Abstract
Vaccination is proven to be effective in controlling many infections including small pox, influenza and hepatitis, but strain-specific factors may limit vaccine efficacy. All of these vaccines work through the generation of neutralizing antibodies but for some pathogens there may be roles for serotype-independent immunity. Recently several groups using murine vaccine models have shown that induced T helper cell responses including Th17 responses have shown the potential for CD4+ T-cell dependent vaccine responses. Th17 mediated protective responses involve the recruitment of neutrophils, release of anti-microbial peptides and IL-17-driven Th1 immunity. These effector mechanisms provide immunity against a range of pathogens including the recently described antibiotic-resistant metallo-beta-lactamase 1 Klebsiella pneumoniae. Continued elucidation of the mechanism of Th17 responses and identification of effective adjuvants for inducing robust non pathogenic Th17 responses may lead to successful Th17 based vaccines. Here we summarize the recent advances in understanding the role of Th17 in vaccine induced immunity. We also discuss the current status and future challenges in Th17-based mucosal vaccine development.
Introduction
T cells and B cells, the two major lymphocyte populations, mediate the generation of adaptive immunity. Activation of naïve T cells by their cognate antigens presented by antigen presenting cells (APCs) in the presence of various cytokines leads to the generation of distinct effector T helper (Th) cell-subsets including Th1, Th2 and Th17 [1]. In general, the Th1 subset regulates IFNγ-dependent immunity against most intracellular pathogens. Th1 cell differentiation can be inhibited by IL-4, which subsequently induces another T cell subset: Th2. Th2 cells produce IL-4, IL-5, IL-13 and are required for protection against helminth infection [2, 3]. Th17 cell differentiation requires TGFβ and IL-6 in mice, and IL-1 and IL-23 in humans [1]. Th17-derived effector cytokines especially IL-17A, IL-17F and IL-22 are critical for host defense against bacterial and fungal infection [4–7]. In contrast these Th17 cells can play a pathological role in auto-immune diseases [8]. B cell clonal expansion, differentiation and isotype switch responses are result of interaction between cognate antigen specific activated T cells and B cells in the secondary lymphoid organ [9, 10]. Production of IgG2a antibodies are controlled by Th1 responses, whereas Th2 cell responses promote IgG1 and IgE antibody class switching. Recent studies have documented role of Th17 cells in regulating B cells antibody generation, germinal center and ectopic inducible bronchus-associated lymphoid tissue (iBALT) formation [11–13]. Vaccination refers to administrating specific antigenic substance to induce protective immune responses (antibody dependent or independent) against the microbes. Many mucosal vaccination approaches can induce robust Th17 responses, suggesting Th17 cells may be useful targets for vaccine induced immunity. Here, we discuss recent advances in understanding of Th17 cells in vaccination-induced immunity.
Th17 cells and vaccines: where do we stand now?
Most, if not all U.S. Food and Drug Administration (FDA) approved vaccines are antibody based, such as small pox, influenza and hepatitis, and pneumococcal vaccines. These vaccines have successfully eliminated and controlled many infectious diseases. Only a limited number of vaccines mediate protection via T-cell dependent immunity. Both Th1 responses induced by whole cell pertussis vaccines and Th2-biased responses induced by pertussis acellular vaccines protect against Bordetella pertussis infection. Protection by immunization with whole cells pertussis vaccine could also be mediated by enhancement of Th17 dependent bactericidal activity of macrophages [14]. Antibody based vaccines usually have limited protective spectrum since the responses are restricted to the vaccination strains. For example, a recent clinical trial (conducted in Europe, USA, Philippines and South Africa) showed the efficacy of 7-valent, 9-valent or 11-valent pneumococcal conjugate vaccine (PCV) in preventing invasive pneumococcal and World Health Organization (WHO) radiographically defined pneumonia is approximately 80% and 27% respectively [15]. Protection is predominantly mediated by T-cell independent antibody responses, but failure to mount antibody response to some serogroup (PCV7 is minimally effective against 6B and 19F serotype) could be the reason for the poor efficacy or inconsistent response of vaccine [16]. Furthermore, in order to enhance immunogenicity against pneumococcal strains that cause meningitis or pneumonia, polysaccharide conjugate (with Diphtheria proteins) vaccines have been engineered. The role of T cell immunity induced by these conjugate vaccines remains to be determined but clearly these conjugate vaccines can elicit strong T-cell responses [17]. In contrast, T cell based vaccines have the potential to provide serotype-independent protection by recognizing antigens conserved cross species and thus have been investigated by many researchers most recently [18].
