Abstract
Aims
Intraventricular conduction defects (IVCDs) can impair prognosis of heart failure (HF), but their specific impact is not well established. This study aimed to analyse the clinical profile and outcomes of HF patients with LBBB, right bundle branch block (RBBB), left anterior fascicular block (LAFB), and no IVCDs.
Methods and results
Clinical variables and outcomes after a median follow-up of 21 months were analysed in 1762 patients with chronic HF and LBBB (n = 532), RBBB (n = 134), LAFB (n = 154), and no IVCDs (n = 942). LBBB was associated with more marked LV dilation, depressed LVEF, and mitral valve regurgitation. Patients with RBBB presented overt signs of congestive HF and depressed right ventricular motion. The LAFB group presented intermediate clinical characteristics, and patients with no IVCDs were more often women with less enlarged left ventricles and less depressed LVEF. Death occurred in 332 patients (interannual mortality = 10.8%): cardiovascular in 257, extravascular in 61, and of unknown origin in 14 patients. Cardiac death occurred in 230 (pump failure in 171 and sudden death in 59). An adjusted Cox model showed higher risk of cardiac death and pump failure death in the LBBB and RBBB than in the LAFB and the no IVCD groups.
Conclusion
LBBB and RBBB are associated with different clinical profiles and both are independent predictors of increased risk of cardiac death in patients with HF. A more favourable prognosis was observed in patients with LAFB and in those free of IVCDs. Further research in HF patients with RBBB is warranted.
Keywords: Heart failure, Bundle branch block, Outcomes, Prognosis
Introduction
Intraventricular conduction defects (IVCDs) can probably impair the clinical course and outcomes of patients with chronic heart failure (HF) since delayed activation of either the right or left ventricle shortens the duration of the ventricular diastolic filling period, and this in turn reduces the stroke volume and cardiac output.1,2 Moreover, the systolic and diastolic ventricular asynchrony originating due to the abnormal cardiac activation sequence3,4 will worsen the already depressed cardiac output and favour further ventricular volume remodelling.5,6
Clinical registries that have analysed the prognostic implications of bundle branch block in patients with HF7–12 reported different results. Whereas the presence of LBBB emerges as an independent prognostic marker in some studies,7,9,10 others found a higher mortality linked to the presence of right bundle branch block (RBBB).11,12 Differences in the length of the follow-up period (from 1 up to 5 years)7,10 may account to some extent for the reported prognostic differences, although the categorization of patients and the analysis of the causes of death would certainly play an important role. Indeed, vital status or all-cause mortality instead of a more precise assessment of different causes of cardiac death are presently determined in most studies. On the other hand, the clinical characteristics associated with different IVCDs cannot be well established because echocardiographic data are not reported in all studies and, moreover, they lack information on relevant features such as LV and left atrial size, or mitral valve assessment.7,10,12,13
This study was therefore undertaken to determine the clinical characteristics and 21-month follow-up outcomes of chronic HF in relation to the presence of LBBB, RBBB, and LAFB, and the absence of IVCDs.
Methods
Study population
We screened 2254 patients with chronic HF entered in the Spanish Network for the Study of Heart Failure (REDINSCOR).14 Patients were consecutively recruited between January 2007 and January 2011 at HF units in 18 hospitals. Inclusion criteria were: (i) age older than 18 years; and (ii) prior hospital admission (>24 h) due to HF and at least one echocardiographic abnormality (LVEF ≤40%, LV end-diastolic diameter ≥60 mm, altered LV relaxation indicating diastolic dysfunction, or thickness of interventricular septum/LV posterior wall ≥14 mm). All patients were symptomatic (functional NYHA class II–IV) and were treated according to the established clinical guidelines. Exclusion criteria were: (i) reversible acute HF; (ii) severe valvular disease amenable to surgical repair; (iii) right HF secondary to chronic cor pulmonale; and (iv) concomitant terminal disease. The investigation conforms with the principles outlined in the Declaration of Helsinki. The protocol was approved by the ethics committees of all centres, and all patients gave written informed consent to participate in the study.
Study variables
Data were collected using specifically designed web forms, and quality control was carried out every month. We recorded 93 clinical variables at study inclusion, and standard criteria were used to define them. Cardiovascular risk factors were eventually coded as dichotomous variables (yes/no). Central obesity was defined as abdominal circumference ≥88 cm in women and ≥102 cm in men.15 Body mass index (kg/m2) was entered as a continuous variable. Anaemia was defined as haemoglobin <120 g/L for women and <130 g/L for men.16 The plasma levels of NT-proBNP and BNP were dichotomized for a cut-off value of >118.2 pmol/L and >43.41 pmol/L, respectively.17 The LV mass was determined according to previously published methods.18 The LV relaxation parameters were calculated according to transmitral flow patterns.19 Right ventricular (RV) function was estimated by tricuspid annular plane systolic excursion (TAPSE).20 The estimated glomerular filtration rate (GFR) was calculated using the MDRD (Modification of Diet in Renal Disease) method.21 Plasma levels of cardiac troponin I or T were considered ‘high’ according to the reference cut-off value for each hospital.
Left bundle branch block was defined by a prolonged QRS duration of ≥0.12 s associated with a broad, notched R wave without q waves in leads I, aVL, and V6, and an rS pattern in lead V1. RBBB was characterized by prolonged QRS duration of ≥0.12 s associated with an R, rSR', or qR wave in lead V1; wide, slurred S waves in leads I, aVL, V5, and V6; and a wide terminal r wave in aVR. LAFB was defined by leftward axis deviation of –45° or more associated with qR wave in leads I and aVL and an rS pattern in leads II, III, and aVF.
