Abstract
Aims
Central sleep apnoea/Cheyne–Stokes respiration (CSA/CSR) is a risk factor for increased mortality and morbidity in heart failure (HF). Adaptive servo-ventilation (ASV) is a non-invasive ventilation modality for the treatment of CSA/CSR in patients with HF.
Methods
SERVE-HF is a multinational, multicentre, randomized, parallel trial designed to assess the effects of addition of ASV (PaceWave™, AutoSet CS™; ResMed) to optimal medical management compared with medical management alone (control group) in patients with symptomatic chronic HF, LVEF ≤45%, and predominant CSA. The trial is based on an event-driven group sequential design, and the final analysis will be performed when 651 events have been observed or the study is terminated at one of the two interim analyses. The aim is to randomize ∼1200 patients to be followed for a minimum of 2 years. Patients are to stay in the trial up to study termination. The first patient was randomized in February 2008 and the study is expected to end mid 2015. The primary combined endpoint is the time to first event of all-cause death, unplanned hospitalization (or unplanned prolongation of a planned hospitalization) for worsening (chronic) HF, cardiac transplantation, resuscitation of sudden cardiac arrest, or appropriate life-saving shock for ventricular fibrillation or fast ventricular tachycardia in implantable cardioverter defibrillator patients.
Perspectives
The SERVE-HF study is a randomized study that will provide important data on the effect of treatment with ASV on morbidity and mortality, as well as the cost-effectiveness of this therapy, in patients with chronic HF and predominantly CSA/CSR.
Trial registration
ISRCTN19572887
Keywords: Heart failure, Sleep-disordered breathing, Central sleep apnoea, Adaptive servo-ventilation, Randomized controlled trial
Introduction
Up to 2–3% of the populations in many industrialized countries have chronic heart failure (HF). Despite recent advances in pharmacological treatment, HF continues to cause debilitating symptoms, frequent hospital admissions, and high mortality. Although guidelines recommend therapy with beta-blockers, ACE inhibitors, and other pharmacological agents, as well as device-based management such as CRT,1 many patients with HF have persistent symptoms and most will eventually die from cardiovascular causes, often from progressive HF.
Therefore, there is a need for new interventions that reduce symptoms, increase quality of life, and reduce hospital admissions and mortality in patients with chronic HF. It is likely that these new interventions will be targeted at specific subgroups of HF patients rather than the entire population. Treatment of sleep-disordered breathing (SDB) may be one such intervention.
Sleep-disordered breathing is very common in patients with HF, with reported prevalence rates of 50–75%.2,3 The presence of SDB is associated with decreased survival in HF patients, while effective treatment of SDB could have beneficial effects.4 Two types of abnormal breathing during sleep predominate in SDB: obstructive sleep apnoea (OSA) and central sleep apnoea (CSA), which may manifest as Cheyne–Stokes respiration (CSR). There are several mechanisms by which SDB may be detrimental to cardiac function. These include tissue hypoxia and repetitive arousal from sleep with increased sympathetic nervous system activity.5,6
Obstructive sleep apnoea is caused by obstruction of the upper airway and is the most common type of sleep apnoea. The main features are repetitive pauses in breathing during sleep (despite breathing efforts) and reduced blood oxygen saturation. OSA is present in 20–45% of chronic HF patients, a proportion that is considerably higher than that in the general population.7,8
Treatment of OSA with continuous positive airway pressure (CPAP) rapidly reduces tissue hypoxia and arousals, and, over a period of months, reduces elevated sympathetic activity to normal.9,10 CPAP has also been shown to reduce blood pressure.11–13 Two studies have demonstrated marked improvements in cardiac function within 1 and 3 months of initiating CPAP treatment of OSA,14,15 and the results of a cohort study suggest improved survival in patients who are compliant with CPAP therapy.16 However, no randomized long-term outcome studies in OSA have yet been published.