Pathogen-specific Th17 vaccines
Th17 cells are described as an initiator of pro-inflammatory responses in many autoimmune disease conditions [8, 19]. More recently, it has been appreciated that Th17 responses can also induce protective immunity against many bacterial and fungal pathogens [6, 20–22]. Indeed, vaccination in many mouse models induced significant Th17 responses in the lung and neutralization of IL-17 or blocking its downstream signaling pathways resulted in higher pathogen burden and mortality [5, 21, 23]. Pneumonia is most common cause of death induced by many infectious agents (CDC). Klebsiella pneumoniae infection commonly occurs in immune-compromised patients and is a concern for increasing resistance to carbapenem antibiotics. K. pneumoniae infection induces IL-17, resulting in production of IL-17-targeted cytokines in the lung [6, 24]. Furthermore, overexpression of IL-17 by adenovirus resulted in enhanced clearance of bacteria [24], suggesting the induction of IL-17 can effectively vaccinate against K. pneumoniae. Indeed, immunization with heat killed K. pneumoniae induces antibody response against capsular polysaccharides as well as a concomitant Th17 response. However, antibody response offers little or no protection against heterologous strains having different polysaccharide serotypes, whereas Th17 cells are sufficient and required for serotype independent heterologous protection [6]. Vaccination with highly conserved outer membrane proteins of K. pneumoniae also elicits a strong Th17 response and provides heterologous protection against a range of different strains including the newly described metallo-beta-lactamase 1 strain [6]. Vaccine-induced immunity against K. pneumonniae required neutrophils, but could also have involved the generation of Th17-dependent anti-microbial proteins. Both mechanisms require a functional heterodimeric IL-17 receptor, formed by IL-17RA and IL-17RC. Further studies using IL-17 receptor conditional knockout mice are useful to explore the molecular mechanism and cellular targets of Th17 mediated immunity against K. pneumoniae.
Both antibody-dependent and -independent mechanisms are involved for immunity against Streptococcus pneumonia. Antibody confers protection against capsular polysaccharide antigens; however, antibody-independent CD4 responses are generated against cell wall polysaccharide antigens [25]. Because polysaccharide antigens are poorly immunogenic in children (< 2 years), newer polysaccharide-based vaccine include a carrier protein (immunogenic non-pneumococcal protein) to induce adaptive immune responses. Moreover, conjugate (covalently attached carrier protein to polysaccharides) pneumococcal vaccine (PCV13) have been developed, and are able to provide protection against prevalent serotypes for use in children 6 weeks to 17 years age; however, other strains of pneumococcus also impose significant public health threats. Recent studies suggest that T-cell responses may also be required for vaccine-induced protection. Indeed, anti-capsular antibody titers did not correlate with experimental pneumococcal carriage [26, 27]. Thus, there is a need to identify surface antigens expressed in all major pathogenic pneumococcal strains, which may be capable of eliciting antibody independent protection against all the pathogenic serotypes. Indeed, antigen specific CD4 T cells limit nasopharyngeal colonization of S. pneumoniae[28]. Furthermore, CD4 T cell-derived IL-17, but not IFNγ or IL-4, is required for the clearance of Pneumococcal colonization [23]. The role of CD4-derived IL-17 in clearance of pneumococcal colonization was further demonstrated in another study, where IL-17 dependent recruitment of monocytes/macrophages and neutrophils limited the bacteria burden [22]. Furthermore, immunization with pneumococcal whole cell antigen and several derivatives provided IL-17-mediated, but not antibody dependent, protection [25, 29]. Indeed, Th17 effector responses provide serotype independent immunity by recruitment of monocytes, macrophages and neutrophils, thus having the potential to be used for designing novel pneumococcal vaccines [22].
Recently its has also been shown that a live attenuated vaccine against Y. pestis induces a robust Th17 response in the lung [21]. In this setting, the majority of IL-17 producing CD4 cells also produce IFNγ and TNFα. Neutralization of IL-17 or IFNγ resulted into reduced survival, suggesting IL-17 contributes to vaccine induced immunity against Y. pestis. Interestingly, IL-17 did not reduce bacterial burden in the lung tissue, and was effective only after booster vaccination. Moreover, in this study IL-17-mediated immunity was independent of neutrophil recruitment to the mucosal site; but was dependent on an IL-17 mediated increase in the antimicrobial protein lipocalin 2 [21].
Pseudomonas aeruginosa is a gram negative rod responsible for many cases of hospital-acquired pneumonia. Primary experimental infection with the lab strain of P. aeruginosa, PA01 resulted in rapid IL-17 induction [30] and neutralizing IL-17 worsened lung pathology and enhanced bacteria burden, suggesting a protective role of IL-17 in this model [30]. Interestingly, fewer neutrophils were recruited to the mucosal sites after anti-IL-17 treatment, although it was not investigated if neutrophils were required for protection in this study. In contrast, Dubin et al found that although both IL-23 and IL-17RA signaling was critical in regulating early neutrophil emigration into the lung in response to PA01 P. aeruginosa infection, both IL-17 and IL-17RA signaling were dispensable for controlling bacterial burden [31]. A live-attenuated vaccine against P. aeruginosa using PA14ΔaroA (an aroA deletion in the PA14 strain), has been shown to induce a robust IL-17 response, and neutralization of IL-17 or studies in IL-17R knockout resulted in less neutrophil recruitment and higher mortality in immunized mice against LPS heterologous strain (PAO1 ExoU+) of P. aeruginosa[32]. A recent study identified several antigens from P. aeruginosa that were capable of inducing Th17 responses, and have demonstrated Th17 dependent immunity after immunization with one of these antigens, PopB [33]. However, a recent study documented a pro-inflammatory role of IL-17 in P. aeruginosa induced in an ulcerative keratitis model [34], suggesting protective or inflammatory roles of Th17 cells may be context dependent.
