Abstract
Stem cells play a critical role in development and in tissue regeneration. The dental pulp contains a small sub-population of stem cells that are involved in the response of the pulp to caries progression. Specifically, stem cells replace odontoblasts that have undergone cell death as a consequence of the cariogenic challenge. Stem cells also secrete factors that have the potential to enhance pulp vascularisation and provide the oxygen and nutrients required for the dentinogenic response that is typically observed in teeth with deep caries. However, the same angiogenic factors that are required for dentine regeneration may ultimately contribute to the demise of the pulp by enhancing vascular permeability and interstitial pressure. Recent studies focused on the biology of dental pulp stem cells revealed that the multipotency and angiogenic capacity of these cells could be exploited therapeutically in dental pulp tissue engineering. Collectively, these findings suggest new treatment paradigms in the field of endodontics. The goal of this review is to discuss the potential impact of dental pulp stem cells to regenerative endodontics.
Key words: Tissue engineering, dental pulp, odontoblasts, angiogenesis, differentiation
INTRODUCTION
The discovery of stem cells in the dental pulp has led to a substantial change in our understanding of the mechanisms involved in the maintenance of dental pulp homeostasis in health and in the pulp response to injury. These cells are intimately involved with the physiology of the dental pulp tissue throughout the entire lifespan of the tooth. It has also been postulated that stem cells are involved in the regulation of pulp angiogenesis in response to cariogenic challenges. More recently, the potential use of stem cells in dental pulp tissue engineering has boosted much interest in the field of regenerative endodontics. Here, we summarise key research findings in the area of dental pulp stem cell biology in light of the potential impact of these research observations to the clinical practice of endodontics in the future.
STEM CELLS IN THE DENTAL PULP
Besides the development and introduction of new techniques, instruments, and medicaments for the clinical management of the dental pulp, the fundamental principles of clinical endodontics today are not drastically different than those of the time when first root canal instruments and gutta-percha were introduced in the 1800s. However, the isolation of clonogenic and highly proliferative stem cells from the dental pulp has the potential to change this scenario. Studies carried out at the National Institutes of Dental and Craniofacial Research (NIDCR) unveiled the stem cells from human exfoliated deciduous teeth (SHED) and dental pulp stem cells (DPSC) from permanent teeth1., 2.. These cells are multipotent, as defined by their ability to undergo odontogenic, angiogenic, adipogenic, chondrogenic, neurogenic, or myogenic differentiation2., 3., 4., 5., 6., 7.. More recent studies have started to explore the possibility of using dental pulp stem cells as an alternative treatment for neurologic and cardiac diseases8., 9.. The fraction of multipotent stem cells in the dental pulp is small10 and the location of these cells is not clearly known, but their phenotype is suggestive of their presence in perivascular niches11. Although both DPSC and SHED cells are originated from the dental pulp, they exhibit significant differences. For example, during osteogenic differentiation, SHED present higher levels of alkaline phosphatase activity and osteocalcin production, and higher proliferative rate than DPSC2., 4., 12.. Notably, both SHED and DPSC cells are capable of regenerating dentine and pulp-like tissues in vivo1., 2., 13., 14..
Stem cells and caries-induced dentinogenesis
The dental pulp is a highly vascularised and innervated connective tissue responsible for maintaining tooth vitality and able to respond to injuries. Dentinogenesis is a unique process, which involves the interaction between odontoblasts, endothelial cells, and nerves15. The odontoblasts, ecto-mesenchymal derived cells, are the first cells to respond to the injury caused by bacterial invasion during caries progression16. The endothelial cells and nerve cells located in the vicinity of the carious lesion modulate the odontoblastic response17., 18., 19..
Primary odontoblasts are induced to secrete a dentine matrix that mineralises as reactionary dentine in response to shallow caries20., 21.. This type of tertiary dentine protects the dental pulp from irritants and maintains dental pulp integrity. Meanwhile, when caries advances more deeply towards the pulp, and more severe injury happens, the odontoblasts can undergo cell death. Cells originated from the dental pulp stem cell pool typically substitute dying odontoblasts. This process depends on a cascade of events that involve stem cell proliferation, migration, and differentiation into odontoblasts. In other words, the pulp healing depends at least in part on the regenerative potential of stem cells from the pulp core that actively migrate towards the carious site, differentiate into odontoblasts, and secrete mineralisable matrices. Stem cell-derived odontoblasts can also contribute to the generation of a dentine bridge in cases of pulp exposure. Dentine bridges are also considered a type of tertiary dentine21., 22..