Central sleep apnoea is characterized by a lack of drive to breathe during sleep, resulting in repetitive periods of insufficient ventilation and compromised oxygen supply. CSR consists of periods of hyperventilation in association with waxing and waning tidal volume alternating with periods of CSA. CSA/CSR is the most common SDB pattern seen in the chronic HF population, with a prevalence of 25–40%.7
Central sleep apnoea/Cheyne–Stokes respiration induces chemical, neural, and haemodynamic changes similar to those seen in OSA.5,6 It is an independent risk factor for death in these patients,17 and one of the key underlying mechanisms for this could be an increased risk for malignant ventricular arrhythmias.18 CSA/CSR can be treated with nasal oxygen, CPAP, or non-invasive ventilation including adaptive servo-ventilation (ASV), in increasing order of effectiveness.19 CPAP and oxygen therapy reduce respiratory events by ∼50%.
Adaptive servo-ventilation is a non-invasive ventilatory therapy that provides positive expiratory airway pressure and inspiratory pressure support, which is servo controlled based on monitoring minute ventilation (MV). It automatically adjusts pressure support to stabilize and reduce ventilation in patients with CSA/CSR. If the patient stops breathing completely, the ventilator will maintain ventilation at an automatically set respiratory rate.
One night of therapy with ASV improved the nocturnal breathing pattern and sleep quality in patients with HF and CSA/CSR.19 Sleep and breathing were better during one night of ASV therapy than during one night of treatment with oxygen or CPAP, and patients preferred ASV over both CPAP and bilevel ventilation. A small study showed beneficial effects in terms of LVEF, disease-specific quality of life, and compliance after 6 months of ASV compared with CPAP.20 ASV has a greater ability to reduce the respiratory event rate and normalize the breathing pattern in many patients.19,20
The only randomized controlled trial investigating mortality in patients with HF and CSA/CSR treated with CPAP was the CANPAP study. The trial was stopped prematurely after enrolment of 258 of the planned 408 patients, and data analysis did not show a beneficial effect of CPAP treatment.21 However, a post-hoc analysis suggested that morbidity and mortality might be improved if SDB was well controlled.22
Although clinical experience with ASV is extensive, there is a limited amount of published literature available. Several small studies have shown improvements in symptoms and measures of cardiac function, exercise tolerance, and quality of life with ASV therapy.23–25 Furthermore, a recent meta-analysis reported good control of SDB and a significant improvement in LVEF in patients with CSA/CSR treated with ASV.26
Studies to date have not been of adequate size or duration to determine whether therapy with ASV is associated with significant reductions in morbidity and mortality in patients with HF and CSA/CSR. The SERVE-HF study was designed to address these issues.
Methods
Study design
SERVE-HF is a multinational, multicentre, randomized, parallel group study. The trial is registered with Current Controlled Trials (www.controlled-trials.com; ISRCTN19572887). Patients are being randomized to optimal medical management (control group) or optimal medical treatment plus ASV [PaceWave™, Auto Set CS™; ResMed] in a 1:1 ratio. There is no plan to provide sham positive airway pressure treatment in the control arm. The study is event driven, with an anticipated sample size of ∼1200. The first patient was randomized in February 2008 and the study is expected to finish mid 2015. Patients will be followed up on average for a period of ∼54 months. Minimum follow-up time will be 24 months, and maximum ∼84 months. The average follow-up time will depend on the speed of patient recruitment. There will be a final assessment for each patient at the end of the study.
Objectives
The primary study endpoint (1a) is the time to first event: all-cause death, unplanned hospitalization (or unplanned prolongation of a planned hospitalization) for worsening chronic HF, cardiac transplantation, resuscitation of sudden cardiac arrest or appropriate life-saving shock for ventricular fibrillation and fast ventricular tachycardia in implantable cardioverter defibrillator (ICD) patients, as assessed by the Endpoint Review Committee (ERC). Two further co-primary endpoints are 1b (as for 1a, but with cardiovascular death instead of all-cause death) and 1c (as for 1a, but with all-cause unplanned hospitalization).
Secondary endpoints are as follows: time to death (non-cardiovascular or cardiovascular); time to unplanned hospitalization; proportion of follow-up days (%) during which patients are alive and are not hospitalized; changes in general and disease-specific quality of life and in HF symptoms; and change in 6 min walk distance. In addition, a health economic analysis will be conducted to determine the cost-effectiveness of ASV treatment.