Other microbes for which Th17 cells are protective in vaccine-induced mucosal immunity include Mycobacterium tuberculosis, Bordetella pertussis, Helicobactor pylori and influenza virus. The protective role of IL-17 during the memory recall phase of M. tuberculosis infection has been thoroughly investigated and reviewed elsewhere [35]. Vaccination with the mycobacterial peptide (ESAT-6(1–20) followed by a subsequent aerosol M. tuberculosis challenge resulted in the early generation of IL-17-producing CD4+ T cells and late generation of IFNγ producing CD4 T cells. Interestingly, the early IL-23/IL-17 axis is required for the recruitment of IFNγ producing T cells by chemokines (CXCL9, CXCL10 and CXCL11 [36]. The World Health Organization has estimated the prevalence of H. pylori infection in fifty percent of World and 70% of Asian populations, which poses a significant health care and economic burden. Current treatment includes usage of proton-pump inhibitors and antibiotics. Recombinant H pylori antigens such as VacA, CagA and NAP are currently in clinical trials. In an experimental animal model, vaccination with Hp-SS1 lysate with cholera toxin adjuvant confers protection against H. pylori[37]. Furthermore, elevated levels of IL-17 have been reported in the gastric mucosa of H. pylori infected patients [38], suggesting that IL-17 is a physiological response to H. pylori colonization. Indeed, immunization with H.pylori lysate, confers IL-17 and neutrophil dependent protection against a subsequent infectious challenge [37]. Furthermore, IL-17 dependent protection has also been reported in a mouse model of immunization with H. pylori urease [39], although this study did not specify the underlying mechanism of IL-17-dependent protection.
Th17 responses also provide protective immunity against fungal pathogens. A dominant stable antigen specific Th17 recall response is required for clearance of fungus Candida albicans [7]. Interestingly, both Th1 and Th17 response are induced during primary fungal infection but mice lacking IL-17 receptor are more susceptible to Candida infection, suggesting Th17 cells plays a dominant role in anti-fungal immunity [20]. Interestingly, IL-23 driven IL-17 response have also been reported to promote inflammation during infections with Candida albicans or Aspergillus fumigatus[40]. A recent study shows that Th17 mediated immunity is required for the protection against Blastomyces dermatitidis, C. posodasii and H. Capsulatum[5]. They further show that IL-17 mediated immunity is conferred by the recruitment and activation of PMN and macrophages [5]. To date, only a limited number of studies implicate the direct role of antigen specific memory Th17 cells in clearance of these fungal pathogens. Future studies require the understanding of plastic nature of Th17 cells in vivo during pathogenesis of disease. It is possible that although Th17 cells confer robust protection in most of the diseases, the inflammatory milieu may result in conversion to other T helper subsets.
Mechanisms of protection
Th17-mediated host defense against microorganisms can be classified into many mechanisms and are depicted in Figure 1, including initiating the release of antimicrobial peptides by epithelial cells, recruitment of neutrophils and/or macrophages, initiation of humoral immunity, and augmenting other T helper subsets. Vaccine induced protection can be achieved in a serotype dependent manner through T-cell independent B-cell/antibody responses (Figure 1A). In contrast, recent studies have shed light on vaccine induced serotype independent protection mediated by Th17 cells (Figure 1B). Receptors for Th17-derived cytokines are mainly expressed by epithelial cells and fibroblasts. IL-17 targeted genes include G-CSF, CXCL-1, CCL2 and various other chemokines, resulting in the recruitment of neutrophils and macrophages to mucosal sites [41]. Importance of IL-17 dependent neutrophil recruitment was further demonstrated by adoptive transfer and depletion strategy, demonstrating that IL-17-dependent neutrophil recruitment is required for immunity against pneumococcus [23] and H. pylori infection [39].
Figure 1. Proposed model of serotype dependent and independent immunity.
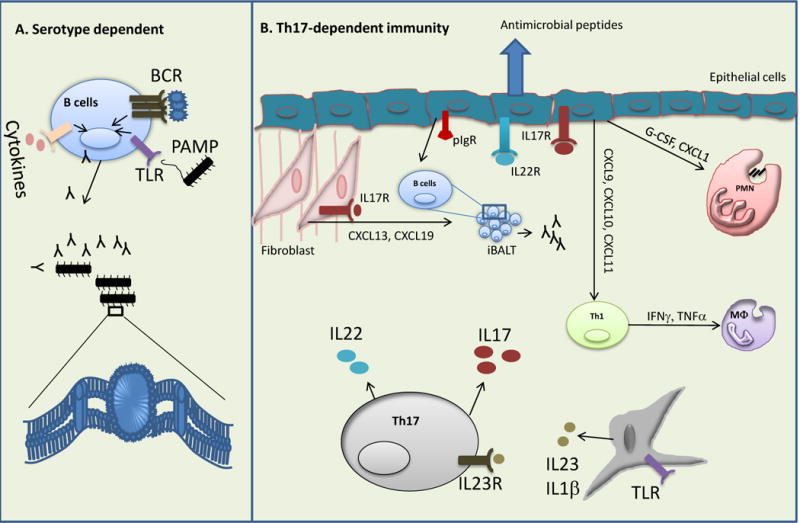
Vaccination can induce both T cells dependent and independent immune responses. A) T cells independent antibody response generated by strong B cells receptor (BCR) signal by antigens and activation of both Toll-like receptor (TLR) and proinflammatory cytokine response. B) Dendritic cells derived IL-23 and IL-1β induce robust Th17 responses. IL-17 and IL-22 produced by Th17 cells activate epithelial cells mediated immune responses. Th17 targeted cytokines and chemokines (G-CSF and CXCL1) released from epithelial cells regulate neutrophils recruitment to the mucosal sites. Th17-mediated neutrophils response is reported to be essential for the protection against a range of pathogens. Epithelial cells also directly participate in limiting pathogens multiplication by the release of Th17 related cytokines regulated antimicrobial peptides. Apart from lymphotoxin signaling, Th17 cells involve in iBALT formation in the lung tissue by activating chemokines (CXCL13, CXCL19) released from the fibroblast. iBALT mediated local immune responses (both B and T cells response) are critical for clearance of influenza virus. Th17 cells also regulate late Th1 responses by modulating chemokines especially CXCL9, CXCL10 and CXCL11 response from epithelial cells. Th1-regulated cytokines (IFNγ or TNFα) and activation of macrophages are required for the immunity against M. tuberculosis. In addition, Th17 cells regulate polymeric immunoglobulin receptor (pIgR) expression on epithelial cells, which is essential for influx of B cells to the mucosal sites, however, the relevance of this pathway in Th17 mediated immunity is yet to determine.
