;77:141–3. doi: 10.1016/s0301-2115(97)00245-5. [DOI] [PubMed] [Google Scholar]
- 2.Malak TM, Ockleford CD, Bell SC, Dalgleish R, Bright N, Macvicar J. Confocal immunofluorescence localization of collagen types I, III, IV, V and VI and their ultrastructural organization in term human fetal membranes. Placenta. 1993;14:385–406. doi: 10.1016/s0143-4004(05)80460-6. [DOI] [PubMed] [Google Scholar]
- 3.Cawston TE. Metalloproteinase inhibitors and the prevention of connective tissue breakdown. Pharmacol Ther. 1996;70:163–82. doi: 10.1016/0163-7258(96)00015-0. [DOI] [PubMed] [Google Scholar]
- 4.Wenstrom KD, Andrews WW, Hauth JC, Goldenberg RL, DuBard MB, Cliver SP. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol. 1998;178:546–50. doi: 10.1016/s0002-9378(98)70436-3. [DOI] [PubMed] [Google Scholar]
- 5.Romero R, Gomez R, Ghezzi F, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179:186–93. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 6.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 7.Maymon E, Romero R, Pacora P, Gomez R, Athayde N, Edwin S, et al. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;183:94–9. doi: 10.1067/mob.2000.105344. [DOI] [PubMed] [Google Scholar]
- 8.Maymon E, Romero R, Chaiworapongsa T, Kim JC, Berman S, Gomez R, et al. Value of amniotic fluid neutrophil collagenase concentrations in preterm premature rupture of membranes. Am J Obstet Gynecol. 2001;185:1143–8. doi: 10.1067/mob.2001.118166. [DOI] [PubMed] [Google Scholar]
- 9.Maymon E, Romero R, Chaiworapongsa T, Berman S, Conoscenti G, Gomez R, et al. Amniotic fluid matrix metalloproteinase-8 in preterm labor with intact membranes. Am J Obstet Gynecol. 2001;185:1149–55. doi: 10.1067/mob.2001.118165. [DOI] [PubMed] [Google Scholar]
- 10.Royston P. Estimating departure from normality. Stat Med. 1991;10:1283–93. doi: 10.1002/sim.4780100811. [DOI] [PubMed] [Google Scholar]
- 11.Ramsey PS, Shinetugs B, Goldenberg RL, Cliver SP, Wenstrom KD. Midtrimester amniotic fluid collagen metabolism and the subsequent development of preterm premature rupture of membranes. J Soc Gynecol Invest. 2002;9:85A. [Google Scholar]
- 12.Ramsey PS, Shinetugs B, Goldenberg RL, Cliver SP, Wenstrom KD. Midtrimester serum markers of collagen metabolism are not associated with preterm premature rupture of membranes. J Soc Gynecol Invest. 2002;9:97A. [Google Scholar]
- 13.Romero R, Chaiworapongsa T, Espinoza J. Micronutrients and intrauterine infection, preterm birth and the fetal inflammatory response syndrome. J Nutr. 2003;133:1668S–73S. doi: 10.1093/jn/133.5.1668S. [DOI] [PubMed] [Google Scholar]
- 14.Romero R, Chaiworapongsa T, Gomez R, Yoon B, Mazor M, Maymon E, et al. Does the human fetus rupture the membranes in preterm PROM? Am J Obstet Gynecol. 2001;185:S71. [Google Scholar]
- 15.Yoon BH, Oh SY, Romero R, Shim SS, Han SY, Park JS, et al. An elevated amniotic fluid matrix metalloproteinase-8 level at the time of mid-trimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol. 2001;185:1162–7. doi: 10.1067/mob.2001.117678. [DOI] [PubMed] [Google Scholar]