For the substudy, the primary endpoint is the change in LVEF measured by echocardiography from baseline to 12 months. Secondary substudy objectives are: ventricular remodelling assessed using echocardiography; ventricular remodelling assessed using cardiac magnetic resonance imaging (MRI); changes in sleep and respiratory parameters; changes in disease-specific quality of life, cognitive function, and depression [assessed using the Kansas City Cardiomyopathy Questionnaire,27 the Mini-Mental State Examination,28 the Patient Health Questionnaire-9,29 and the Generalised Anxiety Disorder Questionnaire (GAD-7)];30 and changes in levels of NT-proBNP and other biomarkers.
Patients
The intended population for this study is patients with HF and predominant CSA. Consecutive, eligible subjects meeting the detailed inclusion and exclusion criteria (Table 1) will be considered for the study.
Table 1.
Trial inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Age >21 years | Significant COPD with FEV1 <50% of predicted (European Respiratory Society criteria) in the 4 weeks before randomization |
| Chronic HF (≥12 weeks since diagnosis) according to current European Society of Cardiology guidelines1 | Oxygen saturation ≤90% at rest during the day |
| LV systolic dysfunction (LVEF ≤45% determined using echocardiography, radionuclide angiography, left ventriculography, or cardiac magnetic resonance imaging) documented <12 weeks before randomization | Current use of PAP therapy |
| NYHA class III or IV, or NYHA class II with ≥1 hospitalization for HF in the previous 24 months | Life expectancy <1 year for diseases unrelated to chronic HF |
| No hospitalization for HF in the 4 weeks prior to enrolment | Cardiac surgery, PCI, MI, or unstable angina within the previous 6 months |
| Optimized medical treatment according to applicable guidelines with no new class of disease-modifying drug for ≥4 weeks prior to randomization. Where there was no treatment with beta-blockers or ACE inhibitors/ARBs, then the reasons must be documented | CRT implantation scheduled or performed within 6 months prior to randomization |
| Predominant central SDB was defined as an AHI >15 events/h with ≥50% central events and a central AHI ≥10 events/h, derived from PG or PSG and based on total recording time, documented within 4 weeks of randomization, with flow measurement performed using a nasal cannula | TIA or stroke within the previous 3 months |
| Primary haemodynamically significant uncorrected valvular heart disease (obstructive or regurgitant) or any valvular disease expected to require surgery during the trial | |
| Acute myocarditis/pericarditis within the previous 6 months | |
| Untreated or therapy-refractory RLS | |
| Contraindication to the use of AutoSet CS because of symptomatic hypotension or significant intravascular volume depletion or pneumothorax or pneumomediastinum | |
| Pregnancy |
AHI, apnoea–hypopnoea index; FEV1, forced expiratory volume in 1 s; HF, heart failure; MI, myocardial infarction; PAP, positive airway pressure; PG, polygraphy; PSG, polysomnography; RLS, restless legs syndrome; SDB, sleep-disordered breathing; TIA, transient ischaemic attack.