Studies have indicated the role of IL-17 in activating B cell antibody response and germinal center formation [11, 12]. Blockade of IL-17 signaling resulted in inhibition of antibody class switch [13]. Furthermore, IL-17 is responsible for lymphotoxin independent inducible bronchus-associated lymphoid tissue (iBALT) formation in the lung [42], promoting B cell and T cell interactions [43]. In addition, Th17-cells enhance influx of B cells in the lung and promote polymeric immunoglobulin receptor (pIgR) expression on bronchial epithelial cells [44]. pIgR regulates the transport of IgA and IgM into the airway lumen [44]. Despite these findings, there is no direct evidence of IL-17 dependent antibody responses after vaccination. Interestingly, conventional influenza vaccines appear less effective for the prevention of clinical illness in the elderly population [45] and circulating IL-17 levels were found decreasing with age [46], suggesting that IL-17 might be involved in influenza vaccine induced antibody responses.
Th17 cells are maintained as effector memory cells in mucosal tissue for a very long period [47]. Indeed, superantigens from S. aureus induce a robust IL-17 response from memory Th17 cells in adult human but not from naïve T cells [48]. Interestingly, CD4+ T cells isolated from cord blood or from infants were poor producers of IL-17 in vitro, however authors did not provide molecular mechanism for this poor T-cells response [48]. Th17 cells are highly plastic in nature, and the cytokine milieu at mucosal cites is capable of transforming Th17 cells into either the Th1 or Th2 lineage. For example, IFNγ or IL-12 can convert Th17 cells into IFNγ producing Th1 cells [49]. Although Th17 cells can be unstable under Th1 inflammatory conditions, stable long lived memory Th17 cells are induced following vaccination in the absence of inflammation [50].
Adjuvants
Adjuvants are inorganic or organic additives to vaccines for generating optimal immune responses to antigens. Aluminum-based adjuvants are the most commonly used additive in FDA approved vaccines. Hepatitis A, hepatitis B, Haemophilus influenza type B, pneumococcal and diphtheria-tetanus-pertussis vaccine immune responses are boosted by use of alum salts (CDC). Aluminum-based adjuvants have been used in vaccine formulation for more than 90 years; however, the mechanism of actions of adjuvants is still poorly understood. Possible mechanism of adjuvants are enhanced antigen uptake and presentation by activated antigen presenting cells (APCs), which leads to initiation of antigen specific T cells responses [51]. Activation of APCs such as macrophages and dendritic cells involves activation of Toll-like receptors (TLRs) and inflammasome pathways [52–54]. In addition, Th2 cell and their specific B cells antibody response (IgG1 and IgE) are preferentially induced by alum [55]. To date, how inflammasome activation contributes to alum-based Th2 response is ill-defined. Interestingly, recent findings report that alum adjuvant can enhance the release of uric acid, which subsequently activates the inflammasome via both direct and indirect mechanisms [56, 57]. A recent study documented the role of uric acid in inflammasome-dependent Th17 differentiation [58], suggesting a possible mechanism that Th17 cells are promoted in alum formulated vaccines. Furthermore, activation of the inflammasome has been shown to induce IL-1β and IL-18 production from APCs and both of these cytokines are known to enhance Th17 responses [59]. Interestingly, non-alum based adjuvants such as a nanoemulsion, incomplete Freund’s adjuvants, monophospho lipid A (MPL)-trehalose dimycolate (TDM), heat labile enterotoxin and cholera toxin have been reported to induce Th17 responses in experimental models [36, 60–65]. MPL-TDM is stable oil in water emulsion derived from cell wall component of bacterial and mycobacterium, thus capable of inducing strong adaptive Th1 and Th17 immune responses via activation of TLR4 on innate immune cells [36, 63, 66]. E. coli heat labile enterotoxin have also been reported to induce protective Th17 immune responses against B. pertussis[67] and M. tuberculosis[65]. Heat labile enterotoxin induced Th17 responses appeared to be mediated through activation of inflammasome and IL-1 and IL-23 release from dendritic cells [67]. Similarly, cholera toxin have been reported to induce Th17 responses by dendritic cells through cAMP dependent secretion of IL-1β [61]. However, the major limitation of non-alum based adjuvants is concern for toxicity. A strong Th17 cell response could be induced or enhanced by co-administration of antigen and recombinant protein such as IL-1β, IL-6 or IL-23 as adjuvants. Indeed, this strategy has been successfully utilized in pre-clinical models to increase the efficacy of helper function of young and aged CD4 T cells [68].