Follow-up
Follow-up data were obtained from the outpatient annual visits or from the readmission and event reports. Total mortality included all cardiac and non-cardiac deaths. Cardiac mortality included death due to pump failure and sudden death. Pump failure death included patients dying because of refractory HF and patients undergoing urgent cardiac transplantation. Sudden death was defined as an unexpected natural death with no apparent cause occurring < 1 h after the onset of symptoms. Patients lost to follow-up and those submitted to non-emergency heart transplant were censured for the analysis. The reported deaths were reviewed by an ad hoc committee.
Statistical analysis
Continuous variables were expressed as the mean ±standard deviation (SD) and the categorical variables are presented as frequency and percentage. Differences in the categorical variables were assessed by the χ2 test or Fisher's exact test, and differences in continuous variables were analysed by analysis of variance (ANOVA). A multivariate analysis (Cox model) was built to assess the influence of the different IVCDs on survival, and a Cox proportional hazard regression model was used to identify independent predictors of readmissions and cardiac death for each IVCD. Variables showing a significant level in the univariate model (P < 0.1) were thereafter included in the multivariate Cox model following a backward stepwise approach. The final model was adjusted for those variables categorized as clinically relevant. Moreover, confounding variables were included when they carry a change of the effect on the hazard ratio >10%.22 The proportionality assumption of the models was verified using time-dependent variables. Variables with >10% of missing data were not included in the Cox models, and a multivariate regression imputation was applied, whenever necessary.23 A two-sided P < 0.05 was considered statistically significant. All analyses were performed using SPSS (v 19.0) software.
Results
Clinical characteristics
Among the 2254 patients screened, 532 (23.6%) presented LBBB, 134 (6%) RBBB, 154 (6.8%) LAFB, and 942 (41.8%) no IVCDs at inclusion. The remaining 492 patients (21.8%) presented left posterior fascicular block (n = 14), combined BBB (n = 87), non-specific intraventricular conduction (n = 131), and ventricular pacing rhythm (n = 260), and they were not included in the analysis. Thus, the final study population consisted of 1762 patients (mean age 66 years, 68% men, 57% in NYHA class III–IV, mean LVEF of 36%).
As shown in Table 1, there were significant clinical differences between the study groups. Patients with LBBB had a more frequent history of dilated cardiomyopathy and presented with the most dilated and weighted LV, more advanced mitral valve regurgitation, largest QRS duration, and most depressed LVEF. Patients with RBBB had a more frequent history of ischaemic heart disease and prior myocardial infarction, a greatest proportion of central obesity, signs of left and right HF, and abnormal RV motion at echocardiography. Patients with LAFB presented an intermediate proportion of risk factors and degree of structural and functional cardiac involvement, and had a lower prevalence of previous myocardial infarction than patients with LBBB or RBBB. However, deterioration of the NYHA functional class and presence of signs of left and right HF remained highly expressed in patients with LAFB. Patients free of IVCDs were more often women, with less enlarged left ventricles and less depressed LVEF. The mean haemoglobin values were normal, but anaemia was present in 673 patiens (38%). Anaemic patients were distributed similarly among the study groups, and had lower GFR (61 vs. 73 mL/min/1.73 m2) and received more antithrombotics (63% vs. 49%) and diuretics (88% vs. 81%) than non-anaemic patients (P < 0.001). Erythropoietin-stimulating agents were administered in 13 patients (0.73%).
Table 1.
Baseline clinical characteristics of 1762 patients with and without intraventricular conduction defects
| Variables | LBBB (n = 532, 30.2%) | RBBB (n = 134, 7.6%) | LAFB (n = 154, 8.7%) | No IVCDs (n = 942, 53.5%) | P-value |
|---|---|---|---|---|---|
| Age, years | 66.8 ± 11.6 | 67.6 ± 12.6 | 68.5 ± 13.8 | 64.7 ± 13.7 | <0.001 |
| Sex, male | 380 (71%) | 101 (75%) | 108 (70%) | 610 (65%) | 0.011 |
| Diabetes mellitus | 209 (39%) | 58 (43%) | 53 (34%) | 406 (43%) | 0.146 |
| Hypertension | 351 (66%) | 98 (73%) | 111 (72%) | 639 (68%) | 0.282 |
| Prior AMI | 176 (33%) | 59 (44%) | 45 (29%) | 352 (37%) | 0.023 |
| Ischaemic heart disease | 228 (43%) | 70 (52%) | 61 (40%) | 450 (48%) | 0.047 |