Study plan, investigations, and treatment
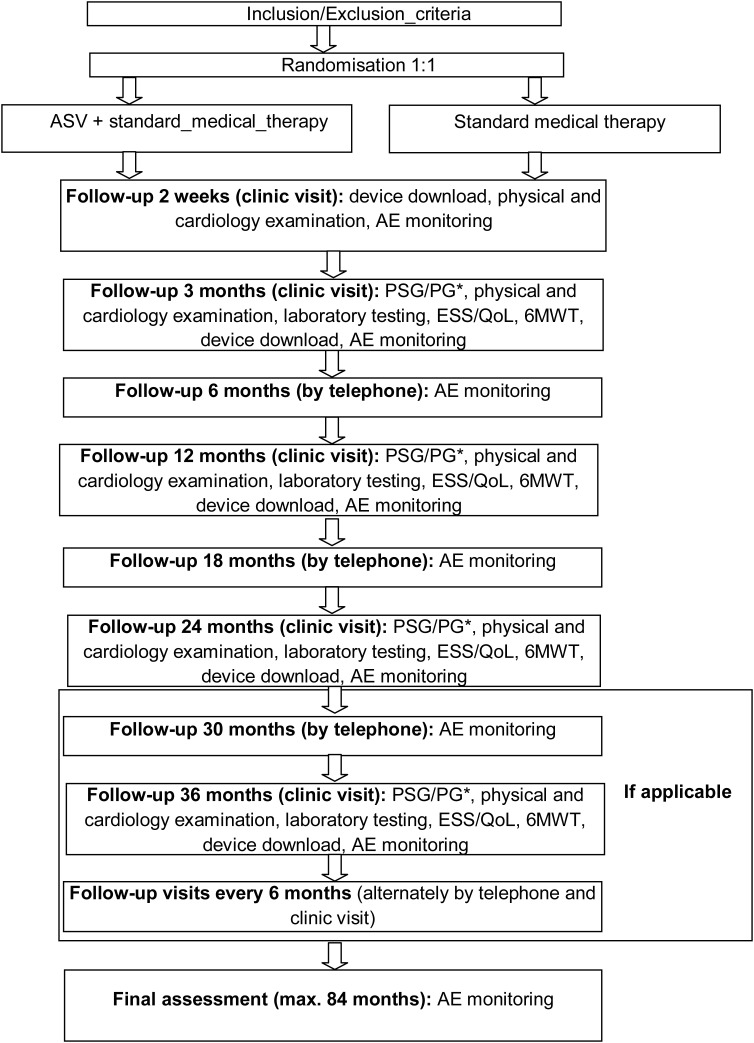
The study schedule is shown in Figure 1. Clinic visits are scheduled for all patients at study entry, after 2 weeks, after 3 and 12 months, and every 12 months thereafter until the end of the study. Each of these visits includes a blood sample (for measurement of haemoglobin, serum creatinine, leucocytes and haematocrit), quality of life assessment using the Minnesota Living with Heart Failure questionnaire (MLHFQ) and the Euroqol questionnaire (EQ5D), determination of sleepiness using the Epworth Sleepiness Scale (ESS), a 6 min walk test (6MWT), and an ECG. Additional assessments include a general history, medication history and event history for the time since the last visit, a physical examination, and determination of NYHA class. Furthermore, patients in the ASV group undergo polygraphy (PG) or polysomnography (PSG) and a data download from the ASV device. Patients are contacted by telephone at 6 and 18 months, and then every 12 months.
Figure 1.
SERVE-HF Study plan. AE, adverse event; ASV, adaptive servo-ventilation; ESS, Epworth Sleepiness Scale; 6MWT, 6 min walk test; PG, polygraphy; PSG, polysomnography; QoL, quality of life (* = only performed in patients in the ASV group).
Adaptive servo-ventilation is started in hospital. Use of a full face mask is recommended for the initiation of therapy. Treatment is started using standard settings, and pressure levels are adjusted based on the results of respiratory monitoring. The PaceWave™ ASV algorithm sets the MV target to 90% of the patient's own ventilation. Patients are instructed to use the ASV device for at least 5 h each night, 7 days a week. It is recommended that major mask leaks should be avoided if possible. The target is to reduce the apnoea–hypopnoea index (AHI) to <10/h within 14 days of starting ASV. If this is shown not to be the case at clinic visits (based on the data downloaded from the ASV device), then proper mask fitting is again undertaken and device settings adjusted for each patient individually.
SERVE-HF major substudy
The aim of the substudy is to assess ventricular remodelling, and changes in left and right ventricular function, sleep, breathing, cognitive function, anxiety, and depression. Evaluations to collect data for the substudy (echocardiography, cardiac MRI, PSG, and questionnaires) will be performed at baseline and at 3 and 12 months after randomization.
Statistical considerations
Statistical methodology
Randomization will be stratified by site and by participation in the major substudy, and is performed by the computerized electronic case record form management system.
The primary analysis will be performed on adjudicated endpoints in the intention-to-treat population (all randomized patients), following a group sequential design with O'Brien–Fleming stopping boundaries. The two interim analyses and the final analysis will be performed when 50, 75, and 100% of the information has been collected, and will consist of a two-sided log-rank test comparing the control and intervention group, stratified by country (to account for possible differences in event rates in the control group between countries).