Vaccine Safety
Despite the requirement of IL-17 in vaccine induced immunity in experimental animal models, its role in vaccination induced protection in humans remains ill-defined. The pro-inflammatory role of IL-17 is well documented in auto-immune diseases [8, 19]. Thus, induction of Th17 responses in autoimmune patients may aggravate underlying disease conditions. IL-17 has also been shown to be responsible for the immunopathology seen in influenza infected mice [69]. Thus, elevated IL-17 production could be harmful during an active flu infection. Another Th17-derived cytokine, IL-22, has been reported to induce psoriasis like skin inflammation and can be a safety concern for Th17 based vaccines [70]. Indeed, mucosal IL-22 producing Th17 cells are elevated in heat-killed K. pneumoniae immunized mice [6], whether these cells are pathogenic in autoimmune disease settings has not been investigated. IL-22 has also been reported to play a critical role in vaccine induced immunity against Mycobacterium [71], its protective or inflammatory role in other vaccination model has to be determined. Aside from the concern of the pro-inflammatory nature of Th17 effector cytokines, Th17 promoting adjuvants also raise safety concerns. Many experimental adjuvants such as cholera toxin, pertussis toxin and complete Freund’s adjuvants induce potent Th-17 responses [61, 72, 73], but underlying toxicity may limit the use of these adjuvants. Recently, TLRs have been shown to respond to endogenous host molecules and trigger inflammatory responses in addition to recognizing pathogens [74]. Thus, adjuvants that may induce endogenous TLR ligands from the host should be carefully examined. Candidate antigens for Th17 based vaccines should also undergo stringent selection processes. Antigens or their homologs found in commensal species may need to be excluded due to the concern of autoimmunity. One recent study showed that not all IL-17 producing Th17 cells can induce pathogenic responses. Endogenous TGFβ3 produced by developing Th17 cells can drive them to a pathogenic IL-17 producing Th17 subset, and the presence of IL-23 was necessary for induction of autoimmunity by this subset [75]. Our increasing knowledge of what controls pathogenic versus protective Th17 responses will greatly aid vaccine design.
Concluding remarks
Generating antibody dependent immune responses by vaccination had saved and is saving millions of lives every year. However, this strategy might fail to provide serotype independent heterologous protection. Recent advances in Th17 biology, especially antibody independent heterologous protection provided by Th17 cells, open up a new avenue for vaccine development. In experimental models, Th17 cells are effective in providing vaccination induced immunity against a range of pathogens. Identification of Th17 specific antigens for common prevalent pathogens will help to formulate a serotype independent effective vaccination strategy. Modulation of toxic properties and identification of new Th17 specific adjuvants will also have tremendous impact on designing safe and effective vaccines.
Highlights.
Th17 responses show protection against both bacterial and fungal pathogens
Mucosal and systemic vaccines can elicit protective Th17 responses
Th17 responses can mediate serotype immunity against clades of organisms
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dardalhon V, Korn T, Kuchroo VK, Anderson AC. Role of Th1 and Th17 cells in organ-specific autoimmunity. Journal of Autoimmunity. 2008;31:252–256. doi: 10.1016/j.jaut.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coffman RL, Seymour BW, Hudak S, Jackson J, Rennick D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science. 1989;245:308–310. doi: 10.1126/science.2787531. [DOI] [PubMed] [Google Scholar]
- 3.Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. The Journal of Experimental Medicine. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wüthrich M, Gern B, Hung CY, Ersland K, Rocco N, Pick-Jacobs J, Galles K, Filutowicz H, Warner T, Evans M, et al. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. The Journal of Clinical Investigation. 2011;121:554–568. doi: 10.1172/JCI43984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6**.Chen K, McAleer Jeremy P, Lin Y, Paterson David L, Zheng M, Alcorn John F, Weaver Casey T, Kolls Jay K. Th17 Cells Mediate Clade-Specific, Serotype-Independent Mucosal Immunity. Immunity. 2011;35:997–1009. doi: 10.1016/j.immuni.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6**.The authors demonstrate that vaccine induced mucosal Th17 cells recoganize a broad spectrum of pathogens unlike serotype restricted B cells/antibodies and confer protection independent of capsular serotypes. Interestingly, the authors demonstrates memory Th17 cells are long lived and can be detected weeks after vaccination.