| Dilated myocardiopathy | 210 (39%) | 21 (16%) | 49 (32%) | 215 (23%) | <0.0001 |
| BMI, kg/m2 | 28.3 ± 4.6 | 29.3 ± 5.8 | 28.7 ± 4.2 | 29.2 ± 5.4 | 0.011 |
| Central obesity | 299 (56%) | 91 (68%) | 87 (56%) | 586 (62%) | 0.025 |
| NYHA class, III–IV | 316 (59%) | 75 (56%) | 98 (64%) | 512 (54%) | 0.081 |
| Orthopnoea | 222 (42%) | 64 (48%) | 76 (49%) | 348 (37%) | 0.004 |
| Nocturnal dyspnoea | 102 (19%) | 38 (28%) | 36 (23%) | 176 (19%) | 0.042 |
| Third heart sound | 95 (18%) | 23 (17%) | 14 (9%) | 104 (11%) | <0.001 |
| Lower limb oedema | 137 (26%) | 58 (43%) | 54 (35%) | 270 (29%) | <0.001 |
| Jugular ingurgitation | 129 (24%) | 37 (28%) | 43 (28%) | 168 (18%) | <0.001 |
| Hepatomegaly | 76 (14%) | 32 (24%) | 17 (11%) | 108 (11%) | <0.001 |
| Hepatojugular reflex | 91 (17%) | 27 (20%) | 33 (21%) | 124 (13%) | 0.011 |
| Ascites | 27 (5%) | 16 (12%) | 9 (6%) | 48 (5%) | 0.013 |
| RR interval, ms | 826 ± 168 | 801 ± 169 | 817 ± 162 | 808 ± 175 | 0.223 |
| QRS duration, ms | 148 ± 28 | 135 ± 28 | 111 ± 25 | 98 ± 20 | <0.0001 |
| AF/flutter | 125 (23%) | 44 (33%) | 37 (24%) | 260 (28%) | 0.098 |
| LVEDD, mm | 65 ± 10 | 57 ± 10 | 61 ± 11 | 58 ± 10 | <0.0001 |
| Indexed LVEDD, mm/m2 | 34 ± 6 | 30 ± 6 | 32 ± 6 | 30 ± 6 | <0.0001 |
| RVEDD, mma | 31 ± 7 | 32 ± 8 | 32 ± 7 | 31 ± 7 | 0.190 |
| Indexed RVEDD, mm/m2a | 16 ± 4 | 17 ± 5 | 17 ± 4 | 16 ± 4 | 0.279 |
| LVEF, % | 29 ± 10 | 39 ± 15 | 36 ± 16 | 39 ± 16 | <0.0001 |
| LV mass, g | 404 ± 123 | 351 ± 103 | 372 ± 119 | 348 ± 121 | <0.0001 |
| LV mass index, g/m2 | 212 ± 62 | 182 ± 55 | 196 ± 60 | 181 ± 64 | <0.0001 |
| LA, mm | 48 ± 9 | 49 ± 9 | 48 ± 7 | 46 ± 8 | <0.001 |
| Indexed LA, mm/m2 | 25 ± 5 | 25 ± 6 | 25 ± 5 | 24 ± 5 | <0.001 |
| Septal thickness, mm | 11 ± 3 | 12 ± 3 | 12 ± 3 | 12 ± 3 | <0.0001 |
| LV posterior wall, mm | 11 ± 2 | 11 ± 3 | 11 ± 2 | 11 ± 2 | 0.126 |
| Mitral regurgitation, III/IV | 141 (27%) | 18 (13%) | 20 (13%) | 145 (15%) | <0.0001 |
| Normal RV motion | 393 (74%) | 93 (69%) | 111 (72%) | 764 (81%) | <0.001 |
| Abnormal LV relaxation | 157 (36%) | 41 (34%) | 46 (37%) | 337 (40%) | 0.351 |
| Pseudonormal LV relaxation | 66 (15%) | 18 (15%) | 14 (11%) | 114 (13%) | 0.696 |
| Restrictive LV relaxation | 101 (23%) | 25 (21%) | 25 (20%) | 166 (20%) | 0.587 |
| Haemoglobin, g/L | 131 ± 20 | 131 ± 21 | 130 ± 18 | 132 ± 21 | 0.779 |
| eGFR (mL/min/1.73 m2) | 67 ± 25 | 68 ± 29 | 66 ± 27 | 70 ± 27 | 0.052 |
| NT-proBNP or BNP highb | 292 (70%) | 69 (64%) | 83 (72%) | 438 (60%) | 0.003 |
| ACE inhibitor | 356 (67%) | 93 (69%) | 107 (69%) | 618 (66%) | 0.684 |
| ARB | 118 (22%) | 27 (20%) | 29 (19%) | 206 (22%) | 0.799 |
| RAAS blockade | 462 (87%) | 117 (87%) | 135 (88%) | 805 (85%) | 0.792 |
| Beta-blockers | 442 (83%) | 102 (76%) | 115 (75%) | 755 (80%) | 0.067 |
| Aldosterone antagonists | 332 (62%) | 71 (53%) | 94 (61%) | 467 (50%) | <0.0001 |
| Digoxin | 132 (25%) | 36 (27%) | 43 (28%) | 191 (20%) | 0.042 |
| Loop diuretics | 468 (88%) | 116 (87%) | 141 (92%) | 748 (79%) | <0.0001 |
| Antithrombotics | 271 (51%) | 79 (59%) | 71 (46%) | 542 (58%) | 0.009 |
| Anticoagulants | 226 (42%) | 62 (46%) | 74 (48%) | 368 (39%) | 0.090 |
| Erythropoietin stimulation | 2 (0.37%) | 1 (0.74%) | 3 (1.94%) | 7 (0.74%) | 0.257 |
| ICD | 59 (11%) | 12 (9%) | 13 (8%) | 70 (7%) | 0.125 |
| CRT/CRT-D | 26 (5%) | 4 (3%) | 9 (6%) | 12 (1%) | <0.001 |
Qualitative data are presented as absolute frequencies and percentages. Quantitative data are expressed as mean ± SD.
AMI, acute myocardial infarction; BMI, body mass index; CRT-D, CRT with cardioverter defibrillation function; eGFR, estimated glomerular filtration rate (MDRD method); ICD, implantable cardioverter-defibrillator; IVCD, intraventricular conduction defect; LA, left atrium; LAFB, left anterior fascicular block; LVEDD, left ventricular end-diastolic diameter; RAAS blockade, renin–angiotensin–aldosterone system blockade; RBBB, right bundle branch block; RVEDD, right ventricular end-diastolic diameter.
aValid cases: 1054 (60%).
bValid cases: 1368 (78%).
The percentage of drug prescription varied among the study categories. Patients with LBBB tended to receive more beta-blockers, whereas loop diuretics were used more in patients with RBBB or LAFB. At the end of the follow-up, the total percentage of patients with ACE inhibitors changed from 68% at inclusion to 63% (P < 0.001), aldosterone antagonists from 54% to 51% (P = 0.012), and diuretics from 82% to 78% (P < 0.001), but the percentage of renin–angiotensin–aldosterone system (RAAS) blockers (88%) and beta-blockers (82%) remained the same. Similar trends were observed in all four study groups.