The null hypothesis is that the time to first event in the intervention group is identical to the time to first event in the control group. The alternative hypothesis is that the time to first event in the intervention group is different from the time to first event in the control group. The test level and the rejection boundaries will depend on the interim analysis when the endpoint is analysed. At interim analysis 1, interim analysis 2, and the final analysis based on 325, 488, and 651 first events, significance levels will be 0.0042, 0.0194, and 0.0430, respectively, and the corresponding rejection boundaries of the standardized log-rank statistic will be ±2.863, ±2.337, and ±2.024, keeping an overall two-sided significance level of 5%. To maintain study blinding, the statistician of the Data and Safety Monitoring Board will link the time-to-event data at each of the interim analyses to the randomization code, calculate the log-rank statistic, and compare it with the pre-defined limits.
Following the closed testing procedure of Lehmacher et al.,31 if the null hypothesis for the first primary endpoint (all-cause mortality or unplanned hospitalization for worsening HF) is not rejected, then the testing ceases. Otherwise, testing proceeds to the second primary endpoint (cardiovascular mortality or unplanned hospitalization for worsening HF). If the null hypothesis for the second primary endpoint is not rejected, then the testing ceases. Otherwise, testing proceeds to the third primary endpoint (all-cause mortality or all-cause unplanned hospitalization). Kaplan–Meier curves will be used to visualize survival data.
Secondary analysis
Secondary endpoints will be analysed according to type of scale: time-to-event endpoints will be analysed in the same ways as the primary endpoint; dichotomous variables (improvement in NYHA class) will be analysed by a likelihood χ2 test; continuous endpoints will be analysed by analysis of covariance (ANCOVA) including the baseline value as a covariate if available; variables with right-skewed distributions within random groups will be log-transformed prior to analysis. All secondary endpoint comparisons will be performed at α = 0.05 without adjustment for multiplicity.
Extended analyses will be conducted using regression models (linear, logistic, or Cox proportional hazards) to explore further the influence of patient characteristics and clinical conditions on outcome. The list of covariates includes age, gender, country, site, co-morbidities, severity of SDB (AHI 15–30 events/h, AHI ≥30 events/h), NYHA class, ischaemic/non-ischaemic HF, and CSR <20%/20–50%/>50% of recording time to be determined at or immediately after inclusion.
Sample size
The study was designed to demonstrate a 20% reduction in the primary endpoint with an overall type 1 error rate of 5% two-sided including two interim analyses and with a power of 80%. For this purpose, a maximum of 651 events are to be observed if the study will not be stopped at one of the interim analyses. To reach this goal, that remained unchanged during the trial, a wide range of possible recruitment and follow-up scenarios were considered at study start. Eventually the study began with an assumption of a 35% control group event rate per year and a recruitment goal of 70 patients/month. These figures turned out to be not realistic. After a pre-specified blind interim analysis of pooled data shortly before the scheduled end of recruitment, a new sample size calculation was performed, with the result that at least 1193 patients will be recruited over a period of 60 months.
For the major substudy, a sample size of 300 patients was calculated based on the assumption that ASV treatment will increase LVEF by 4% over 12 months compared with baseline, with no change in LVEF in the control group.
Ethics and monitoring
The Executive Steering Committee is responsible for the clinical and scientific conduct of the study and publication of the results. An independent Endpoint Review Committee reviews and adjudicates all pre-specified events according to established definitions. Both committees only have access to blinded data while the study is underway. There is also an independent Data Safety Monitoring Board supervising the trial. Members of the committees are listed in Appendix 1. The trial design was approved by the local ethics committees. The trial is being conducted in accordance with national laws, Good Clinical Practice, and the Declaration of Helsinki 2002.
Discussion
Sleep-disordered breathing is common in patients with chronic HF and is associated with adverse prognosis. Therefore, a treatment that can control SDB has the potential to improve outcomes, as well as quality of life, in patients with chronic HF. Such an overall improvement in the HF symptom burden by targeting one co-morbidity is an attractive proposition.
Continuous positive airway pressure treatment for OSA in cardiovascular disease is often recommended based on evidence of the beneficial effects of CPAP on OSA in the general population.32,33 Although there is some evidence for cardiovascular benefit of the treatment of OSA,14,16,34 it is not yet known whether treatment of CSA/CSR improves outcomes in patients with HF.