- 7.Hernandez-Santos N, Huppler AR, Peterson AC, Khader SA, McKenna KC, Gaffen SL. Th17 cells confer long-term adaptive immunity to oral mucosal Candida albicans infections. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of Immune Induction of Collagen-Induced Arthritis in IL-17-Deficient Mice. The Journal of Immunology. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 9.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of Specific B and T Lymphocyte Interactions in the Lymph Node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 10.Smith KM, Pottage L, Thomas ER, Leishman AJ, Doig TN, Xu D, Liew FY, Garside P. Th1 and Th2 CD4+ T Cells Provide Help for B Cell Clonal Expansion and Antibody Synthesis in a Similar Manner In Vivo. The Journal of Immunology. 2000;165:3136–3144. doi: 10.4049/jimmunol.165.6.3136. [DOI] [PubMed] [Google Scholar]
- 11.Hsu H-C, Yang P, Wang J, Wu Q, Myers R, Chen J, Yi J, Guentert T, Tousson A, Stanus AL, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 12.Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont M-C, Ranchin B, Fabien N, Cochat P, Pouteil-Noble C, Trolliet P, et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 13.Mitsdoerffer M, Lee Y, Jäger A, Kim H-J, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proceedings of the National Academy of Sciences. 2010;107:14292–14297. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins SC, Jarnicki AG, Lavelle EC, Mills KHG. TLR4 Mediates Vaccine-Induced Protective Cellular Immunity to Bordetella pertussis: Role of IL-17-Producing T Cells. The Journal of Immunology. 2006;177:7980–7989. doi: 10.4049/jimmunol.177.11.7980. [DOI] [PubMed] [Google Scholar]
- 15.Lucero MG, Dulalia VE, Nillos LT, Williams G, Parreno RA, Nohynek H, Riley ID, Makela H. Pneumococcal conjugate vaccines for preventing vaccine-type invasive pneumococcal disease and X-ray defined pneumonia in children less than two years of age. Cochrane Database Syst Rev. 2009:CD004977. doi: 10.1002/14651858.CD004977.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park SY, Van Beneden CA, Pilishvili T, Martin M, Facklam RR, Whitney CG. Invasive Pneumococcal Infections among Vaccinated Children in the United States. The Journal of Pediatrics. 2010;156:478–483. e472. doi: 10.1016/j.jpeds.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 17.McCool TL, Harding CV, Greenspan NS, Schreiber JR. B- and T-Cell Immune Responses to Pneumococcal Conjugate Vaccines: Divergence between Carrier- and Polysaccharide-Specific Immunogenicity. Infection and Immunity. 1999;67:4862–4869. doi: 10.1128/iai.67.9.4862-4869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18**.Malley R, Anderson PW. Serotype-independent pneumococcal experimental vaccines that induce cellular as well as humoral immunity. Proceedings of the National Academy of Sciences. 2012;109:3623–3627. doi: 10.1073/pnas.1121383109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. The Journal of Experimental Medicine. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. The Journal of Experimental Medicine. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin J-S, Kummer LW, Szaba FM, Smiley ST. IL-17 Contributes to Cell-Mediated Defense against Pulmonary Yersinia pestis Infection. The Journal of Immunology. 2011;186:1675–1684. doi: 10.4049/jimmunol.1003303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22**.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. The Journal of Clinical Investigation. 2009;119:1899–1909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22**.The authors show that TLR2-dependent Th17 cells are required for the monocyte/macrophages and neutrophils dependent clearence of pneumococcal colonization.
- 23.Lu Y-J, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, Kolls JK, Srivastava A, Lundgren A, Forte S, et al. Interleukin-17A Mediates Acquired Immunity to Pneumococcal Colonization. PLoS Pathog. 2008;4:e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and Lung Host Defense againstKlebsiella pneumoniae Infection. American Journal of Respiratory Cell and Molecular Biology. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 25.Malley R, Srivastava A, Lipsitch M, Thompson CM, Watkins C, Tzianabos A, Anderson PW. Antibody-Independent, Interleukin-17A-Mediated, Cross-Serotype Immunity to Pneumococci in Mice Immunized Intranasally with the Cell Wall Polysaccharide. Infection and Immunity. 2006;74:2187–2195. doi: 10.1128/IAI.74.4.2187-2195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCool TL, Cate TR, Moy G, Weiser JN. The Immune Response to Pneumococcal Proteins during Experimental Human Carriage. The Journal of Experimental Medicine. 2002;195:359–365. doi: 10.1084/jem.20011576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCool TL, Weiser JN. Limited Role of Antibody in Clearance of Streptococcus pneumoniae in a Murine Model of Colonization. Infection and Immunity. 2004;72:5807–5813. doi: 10.1128/IAI.72.10.5807-5813.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trzciński K, Thompson CM, Srivastava A, Basset A, Malley R, Lipsitch M. Protection against Nasopharyngeal Colonization by Streptococcus pneumoniae Is Mediated by Antigen-Specific CD4+ T Cells. Infection and Immunity. 2008;76:2678–2684. doi: 10.1128/IAI.00141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moffitt KL, Malley R, Lu Y-J. Identification of Protective Pneumococcal TH17 Antigens from the Soluble Fraction of a Killed Whole Cell Vaccine. PLoS ONE. 2012;7:e43445. doi: 10.1371/journal.pone.0043445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Feng Y, Yang K, Li Q, Ye L, Han L, Wan H. Early production of IL-17 protects against acute pulmonary Pseudomonas aeruginosa infection in mice. FEMS Immunology & Medical Microbiology. 2011;61:179–188. doi: 10.1111/j.1574-695X.2010.00764.x. [DOI] [PubMed] [Google Scholar]
- 31.Dubin PJ, Martz A, Eisenstatt JR, Fox MD, Logar A, Kolls JK. Interleukin-23-Mediated Inflammation in Pseudomonas aeruginosa Pulmonary Infection. Infection and Immunity. 2012;80:398–409. doi: 10.1128/IAI.05821-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Priebe GP, Walsh RL, Cederroth TA, Kamei A, Coutinho-Sledge YS, Goldberg JB, Pier GB. IL-17 Is a Critical Component of Vaccine-Induced Protection against Lung Infection by Lipopolysaccharide-Heterologous Strains of Pseudomonas aeruginosa. The Journal of Immunology. 2008;181:4965–4975. doi: 10.4049/jimmunol.181.7.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Wu W, Huang J, Duan B, Traficante DC, Hong H, Risech M, Lory S, Priebe GP. Th17-stimulating Protein Vaccines Confer Protection against Pseudomonas aeruginosa Pneumonia. American Journal of Respiratory and Critical Care Medicine. 2012;186:420–427. doi: 10.1164/rccm.201202-0182OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Using P.aeruginosa protein library, the authors identfied several key antigens for inducing Th17 responses both in vitro and in vivo, and demonstrated Th17 dependent protection using one of the antigens, PopB, which could be a potential antigen candidate for vaccine development.