An implantable cardioverter-defibrillator (ICD) was inserted in 154 patients before inclusion (Table 1), and 70 devices (Table 2) were implanted during the follow-up (65 new implants and 5 reimplants). The proportion of implanted ICDs among the four groups did not differ significantly. CRT was applied to 140 patients (51 before inclusion and 89 during the follow-up), and in 117 of them (83%) an ICD was added to the CRT (CRT-D). Thus, the total number of patients with ICD either alone or in combination with CRT was 330 (19% of the study cohort). The percentage of implantation of a CRT/CRT-D was 18% in LBBB, 7% in RBBB, 7% in LAFB, and 2% in the no IVCD group (P < 0.001). In 69% of cases, the ICD was implanted for primary prevention and 18% of patients received appropriate shocks to treat severe ventricular arrhythmias.
Table 2.
Readmissions and mortality rates in heart failure patients with and without intraventricular conduction defects after a median follow-up of 21 months
| Variables | LBBB (n = 532, 30.2%) | RBBB (n = 134, 7.6%) | LAFB (n = 154, 8.7%) | No IVCDs (n = 942, 53.5%) | P-value |
|---|---|---|---|---|---|
| Readmissionsa | |||||
| All-cause readmission | 219 (41%) | 52 (39%) | 46 (30%) | 282 (30%) | <0.0001 |
| Heart failure | 128 (24%) | 36 (27%) | 37 (24%) | 197 (21%) | 0.289 |
| Myocardial ischaemia | 35 (7%) | 12 (9%) | 6 (4%) | 59 (6%) | 0.370 |
| Arrhythmias | 102 (19%) | 16 (12%) | 13 (8%) | 63 (7%) | <0.0001 |
| ICD | 23 (4%) | 4 (3%) | 7 (5%) | 36 (4%) | 0.873 |
| CRT/CRT-D | 72 (14%) | 5 (4%) | 2 (1%) | 10 (1%) | <0.0001 |
| Cardiac transplantation | 23 (4%) | 5 (4%) | 7 (5%) | 23 (2%) | 0.190 |
| Mortality | |||||
| All-cause mortality | 125 (23%) | 36 (27%) | 31 (20%) | 140 (15%) | <0.0001 |
| Cardiovascular death | 99 (19%) | 27 (20%) | 25 (16%) | 106 (11%) | <0.001 |
| Cardiac death | 94 (18%) | 24 (18%) | 21 (14%) | 91 (10%) | <0.0001 |
| Pump failure | 66 (12%) | 23 (17%) | 18 (12%) | 64 (7%) | <0.0001 |
| Sudden | 28 (5%) | 1 (1%) | 3 (2%) | 27 (3%) | 0.019 |
| Vascular | 5 (1%) | 3 (2%) | 4 (3%) | 15 (2%) | 0.308 |
| Extravascular | 21 (4%) | 5 (4%) | 6 (4%) | 29 (3%) | 0.750 |
| Unknown cause | 5 (0.9%) | 4 (3.0%) | 0 (0%) | 5 (0.5%) | 0.037 |
aOne or more readmissions for the same cause.
CRT-D, cardiac resynchronization therapy with cardioverter defibrillation function; ICD, implantable cardioverter-defibrillator; IVCD, intraventricular conduction defect; LAFB, left anterior fascicular block; RBBB, right bundle branch block.
Outcomes
Patients were followed for a median of 21 months (interquartile range 11–33) and 15 of them (0.9%) were lost to follow-up. Non-emergency heart transplant was performed in 43 patients (2.4%). There were 666 readmissions due to decompensated HF, 148 due to myocardial ischaemia, and 222 due to arrhythmias. Death occurred in 332 patients (interannual mortality rate of 10.8%). Causes of death were: cardiovascular in 257 (77.4%), extravascular in 61 (18.4%), and of unknown origin in 14 (4.2%) patients. Among the cardiac deaths, 171 were due to pump failure and 59 occurred suddenly. As shown in Table 2, patients with RBBB or LBBB had the highest rates of readmissions and mortality, followed by patients with LAFB, and finally by patients free of IVCDs. After adjustment for age, prior myocardial infarction, diabetes, central obesity, mitral valve regurgitation, signs of left and right HF, LVEF, left atrial size, LV mass, haemoglobin, renal function, ICD, CRT, and drug therapy, the Cox model showed that RBBB and LBBB but not LAFB continued to carry a 21-month mortality risk higher than that of patients free of IVCDs (Figure 1, Table 3). Although not significant, the hazard ratio for readmission, cardiac death, and pump failure death tended to be higher in the RBBB than in the LBBB group. In the multivariate analysis, the most prevailing predictors of these events were diabetes mellitus, prior myocardial infarction, presence of signs of left and right HF, anaemia, and decreased GFR (Supplementary material, Table S1). The beneficial effect of ICD and CRT on cardiac death and pump failure death did not reach statistical significance. In contrast, prescription of beta-blockers and RAAS blockers predicted significant benefit on mortality risk (Supplementary material, Table S1).
Figure 1.
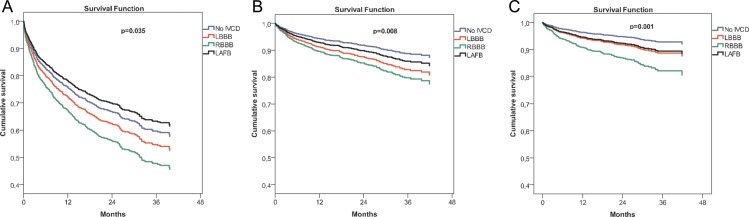
Adjusted Cox model survival curves for readmissions (A), cardiac death (B), and pump failure death (C) in patients with chronic heart failure with and without intraventricular conduction defects (IVCDs) after a median follow-up of 21 months. LAFB, left anterior fascicular block; LBBB, left bundle branch block; RBBB, right bundle branch block.
Table 3.