The CANPAP study is the only trial to date that investigated the impact of positive airway pressure treatment (CPAP) on outcomes (heart transplant-free survival) in patients with predominant CSA/CSR.21 There was no overall survival benefit, but the results of a post-hoc analysis suggested a potential benefit in patients for whom CPAP was the most effective at attenuating CSA/CSR.22
Adaptive servo-ventilation therapy is currently the most effective treatment for CSA/CSR and is well tolerated. Use of ASV has been shown to improve multiple intermediate cardio-respiratory endpoints in small groups of chronic HF patients.26 When comparing ASV and CPAP, the improvements seen in ASV recipients were considerably greater than those in patients treated with CPAP.20 Two observational cohort studies have recently reported that positive airway pressure therapy for treatment of SDB in HF has the potential to improve morbidity and mortality.4,35 Neither of them reported the potentially different treatment effects of CPAP and ASV. The German group only used ASV when CPAP therapy failed to suppress CSA,4 whereas the French group switched from using CPAP to ASV for the treatment of all patients with CSA mid-way though their series of patients.35 However, apart from the ongoing SERVE-HF trial, no prospective randomized outcome study has been performed, and translation of reported improvements to benefits in terms of mortality and morbidity in patients with chronic HF has yet to be documented.
Conclusions
SERVE-HF is an important randomized controlled trial that will assess, for the first time, whether treatment of predominant CSA/CSR with ASV can reduce morbidity and mortality in patients with chronic HF. The results of the SERVE-HF study should provide further clarification of the benefits of effectively treating CSA/CSR in patients with HF. In addition, the findings might have important implications for individualized therapeutic strategies targeted at reducing the morbidity and mortality, and perhaps even the economic burden, of HF.
Funding
ResMed Ltd, Sydney, Australia.
Conflict of interest: M.-P.dO. is currently conducting research sponsored by ResMed and has received honararia for symposia during the period 2009–2012. C.A. is a member of the Steering Committee of the SERVE-HF study and receives honoraria from ResMed; she is a speaker for ResMed at scientific symposia for which she received honaria annd is currently conducting research for this company. M.R.C. is currently conducting research sponsored by ResMed. He has received honoraria and travel expenses from ResMed. A.S. has received a research grant from ResMed, unrelated to the trial described in this paper. F.Z. is a Steering Committee member and has received honoraria from ResMed. V.K.S. served as a consultant for ResMed, Respicardia, Apnex Medical, Deshum, Johnson & Johnson, Sova Pharmaceutical, Neu Pro, and Medtronic. The Mayo Foundation has received a gift from Phillips-Respironics for the study of sleep apnoea and cardiovascular disease. Also working with Mayo Health Solutions on intellectual property related to sleep and obesity. E.E. is a member of the Steering Committee of the SERVE-HF study sponsored by ResMed and has received reimbursement of travel and other expenses. H.W. is an employee of ResMed. H.T. is currently conducting research sponsored by ResMed and has received travelling expenses and a speakers honorarium in the past from ResMed. P.L. and K.W. have no conflicts to declare.
Acknowledgements
Nicola Ryan provided English language medical writing support funded by ResMed. M.R.C. and A.S. receive salary support from the National Institute for Health Research Biomedical Research Units at the Royal Brompton Hospital.
Appendix 1. Committee and Board Members
Executive Steering Committee
Martin Cowie (UK) Co-Chair, Helmut Teschler (Germany) Co-Chair, Christiane Angermann (Germany), Marie-Pia d'Ortho (France), Erland Erdmann (Germany), Patrick Levy (France), Anita Simonds (UK), Virend Somers (USA), Karl Wegscheider (Germany), Faiez Zannad (France), Holger Woehrle (Germany).
Endpoint Review Committee
Jean N. Trochu (France), Jean M. Davy (France), Ludger Seipel (Germany).
Data and Safety Monitoring Board
Alain Leizorovicz (France), Bernd Lüderitz (Germany), Ian Ford (UK).
Scientific Advisory Board
Heinrich Becker (Germany), Andrew Clark (UK), Till Neumann (Germany), Joachim Ficker (Germany), Luc Hittinger (France), Guillaume Jondeau (France), Barbara Lamp (Germany), Michael Pfeiffer (Germany), Thomas Podszus (Germany).