Stem cells and pulp angiogenesis
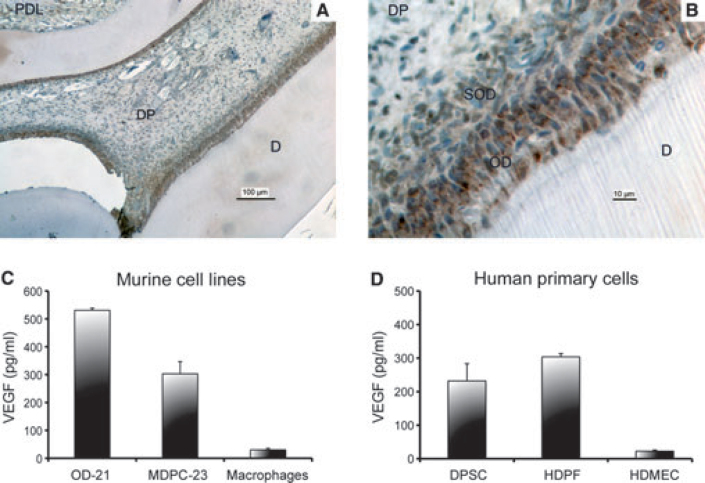
The spatial proximity between odontoblasts and blood vessels suggests the existence of an active interchange of signalling molecules during dentinogenesis23. Vascular endothelial growth factor (VEGF) is a potent inducer of endothelial cell differentiation and survival, and it is the most effective angiogenic factor characterised to date24., 25., 26.. VEGF also plays a critical role in the control of vascular permeability during physiological and pathological events26. VEGF is strongly expressed by odontoblasts and in the sub-odontoblastic layer in vivo (Figure 1)27., 28., 29.. Indeed, VEGF is potently expressed in dental pulp tissues of teeth undergoing caries-induced pulpitis, as demonstrated by immunohistochemical studies30. Among its receptors, VEGFR2 appears to be the most intimately associated with the angiogenic potential of endothelial cells31. Notably, VEGFR2 is expressed in the dental pulp of permanent and primary teeth, which is consistent with the ability of pulp cells to respond to VEGF-induced signalling32. We have recently performed a pilot study to evaluate the difference in VEGF expression in carious teeth, using non-carious teeth from the same patient as controls. Initial data analysis revealed a significant increase in VEGF expression in teeth with caries as compared to sound teeth (Figure 2). We have also observed an increase in VEGFR2 expression in the carious tooth of one of the patients examined, but not in the other patient, in these pilot studies (Figure 2).
Figure 1.
Expression of VEGF by dental pulp cells. Photomicrographs at low (A) and high (B) magnification of VEGF immunohistochemistry from the rat dental pulp. Intense VEGF staining is observed in the odontoblastic and sub-odontoblastic layers. Legends: periodontal ligament (PDL), dental pulp (DP), dentine (D), odontoblastic layer (OD), sub-odontoblastic layer (SOD). (C) Baseline VEGF expression in murine cell lines: undifferentiated dental pulp cells (OD-21), odontoblasts-like cells (MDPC-23), and macrophages. (D) Baseline VEGF expression in human primary cells: dental pulp stem cells from permanent teeth (DPSC), human dental pulp fibroblasts (HDPF), and human dermal microvascular endothelial cells (HDMEC).
Figure 2.
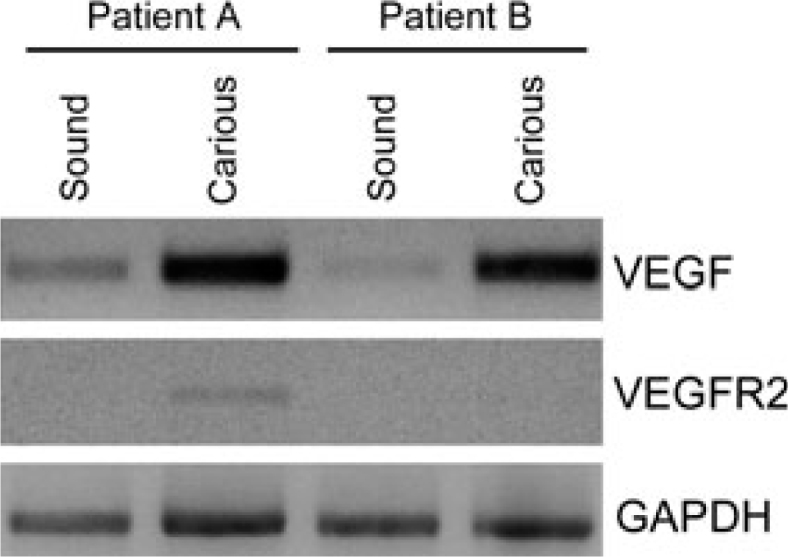
Pilot study on VEGF and VEGFR2 expression in the pulp of sound and carious teeth. VEGF and VEGFR2 gene expression was analysed by RT-PCR in the dental pulp of two patients (patient A and B). Each pair consists of one tooth with a deep caries lesion and a non-carious tooth. VEGF was upregulated in the pulps of carious teeth as compared to the pulps from non-carious teeth. VEGFR2 expression was low in the pulp from one tooth with deep caries (patient A), and undetectable by RT-PCR in the remaining samples analysed here.