- 34.Zaidi TS, Zaidi T, Pier GB, Priebe GP. Topical Neutralization of Interleukin-17 during Experimental Pseudomonas aeruginosa Corneal Infection Promotes Bacterial Clearance and Reduces Pathology. Infection and Immunity. 2012;80:3706–3712. doi: 10.1128/IAI.00249-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin Y, Slight S, Khader S. Th17 cytokines and vaccine-induced immunity. Seminars in Immunopathology. 2010;32:79–90. doi: 10.1007/s00281-009-0191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 36*.This paper show for the first time that Th17 response is elicited during vaccination prior to protective Th1 response and is required for the Th1-mediated protection against M. tuberculosis.
- 37.DeLyria ES, Redline RW, Blanchard TG. Vaccination of Mice Against H pylori Induces a Strong Th-17 Response and Immunity That Is Neutrophil Dependent. Gastroenterology. 2009;136:247–256. doi: 10.1053/j.gastro.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luzza F, Parrello T, Monteleone G, Sebkova L, Romano M, Zarrilli R, Imeneo M, Pallone F. Up-Regulation of IL-17 Is Associated with Bioactive IL-8 Expression in Helicobacter pylori-Infected Human Gastric Mucosa. The Journal of Immunology. 2000;165:5332–5337. doi: 10.4049/jimmunol.165.9.5332. [DOI] [PubMed] [Google Scholar]
- 39.Velin D, Favre L, Bernasconi E, Bachmann D, Pythoud C, Saiji E, Bouzourene H, Michetti P. Interleukin-17 Is a Critical Mediator of Vaccine-Induced Reduction of Helicobacter Infection in the Mouse Model. Gastroenterology. 2009;136:2237–2246. e2231. doi: 10.1053/j.gastro.2009.02.077. [DOI] [PubMed] [Google Scholar]
- 40.Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, Belladonna ML, Vacca C, Conte C, Mosci P, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. European Journal of Immunology. 2007;37:2695–2706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 41.Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu Rev Physiol. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- 42*.Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, Hwang JY, Kusser K, Hartson L, Kolls JK, Khader SA, Randall TD. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol. 2011;12:639–646. doi: 10.1038/ni.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.This is an interesting article demonstrating IL-17-dependent CXCL13 expression in the lung can induce iBALT formation independent of lymphotoid inducer cells.
- 43.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10:927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 44.Jaffar Z, Ferrini ME, Herritt LA, Roberts K. Cutting Edge: Lung Mucosal Th17-Mediated Responses Induce Polymeric Ig Receptor Expression by the Airway Epithelium and Elevate Secretory IgA Levels. The Journal of Immunology. 2009;182:4507–4511. doi: 10.4049/jimmunol.0900237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Research. 2004;103:133–138. doi: 10.1016/j.virusres.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 46.Alvarez-Rodriguez L, Lopez-Hoyos M, Munoz-Cacho P, Martinez-Taboada VM. Aging is associated with circulating cytokine dysregulation. Cellular immunology. 2012;273:124–132. doi: 10.1016/j.cellimm.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 47.Kryczek I, Zhao E, Liu Y, Wang Y, Vatan L, Szeliga W, Moyer J, Klimczak A, Lange A, Zou W. Human TH17 Cells Are Long-Lived Effector Memory Cells. Science Translational Medicine. 2011;3:104ra100. doi: 10.1126/scitranslmed.3002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Islander U, Andersson A, Lindberg E, Adlerberth I, Wold AE, Rudin A. Superantigenic Staphylococcus aureus Stimulates Production of Interleukin-17 from Memory but Not Naive T Cells. Infection and Immunity. 2010;78:381–386. doi: 10.1128/IAI.00724-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late Developmental Plasticity in the T Helper 17 Lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindenstrøm T, Woodworth J, Dietrich J, Aagaard C, Andersen P, Agger EM. Vaccine-Induced Th17 Cells Are Maintained Long-Term Postvaccination as a Distinct and Phenotypically Stable Memory Subset. Infection and Immunity. 2012;80:3533–3544. doi: 10.1128/IAI.00550-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghimire TR, Benson RA, Garside P, Brewer JM. Alum increases antigen uptake, reduces antigen degradation and sustains antigen presentation by DCs in vitro. Immunology Letters. 2012;147:55–62. doi: 10.1016/j.imlet.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 53.Kool M, Pétrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN, Tschopp J. Cutting Edge: Alum Adjuvant Stimulates Inflammatory Dendritic Cells through Activation of the NALP3 Inflammasome. The Journal of Immunology. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 54.Eisenbarth SC, Colegio OR, O/’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jordan MB, Mills DM, Kappler J, Marrack P, Cambier JC. Promotion of B Cell Immune Responses via an Alum-Induced Myeloid Cell Population. Science. 2004;304:1808–1810. doi: 10.1126/science.1089926. [DOI] [PubMed] [Google Scholar]
- 56*.Kool M, Soullié T, van Nimwegen M, Willart MAM, Muskens F, Jung S, Hoogsteden HC, Hammad H, Lambrecht BN. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. The Journal of Experimental Medicine. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56*.Alum does not activate monocyte and dendtric cells directly. This is an interesting article demonstrating alum-formulated antigens induces endogenous danger signal uric acid from necrotic cells, which leads to activation of T cells by activated monocyte-derived dendritic cells. Furthemore, authors show that uricase treatment abolished T cells activation.