Hazard ratios for readmissions and cardiac mortality in heart failure patients with and without intraventricular conduction defects after a median follow-up of 21 months
| P-value | HR | 95% CI for HR |
||
|---|---|---|---|---|
| Lower | Upper | |||
| All-cause readmissiona | ||||
| LBBB vs. no IVCD | 0.123 | 1.172 | 0.958 | 1.433 |
| RBBB vs. no IVCD | 0.018 | 1.433 | 1.063 | 1.930 |
| LAFB vs. no IVCD | 0.459 | 0.887 | 0.646 | 1.218 |
| Cardiac deathb | ||||
| LBBB vs. no IVCD | 0.005 | 1.579 | 1.149 | 2.169 |
| RBBB vs. no IVCD | 0.007 | 1.894 | 1.192 | 3.008 |
| LAFB vs. no IVCD | 0.336 | 1.266 | 0.783 | 2.049 |
| Pump failure deathc | ||||
| LBBB vs. no IVCD | 0.015 | 1.600 | 1.097 | 2.335 |
| RBBB vs. no IVCD | 0.0001 | 2.620 | 1.603 | 4.283 |
| LAFB vs. no IVCD | 0.139 | 1.492 | 0.878 | 2.535 |
AMI, acute myocardial infarction; CI, confidence interval; eGFR, estimated glomerular filtration rate (MDRD method); HR, hazard ratio; ICD, implantable cardioverter-defibrillator; IVCD, intraventricular conduction defect; LA, left atrial; LAFB, left anterior fascicular block; RAAS blockade: renin–angiotensin–aldosterone system blockade; RBBB: right bundle branch block.
aAdjusted for: age, ICD, CRT, signs of left HF, signs of right HF, LVEF, diabetes mellitus, prior AMI, LV mass index, haemoglobin, eGFR, and loop diuretics.
bAdjusted for: age, ICD, CRT, signs of left HF, signs of right HF, LVEF, diabetes mellitus, prior AMI, LV mass index, indexed LA diameter, mitral valve regurgitation III/IV, haemoglobin, beta-blockers, and RAAS blockade.
cAdjusted for: age, ICD, CRT, signs of left HF, signs of right HF, LVEF, diabetes mellitus, LV mass index, indexed LA diameter, haemoglobin, eGFR, beta-blockers, and RAAS blockade.
During the follow-up, 65 de novo cases of IVCDs were recorded: 26 LBBB, 20 RBBB, and 19 LAFB. Patients with new onset of IVCDs continued to be ascribed to the free of IVCDs group because patient categorization was done according to the inclusion ECG.
Discussion
This study provides integrative information on the clinical and prognostic influence of the most frequent IVCDs in a cohort of patients with chronic HF. Differences in the clinical profile and 21-month risk of readmissions and cause of death were observed among patients with LBBB, RBBB, and LAFB, and in those without IVCDs.
Clinical characteristics
A clinical phenotype characterized by LV dilation with markedly depressed systolic function, advanced mitral valve regurgitation, and history of dilated cardiomyopathy was more often observed in patients with LBBB. In contrast, patients with RBBB presented with overt signs of right and left HF, more depressed RV motion at echocardiography, and more frequently reported a history of coronary heart disease. On the other hand, patients with LAFB showed intermediate degrees of structural LV derangements with respect to LBBB and RBBB, although they still presented marked signs of left and right HF and advanced NYHA functional class. Patients free of IVCDs were more often women with less enlarged left ventricles and less depressed LVEF, suggesting a predominance of a diastolic rather than a systolic dysfunctional substrate. The clinical–ECG associations described in this study have not been previously recognized, due to the lack of studies analysing more than two IVCDs in the same cohort. On the other hand, not all studies assessing the prognosis of both LBBB and RBBB in the same series of patients8,10–12 have provided echocardiographic information, and, moreover, specific data on relevant structural cardiac features such as LV mass, LV diameter, or LA size, or resynchronization therapies were not reported in these studies. However, specific findings such as the observation of a more advanced mitral valve regurgitation in patients with LBBB in one study,8 or the higher prevalence of previous myocardial infarction in HF patients with RBBB reported in three studies8,11,12 are in agreement with the clinical patterns found in our patients. Furthermore, there is a coherence between the pharmacological treatment instituted in our patients and their clinical phenotype, since, in accordance with the predominance of LV dilation in patients with LBBB, they received more CRT and beta-blockers. Likewise, patients with RBBB received more loop diuretics in consonance with their advanced signs of congestive HF and greater percentage of depressed RV motion at echocardiography. It is therefore unlikely that the clinical associations observed in our study were fortuitous as they share clinical and pathophysiological plausibility. The mechanistic foundation for the association between LBBB and LV dilation is provided by experimental observations demonstrating that LBBB itself is able to induce LV dilation.5 On the other hand, LV dilation could secondarily induce LBBB because the increased wall stress linked to the dilated cavity24 may overstretch the left bundle branch fibres and then impair conduction through them. An example of impairment of bundle branch conduction secondary to ventricular dilation is the occurrence of RBBB in the course of pulmonary thrombo-embolism.25
Outcomes