References
- 1.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P ESC Committee for Practice Guidelines (CPG) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. Document Reviewers. [DOI] [PubMed] [Google Scholar]
- 2.Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Topfer V. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9:251–257. doi: 10.1016/j.ejheart.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Schulz R, Blau A, Börgel J, Duchna HW, Fietze I, Koper I, Prenzel R, Schädlich S, Schmitt J, Tasci S, Andreas S working group Kreislauf und Schlaf of the German Sleep Society (DGSM) Sleep apnoea in heart failure. Eur Respir J. 2007;29:1201–1205. doi: 10.1183/09031936.00037106. [DOI] [PubMed] [Google Scholar]
- 4.Jilek C, Krenn M, Sebah D, Obermeier R, Braune A, Kehl V, Schroll S, Montalvan S, Riegger GA, Pfeifer M, Arzt M. Prognostic impact of sleep disordered breathing and its treatment in heart failure: an observational study. Eur J Heart Fail. 2011;13:68–75. doi: 10.1093/eurjhf/hfq183. [DOI] [PubMed] [Google Scholar]
- 5.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52:686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Jaffe LM, Kjekshus J, Gottlieb SS. Importance and management of chronic sleep apnoea in cardiology. Eur Heart J. doi: 10.1093/eurheartj/ehs046. doi:10.1093/eurheartj/ehs046 in press. [DOI] [PubMed] [Google Scholar]
- 7.Levy P, Pepin J-L, Tamisier R, Neuder Y, Baguet J-P, Javaheri S. Prevalence and impact of central sleep apnea in heart failure. Sleep Med Clin. 2007;2:615–621. [Google Scholar]
- 8.Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167:1181–1185. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–865. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 10.Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davison DE, Somers VK. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation. 1999;100:2332–2335. doi: 10.1161/01.cir.100.23.2332. [DOI] [PubMed] [Google Scholar]
- 11.Becker HF, Jerrentrup A, Ploch T, Grote L, Penzel T, Sullivan CE, Peter JH. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 12.Haentjens P, Van Meerhaeghe A, Moscariello A, De Weerdt S, Poppe K, Dupont A, Velkeniers B. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757–764. doi: 10.1001/archinte.167.8.757. [DOI] [PubMed] [Google Scholar]
- 13.Barbé F, Durán-Cantolla J, Capote F, de la Peña M, Chiner E, Masa JF, Gonzalez M, Marín JM, Garcia-Rio F, de Atauri JD, Terán J, Mayos M, Monasterio C, del Campo F, Gomez S, de la Torre MS, Martinez M, Montserrat JM Spanish Sleep and Breathing Group. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718–726. doi: 10.1164/rccm.200901-0050OC. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko Y, Floras JS, Usui K, Plante J, Tkacova R, Kubo T, Ando S, Bradley TD. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233–1241. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 15.Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169:361–366. doi: 10.1164/rccm.200306-752OC. [DOI] [PubMed] [Google Scholar]
- 16.Kasai T, Narui K, Dohi T, Yanagisawa N, Ishiwata S, Ohno M, Yamaguchi T, Momomura S. Prognosis of patients with heart failure and obstructive sleep apnea treated with continuous positive airway pressure. Chest. 2008;133:690–696. doi: 10.1378/chest.07-1901. [DOI] [PubMed] [Google Scholar]
- 17.Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol. 2007;49:2028–2034. doi: 10.1016/j.jacc.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 18.Bitter T, Westerheide N, Prinz C, Hossain MS, Vogt J, Langer C, Horstkotte D, Oldenburg O. Cheyne–Stokes respiration and obstructive sleep apnoea are independent risk factors for malignant ventricular arrhythmias requiring appropriate cardioverter-defibrillator therapies in patients with congestive heart failure. Eur Heart J. 2011;32:61–74. doi: 10.1093/eurheartj/ehq327. [DOI] [PubMed] [Google Scholar]
- 19.Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne–Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164:614–619. doi: 10.1164/ajrccm.164.4.9908114. [DOI] [PubMed] [Google Scholar]