- 57.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Conforti-Andreoni C, Spreafico R, Qian HL, Riteau N, Ryffel B, Ricciardi-Castagnoli P, Mortellaro A. Uric Acid-Driven Th17 Differentiation Requires Inflammasome-Derived IL-1 and IL-18. The Journal of Immunology. 2011;187:5842–5850. doi: 10.4049/jimmunol.1101408. [DOI] [PubMed] [Google Scholar]
- 59.Sutton C, Brereton C, Keogh B, Mills KHG, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. The Journal of Experimental Medicine. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bielinska AU, Gerber M, Blanco LP, Makidon PE, Janczak KW, Beer M, Swanson B, Baker JR., Jr Induction of Th17 cellular immunity with a novel nanoemulsion adjuvant. Crit Rev Immunol. 2010;30:189–199. doi: 10.1615/critrevimmunol.v30.i2.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Datta SK, Sabet M, Nguyen KPL, Valdez PA, Gonzalez-Navajas JM, Islam S, Mihajlov I, Fierer J, Insel PA, Webster NJ, et al. Mucosal adjuvant activity of cholera toxin requires Th17 cells and protects against inhalation anthrax. Proceedings of the National Academy of Sciences. 2010;107:10638–10643. doi: 10.1073/pnas.1002348107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee J-B, Jang J-E, Song MK, Chang J. Intranasal Delivery of Cholera Toxin Induces Th17-Dominated T-Cell Response to Bystander Antigens. PLoS ONE. 2009;4:e5190. doi: 10.1371/journal.pone.0005190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vitoriano-Souza J, Moreira NdD, Teixeira-Carvalho A, Carneiro CM, Siqueira FAM, Vieira PMdA, Giunchetti RC, Moura SAdL, Fujiwara RT, Melo MN, et al. Cell Recruitment and Cytokines in Skin Mice Sensitized with the Vaccine Adjuvants: Saponin, Incomplete Freund’s Adjuvant, and Monophosphoryl Lipid A. PLoS ONE. 2012;7:e40745. doi: 10.1371/journal.pone.0040745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leach S, Clements JD, Kaim J, Lundgren A. The Adjuvant Double Mutant Escherichia coli Heat Labile Toxin Enhances IL-17A Production in Human T Cells Specific for Bacterial Vaccine Antigens. PLoS ONE. 2012;7:e51718. doi: 10.1371/journal.pone.0051718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65*.Gopal R, Rangel-Moreno J, Slight S, Lin Y, Nawar HF, Fallert Junecko BA, Reinhart TA, Kolls J, Randall TD, Connell TD, et al. Interleukin-17-dependent CXCL13 mediates mucosal vaccine-induced immunity against tuberculosis. Mucosal Immunol. 2013 doi: 10.1038/mi.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65*.In this study authors show that instead of IFNγ-dependent immunity, inducing IL-17 response could be strategically used for vaccination against M. tuberculosis.
- 66.Agger EM, Cassidy JP, Brady J, Korsholm KS, Vingsbo-Lundberg C, Andersen P. Adjuvant modulation of the cytokine balance in Mycobacterium tuberculosis subunit vaccines; immunity, pathology and protection. Immunology. 2008;124:175–185. doi: 10.1111/j.1365-2567.2007.02751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brereton CF, Sutton CE, Ross PJ, Iwakura Y, Pizza M, Rappuoli R, Lavelle EC, Mills KHG. Escherichia coli Heat-Labile Enterotoxin Promotes Protective Th17 Responses against Infection by Driving Innate IL-1 and IL-23 Production. The Journal of Immunology. 2011;186:5896–5906. doi: 10.4049/jimmunol.1003789. [DOI] [PubMed] [Google Scholar]
- 68.Maue AC, Eaton SM, Lanthier PA, Sweet KB, Blumerman SL, Haynes L. Proinflammatory Adjuvants Enhance the Cognate Helper Activity of Aged CD4 T Cells. The Journal of Immunology. 2009;182:6129–6135. doi: 10.4049/jimmunol.0804226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kudva A, Scheller EV, Robinson KM, Crowe CR, Choi SM, Slight SR, Khader SA, Dubin PJ, Enelow RI, Kolls JK, et al. Influenza A Inhibits Th17-Mediated Host Defense against Bacterial Pneumonia in Mice. The Journal of Immunology. 2011;186:1666–1674. doi: 10.4049/jimmunol.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 71.Dhiman R, Periasamy S, Barnes PF, Jaiswal AG, Paidipally P, Barnes AB, Tvinnereim A, Vankayalapati R. NK1.1+ Cells and IL-22 Regulate Vaccine-Induced Protective Immunity against Challenge with Mycobacterium tuberculosis. The Journal of Immunology. 2012;189:897–905. doi: 10.4049/jimmunol.1102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hofstetter HH, Grau C, Buttmann M, Forsthuber TG, Gaupp S, Toyka KV, Gold R. The PLPp-specific T-cell population promoted by pertussis toxin is characterized by high frequencies of IL-17-producing cells. Cytokine. 2007;40:35–43. doi: 10.1016/j.cyto.2007.07.192. [DOI] [PubMed] [Google Scholar]
- 73.Chen X, Howard OMZ, Oppenheim JJ. Pertussis Toxin by Inducing IL-6 Promotes the Generation of IL-17-Producing CD4 Cells. The Journal of Immunology. 2007;178:6123–6129. doi: 10.4049/jimmunol.178.10.6123. [DOI] [PubMed] [Google Scholar]
- 74.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 75.Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]