The prognosis of LBBB has been assessed in several clinical registries,7–12,26,27 and although most of them found a worse outcome in patients with LBBB, other studies reported a more unfavourable prognosis linked to RBBB. In a cohort of 5517 patients with congestive HF,7 patients with LBBB (n = 1391) showed a higher 1-year all-cause mortality and sudden death than controls free of LBBB. An unfavourable prognosis of LBBB was also observed in a cohort of patients with acute HF 1 year after admission.9 However, studies simultaneously comparing the prognosis of LBBB and RBBB in the same cohort of patients have not afforded consistent results since some of these studies report a more unfavourable prognosis in LBBB, whereas others found increased mortality risk in patients with RBBB. Indeed, in a series of 9082 hospitalized HF patients with LBBB (n = 1480) and RBBB (n = 651) followed for 5 years, the adjusted risk of death was higher in patients with LBBB than in those with RBBB.10 Likewise, in a cohort of 110 000 subjects free of cardiovascular disease followed up for 9.5 years, 310 subjects developed BBB (LBBB, 112; RBBB, 198), and those with LBBB presented increased prevalence of cardiovascular disease and higher cardiac mortality than age- and sex-matched controls.13 In contrast, a study including 3200 hospitalized patients with acute HF showed that rehospitalization and death occurred more frequently in patients with RBBB (n = 118) than in those with LBBB (n =107).12 Likewise, in a cohort of 1888 patients with HF and LVEF <50%, RBBB (n = 193) had a 4-year all-cause mortality higher than LBBB (n = 306).11 Finally, a comparable all-cause mortality of LBBB and RBBB was observed in patients with decompensated HF 23 months after being admitted to an intensive care unit.8
Over the last 10 years, randomized trials have consistently demonstrated a significant reduction in morbidity and mortality in patients with mild to advanced HF and prolonged QRS complex duration treated with CRT,28,29 particularly when an ICD is added to the CRT.29 In our study, the beneficial effect of CRT on mortality in the Cox model did not reach statistical significance even though 83% of the 140 CRT-implanted patients had an added ICD and, therefore, an optimal therapy. A rate of CRT implantation higher than ours could theoretically mitigate the worse prognosis of IVCD, but this cannot be ascertained because the CRT implantation rate is not reported in the above-reviewed studies on the prognosis of BBB. Moreover, CRT implantation rates were largely heterogeneous across the European countries: in 2006 (our study began in January 2007), >80 devices per million inhabitants were implanted in six countries, whereas <40 devices per million inhabitants were implanted in another four, including Spain.30
In summary, our study reveals that both LBBB and RBBB are associated with a higher 21-month incidence of cardiac death and pump failure death than for patients with LAFB and patients free of IVCDs. Thus, in addition to the advanced therapies that are currently applied to patients with LBBB (i.e. LV resynchronization), a search for more specific therapies in HF patients with RBBB is warranted.
Study limitations
Among the initially screened cohort of 2254 patients, there were 492 (21.8%) patients who presented either combined BBB, non-specific IVC, ventricular pacing rhythm, or left posterior fascicular block. These patients were not included because the objective of the study was to analyse the specific influence of the three most common IVCDs.
This study was conducted in tertiary hospitals and, as compared with patients currently attending primary care services, they probably presented with more advanced stages of the disease, as suggested by >50% of patients in NYHA class III–IV. Therefore, our results apply to patients with well-defined underlying structural derangements and not to patients still in the early stages of the disease. However, knowledge of the prognostic risk factors detected in patients with evolved HF could guide implementation of more appropriate measures to slow the clinical progression of the disease.
Supplementary material
Supplementary material is available at European Journal of Heart Failure online.
Funding
The Spanish Ministry of Science and Innovation, Redes de Investigación del Instituto de Salud Carlos III (REDINSCOR, grant no. RD06/0003); FondoEuropeo de Desarrollo Regional (FEDER).
Acknowledgements
The Spanish Ministry of Science and Innovation, Redes de Investigación del Instituto de Salud Carlos III (REDINSCOR, grant no. RD06/0003); FondoEuropeo de Desarrollo Regional (FEDER).
Conflict of interest: none declared.
Appendix 1. REDINSCOR investigators
Hospital Clínic de Barcelona: J. Brugada, M. Batlle, A. Berruezo, S. Hevia, L. Mont, F. Pérez-Villa; Hospital de la Santa Creu i Sant Pau: J. Cinca, E. Roig, A. Bayés de Luna, X. Borrás, F. Carreras, A. Ferrero, J.M. Guerra, L. Hove-Madsen, E. Jorge, R. Martínez, J. Padró, T. Puig, N. Ribas, X. Viñolas, J. Alvarez-Garcia; Hospital Clínico Universitario de Santiago de Compostela: J.R. González-Juanatey, M. Bandín, S. Eiras, L. Fernández-Hernández, J. García-Acuña, I. Gómez-Otero, L. Grigorian-Shamagian, F. Lago, P. Manzón, M. Moure, F. Otero-Raviña, F. Otero-Santiago, B.K. Rodino Janeiro, J. Rubio, A. Salgado, A. Seoane, A. Varela, P.V. Lear; Hospital Clínico San Carlos–Medicina Interna: A. Fernández-Cruz, A. Alvarez de Arcaya Vicente, M. Avila, E. Bordiu, L. Calle, C. Fernández-Pinilla, D. Gómez-Garre, L. González-Rubio, J. Marco, N. Martell, P. Muñoz-Pacheco, A. Ortega, R. Patiño, J. Pedrajas, L. Reinares; Hospital