- 20.Philippe C, Stoïca-Herman M, Drouot X, Raffestin B, Escourrou P, Hittinger L, Michel PL, Rouault S, d'Ortho MP. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne–Stokes respiration in heart failure over a six month period. Heart. 2006;92:337–342. doi: 10.1136/hrt.2005.060038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradley TD, Logan AG, Kimoff RJ, Sériès F, Morrison D, Ferguson K, Belenkie I, Pfeifer M, Fleetham J, Hanly P, Smilovitch M, Tomlinson G, Floras JS CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–2033. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 22.Arzt M, Floras JS, Logan AG, Kimoff RJ, Series F, Morrison D, Ferguson K, Belenkie I, Pfeifer M, Fleetham J, Hanly P, Smilovitch M, Ryan C, Tomlinson G, Bradley TD CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP) Circulation. 2007;115:3173–3180. doi: 10.1161/CIRCULATIONAHA.106.683482. [DOI] [PubMed] [Google Scholar]
- 23.Pepperell JC, Maskell NA, Jones DR, Langford-Wiley BA, Crosthwaite N, Stradling JR, Davies RJ. A randomized controlled trial of adaptive ventilation for Cheyne–Stokes breathing in heart failure. Am J Respir Crit Care Med. 2003;168:1109–1114. doi: 10.1164/rccm.200212-1476OC. [DOI] [PubMed] [Google Scholar]
- 24.Oldenburg O, Schmidt A, Lamp B, Bitter T, Muntean BG, Langer C, Horstkotte D. Adaptive servoventilation improves cardiac function in patients with chronic heart failure and Cheyne–Stokes respiration. Eur J Heart Fail. 2008;10:581–586. doi: 10.1016/j.ejheart.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Hastings PC, Vazir A, Meadows GE, Dayer M, Poole-Wilson PA, McIntyre HF, Morrell MJ, Cowie MR, Simonds AK. Adaptive servo-ventilation in heart failure patients with sleep apnea: a real world study. Int J Cardiol. 2010;139:17–24. doi: 10.1016/j.ijcard.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 26.Sharma BK, Bakker JP, McSharry DG, Desai AS, Javaheri S, Malhotra A. Adaptive servo-ventilation for treatment of sleep-disordered breathing in heart failure: a systematic review and meta-analysis. Chest. 2012;142:1211–1221. doi: 10.1378/chest.12-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faller H, Steinbüchel T, Schowalter M, Spertus JA, Störk S, Angermann CE. The Kansas City Cardiomyopathy Questionnaire (KCCQ)—a new disease-specific quality of life measure for patients with chronic heart failure. Psychother Psychosom Med Psychol. 2005;55:200–208. doi: 10.1055/s-2004-834597. [DOI] [PubMed] [Google Scholar]
- 28.Vogels RL, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: a systematic review of the literature. Eur J Heart Fail. 2007;9:440–449. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Löwe B, Kroenke K, Herzog W, Grafe K. Measuring depression outcome with a brief self-report instrument: sensitivity to change of the Patient Health Questionnaire (PHQ-9) J Affect Disord. 2004;81:61–66. doi: 10.1016/S0165-0327(03)00198-8. [DOI] [PubMed] [Google Scholar]
- 30.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 31.Lehmacher W, Wassmer G, Reitmeir P. Procedures for two-sample comparisons with multiple endpoints controlling the experimentwise error rate. Biometrics. 1991;47:511–521. [PubMed] [Google Scholar]
- 32.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea–hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 33.Buchner NJ, Sanner BM, Borgel J, Rump LC. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med. 2007;176:1274–1280. doi: 10.1164/rccm.200611-1588OC. [DOI] [PubMed] [Google Scholar]
- 34.Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, Martínez-Alonso M, Carmona C, Barceló A, Chiner E, Masa JF, Gonzalez M, Marín JM, Garcia-Rio F, Diaz de Atauri J, Terán J, Mayos M, de la Peña M, Monasterio C, del Campo F, Montserrat JM Spanish Sleep And Breathing Network. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161–2168. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- 35.Damy T, Margarit L, Noroc A, Bodez D, Guendouz S, Boyer L, Drouot X, Lamine A, Paulino A, Rappeneau S, Stoica MH, Dubois-Rande JL, Adnot S, Hittinger L, d'Ortho MP. Prognostic impact of sleep-disordered breathing and its treatment with nocturnal ventilation for chronic heart failure. Eur J Heart Fail. 2012;14:1009–1019. doi: 10.1093/eurjhf/hfs085. [DOI] [PubMed] [Google Scholar]