Clínico San Carlos–Instituto Cardiovascular: J. Pérez-Villacastín, R. Bover, M. Cobos, J. García-Quintanilla, J. Moreno, N. Pérez-Castellano, M. Pérez-Serrano, I. Vila; Hospital 12 de Octubre: J.F. Delgado, F. Arribas, P. Escribano, A. Flox, C. Jiménez López-Guarch, M. Paradina, J. Ruiz-Cano, C. Sáenz de la Calzada, R. Salguero, V. Sánchez-Sánchez, R. Tello de Meneses, M. Vicente-Hernández; Clínica Puerta de Hierro: L. Alonso-Pulpón, I. Fernández -Lozano, P. García-Pavía, A. García-Touchard, M. Gómez-Bueno, J. Márquez, J. Segovia, L. Silva, M. Vázquez-Mosquera; Hospital Universitario Virgen de la Arrixaca: M. Valdés, A. García-Alberola, I. Garrido, D. A. Pascual-Figal, F.J. Pastor-Pérez, J. Sánchez-Más, P. Tornel; Hospital La Fe: M. Rivera, L. Almenar, R. Cortés, L. Martínez-Dolz, J. Montero, M. Portolés, E. Roselló-Lleti, A. Salvador, V. Vila; Hospital Universitario Virgen de Valme: R. Vázquez, J. Cubero, A. Fernández-Palacín, D. García-Medina, S. García-Rey, E. Laguna, J. Leal del Ojo, F. Miñano, L. Pastor-Torres, R. Pavón, A. Pérez-Navarro, D. Villagómez; Hospital Universitario Puerta del Mar: R. Vázquez, R. Arana, D. Bartolomé, P. Cabeza, G. Calle-Pérez, F. Camacho, L. Cano, A. Carrillo, E. Díaz-Retamino, V. Escolar, R. Fernández-Rivero, S. Gamaza, A. Giráldes, N. Hernández-Vicente, M. Lagares, J. López-Benítez, M. Marante, E. Otero, J. Pedregal, M. Sancho-Jaldón, R. Sevillano, R. Zayas; Gerencia del Ámbito Territorial de Bacelona-Institut Català Salud: J.M. Verdú, S. Aguilar, M. Aizpurúa, F. Alguacil, J. Casacuberta, J. Cerain, M. Domingo, M. García-Lareo, J. Herrero-Melechón, N. López-Pareja, A. Mena, A. Pérez-Orcero, J. Rodríguez- Cristóbal, M. Rozas, J. Sorribes, P. Torán; Hospital Universitario Arnau de Vilanova: F. Worner, L. Barta, C. Bravo, J. Cabau, J. Casanova, B. Daga, I. De la Puerta, I. Hernández-Martín, E. Piñol, E. Pueo, G. Torres, A. Troncoso, D. Viles; Hospital Universitario Joan XXIII: A. Bardají, J. Mercè, E. Sanz-Girgas, P. Valdovinos; Hospital Universitario Virgen Macarena: O. Aramburu, J. Arias; Hospital Guadarrama: C. García-González, M. Alonso, C. Bischofberger, G. Domínguez-De Pablos; Hospital Morales Meseguer: D. Jiménez-Cervantes, I. Ureña; Hospital Son Dureta: A. Grau-Sepúlveda, C. Fiol, P. Pericas, M. Villalonga; Hospital Francesc de Borja de Gandía: P. Orosa, J. Agüero; Hospital Municipal de Badalona: F. Planas-Aymá, J. Grau-Amoros, F. Planas-Comes, L. San Vicente.
References
- 1.Xiao HB, Lee CH, Gibson DG. Effect of left bundle branch block on diastolic function in dilated cardiomyopathy. Br Heart J. 1991;66:443–447. doi: 10.1136/hrt.66.6.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grines CL, Bashore TM, Boudoulas H, Olson S, Shafer P, Wooley CF. Functional abnormalities in isolated left bundle branch block. The effect of interventricular asynchrony. Circulation. 1989;79:845–853. doi: 10.1161/01.cir.79.4.845. [DOI] [PubMed] [Google Scholar]
- 3.Kang SJ, Song JK, Yang HS, Song JM, Kang DH, Rhee KS, Nam GB, Choi KJ, Kim JJ, Kim YH. Systolic and diastolic regional myocardial motion of pacing-induced versus idiopathic left bundle branch block with and without left ventricular dysfunction. Am J Cardiol. 2004;93:1243–1246. doi: 10.1016/j.amjcard.2004.01.068. [DOI] [PubMed] [Google Scholar]
- 4.Li CH, Carreras F, Leta R, Carballeira L, Pujadas S, Pons-Llado G. Mechanical left ventricular dyssynchrony detection by endocardium displacement analysis with 3D speckle tracking technology. Int J Cardiovasc Imaging. 2010;8:867–870. doi: 10.1007/s10554-010-9644-x. [DOI] [PubMed] [Google Scholar]
- 5.Vernooy K, Verbeek XA, Peschar M, Crijns HJ, Arts T, Cornelussen RN, Prinzen FW. Left bundle branch block induces ventricular remodelling and functional septal hypoperfusion. Eur Heart J. 2005;26:91–98. doi: 10.1093/eurheartj/ehi008. [DOI] [PubMed] [Google Scholar]
- 6.Bertini M, Sengupta PP, Nucifora G, Delgado V, Ng AC, Marsan NA, Shanks M, van Bommel RJ, Schalij MJ, Narula J, Bax JJ. Role of left ventricular twist mechanics in the assessment of cardiac dyssynchrony in heart failure. JACC Cardiovasc Imaging. 2009;2:1425–1435. doi: 10.1016/j.jcmg.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Baldasseroni S, Opasich C, Gorini M, Lucci D, Marchionni N, Marini M, Campana C, Perini G, Deorsola A, Masotti G, Tavazzi L, Maggioni AP Italian Network on Congestive Heart Failure Investigators. Left bundle-branch block is associated with increased 1-year sudden and total mortality rate in 5517 outpatients with congestive heart failure: a report from the Italian Network on Congestive Heart Failure. Am Heart J. 2002;143:398–405. doi: 10.1067/mhj.2002.121264. [DOI] [PubMed] [Google Scholar]
- 8.McCullough PA, Hassan SA, Pallekonda V, Sandberg KR, Nori DB, Soman SS, Bhatt S, Hudson MP, Weaver WD. Bundle branch block patterns, age, renal dysfunction, and heart failure mortality. Int J Cardiol. 2005;102:303–308. doi: 10.1016/j.ijcard.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Huvelle E, Fay R, Alla F, Solal A, Mebazaa A, Zannad F. Left bundle branch block and mortality in patients with acute heart failure syndrome: substudy of the EFICA cohort. Eur J Heart Fail. 2010;12:156–163. doi: 10.1093/eurjhf/hfp180. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Qadir HM, Tu JV, Austin PC, Wang JT, Lee DS. Bundle branch block patterns and long-term outcomes in heart failure. Int J Cardiol. 2011;146:213–218. doi: 10.1016/j.ijcard.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Barsheshet A, Goldenberg I, Garty M, Gottlieb S, Sandach A, Laish-Farkash A, Eldar M, Glikson M. Relation of bundle branch block to long-term (four-year) mortality in hospitalized patients with systolic heart failure. Am J Cardiol. 2011;107:540–544. doi: 10.1016/j.amjcard.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Hong SJ, Oh J, Kang SM, Youn JC, Han S, Jeon ES, Cho MC, Kim JJ, Yoo BS, Chae SC, Oh BH, Choi DJ, Lee MM, Ryu KH KorHF Registry. Clinical implication of right bundle branch block in hospitalized patients with acute heart failure: data from the Korean Heart Failure (KorHF) Registry. Int J Cardiol. 2012;157:416–418. doi: 10.1016/j.ijcard.2012.03.155. [DOI] [PubMed] [Google Scholar]
- 13.Fahy GJ, Pinski SL, Miller DP, McCabe N, Pye C, Walsh MJ, Robinson K. Natural history of isolated bundle branch block. Am J Cardiol. 1996;77:1185–1190. doi: 10.1016/s0002-9149(96)00160-9. [DOI] [PubMed] [Google Scholar]
- 14.Alonso-Pulpón L, Borrás X, Brugada J, Cinca J, Fernández Cruz A, González Juanatey JR, Sáenz de la Calzada C, Valdés M, Vázquez R, Pérez Villacastín J Investigadores de REDINSCOR. Clinical and Preclinical Heart Failure Research Network (REDINSCOR) Instituto de Salud Carlos III Cooperative Special Topic Research Networks. Rev Esp Cardiol. 2008;61:76–81. [PubMed] [Google Scholar]
- 15.Hu G, Jousilahti P, Antikainen R, Katzmarzyk PT, Tuomilehto J. Joint effects of physical activity, body mass index, waist circumference, and waist-to-hip ratio on the risk of heart failure. Circulation. 2010;121:237–244. doi: 10.1161/CIRCULATIONAHA.109.887893. [DOI] [PubMed] [Google Scholar]
- 16.Izaks GJ, Westendorp RG, Knook DL. The definition of anemia in older persons. JAMA. 1999;281:1714–1717. doi: 10.1001/jama.281.18.1714. [DOI] [PubMed] [Google Scholar]
- 17.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Strömberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K ESC Committee for Practice Guidelines (CPG) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J 2008. 29:2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 18.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 19.Xie GY, Berk MR, Smith MD, Gurley JC, DeMaria AN. Prognostic value of Doppler transmitral flow patterns in patients with congestive heart failure. J Am Coll Cardiol. 1994;24:132–139. doi: 10.1016/0735-1097(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 20.Kaul S, Tei C, Hopkins JM, Shah PM. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J. 1984;107:526–531. doi: 10.1016/0002-8703(84)90095-4. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Greene T, Kusekl JW, Beck GJ, Group MS. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11 A08028. [Google Scholar]
- 22.Vazquez R, Bayes-Genis A, Cygankiewicz I, Pascual-Figal D, Grigorian-Shamagian L, Pavon R, Gonzalez-Juanatey JR, Cubero JM, Pastor L, Ordonez-Llanos J, Cinca J, de Luna AB MUSIC Investigators. The MUSIC Risk score: a simple method for predicting mortality in ambulatory patients with chronic heart failure. Eur Heart J. 2009;30:1088–1096. doi: 10.1093/eurheartj/ehp032. [DOI] [PubMed] [Google Scholar]
- 23.Haukoos JS, Newgard CD. Advanced statistics: missing data in clinical research—part 1: an introduction and conceptual framework. Acad Emerg Ned. 2007;14:662–668. doi: 10.1197/j.aem.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 24.Sandler H, Dodge HT. Left ventricular tension and stress in man. Circ Res. 1963;13:91–104. doi: 10.1161/01.res.13.2.91. [DOI] [PubMed] [Google Scholar]
- 25.Petrov DB. Appearance of right bundle branch block in electrocardiograms of patients with pulmonary embolism as a marker for obstruction of the main pulmonary trunk. J Electrocardiol. 2001;34:185–188. doi: 10.1054/jelc.2001.25132. [DOI] [PubMed] [Google Scholar]
- 26.Manzano L, Babalis D, Roughton M, Shibata M, Anker SD, Ghio S, van Veldhuisen DJ, Cohen-Solal A, Coats AJ, Poole-Wilson PP, Flather MD SENIORS Investigators. Predictors of clinical outcomes in elderly patients with heart failure. Eur J Heart Fail. 2011;13:528–536. doi: 10.1093/eurjhf/hfr030. [DOI] [PubMed] [Google Scholar]
- 27.Lewinter C, Torp-Pedersen C, Cleland JGF, Køber L. Right and left bundle branch block as predictors of long-term mortality following myocardial infarction. Eur J Heart Fail. 2011;13:1349–1354. doi: 10.1093/eurjhf/hfr130. [DOI] [PubMed] [Google Scholar]
- 28.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L for the Cardiac Resynchronization Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 29.Tang ASL, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, Hohnloser SH, Nichol G, Birnie DH, Sapp JL, Yee R, Healey JS, Rouleau JL for the Resynchronization-Defibrillation for Ambulatory Heart Failure Trial (RAFT) investigators. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–2395. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 30.Swedberg K, Cleland J, Cowie MR, Nieminen M, Priori SG, Tavazi L, van Veldhuisen DJ, Alonso-Pulpon L, Camm J, Dickestein K, Drexler H, Filippatos G, Linde C, Lopez-Sendon J, Santini M, Zannad F. Successful treatment of heart failure with devices requires collaboration. Eur J Heart Fail. 2008;10:1229–1235. doi: 10.1016/j.ejheart.2008.09.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