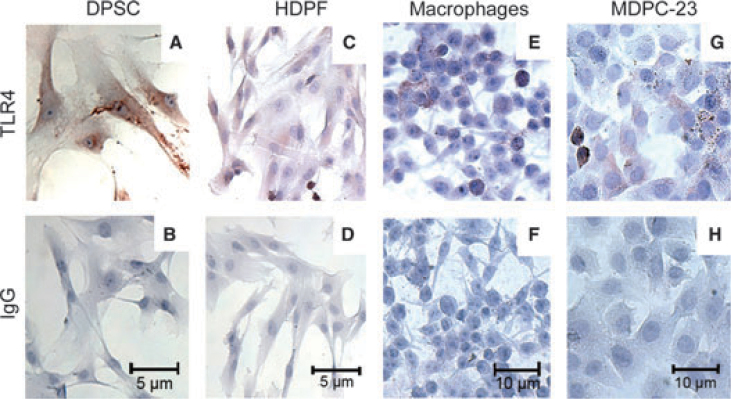
Lipotheicoic acid (LTA) and lipopolysaccharides (LPS) are important toxins associated with gram-positive and gram-negative bacteria, respectively. We have observed that VEGF expression is enhanced in dental pulp cells exposed to LTA or LPS27., 28.. Gram-positive, aerobic or facultative anaerobic bacteria such as Streptococcus mutans and Lactobacillus acidophilus are predominant bacteria in shallow caries lesions. In contrast, gram-negative anaerobic or facultative bacteria as Prevotellas and Porphyromonas are more commonly found in deep caries lesions33. The responses to bacterial stimuli are possible because the odontoblasts express Toll-like receptors. TLR2 is primarily involved in gram-positive and TLR4 in gram-negative bacterial recognition. Previous research showed increased expression of TLR4 in dental pulp cells34 and nociceptive neurons35 during pulpitis. We have shown that TLR2 and TLR4 play a critical role in the regulation of dental pulp angiogenesis in response to bacterial stimuli36., 37.. Notably, dental pulp stem cells (DPSC) express TLR-4 (Figure 3) and exposure to bacterial LPS enhances VEGF expression. In a search for a mechanistic explanation for these results, we observed that LPS triggers intracellular signalling via PKC-ζ and ERK in dental pulp stem cells. This pathway is critical for the regulation of the expression of VEGF by LPS in these cells29. We hypothesise that the ability of odontoblasts and stem cells to sense LPS through TLR signalling contributes to the overall response of the pulp to bacterial infection that is characterised by an increase in vascular density and influx of immune cells.
Figure 3.
Expression of TLR4 in dental pulp cells. Immunohistochemistry for TLR4 gene expression analysis (A, C, E, G) using a non-specific IgG as control (B, D, F, H). TLR4 was observed in the dental pulp stem cells (DPSC), human dental pulp fibroblasts (HDPF), rat odontoblast-like cells (MDPC-23), and mouse macrophages (control).
It is speculated that VEGF plays a key role in the promotion of dentinogenesis by inducing the vascularisation required to sustain the high metabolic demands of odontoblastic cells in active processes of dentine matrix secretion. On the other hand, excessive VEGF might be responsible for irreversible pulpal damage by increasing tissue volume, and perhaps intra-pulpal pressure, which collectively results in additional tissue damage. Deeper knowledge about the effect of VEGF in the dental pulp tissue is necessary before one can fully understand the impact of this potent growth factor to tissue damage and tissue regeneration.
DENTAL PULP TISSUE ENGINEERING
The inspiration for the use of dental pulp stem cells in tooth tissue regeneration was boosted by a key study that demonstrated that these cells are capable of generating complex dental tissues in vivo1. The dental pulp tissue generated by these cells is typically surrounded by a layer of odontoblast-like cells lining mineralised deposits. Notably, odontoblastic processes invading the tubular dentine structures can be observed1. We believe that this landmark study plays a very important role in setting the stage for the field of regenerative endodontics.
Although dental pulp stem cells can be obtained from permanent teeth (e.g. third molars or teeth extracted for orthodontic reasons), primary teeth have become an even more attractive source of mesenchymal stem cells because they constitute perhaps the only truly ‘disposable’ post-natal human tissue. These cells also appear to be highly proliferative, as compared to stem cells from permanent teeth (unpublished observations). The ability of dental pulp stem cells to differentiate into odontoblasts and to regenerate the dental pulp6., 13., 14., 38., 39. has raised the interest towards the use of these cells as a conceptual framework for the development of therapeutic strategies for the revitalisation of the necrotic teeth.
We have recently demonstrated that transplantation of SHED seeded in tooth slice/scaffolds into the subcutaneous space of immunodeficient mice results in the generation of a tissue with morphological characteristics that resemble those observed in human dental pulps38., 39., 40.. The odontoblast-like cells lining the dentine of the tooth slice were positive for dentine sialoprotein (DSP), a marker for odontoblastic differentiation38. Notably, a recent study provided strong evidence for the differentiation of SHED into functional odontoblasts by demonstrating that these cells were able to generate new tubular dentine in vivo, as determined by tetracycline staining13.
Dentine serves as an important reservoir of bioactive molecules that are clearly involved in the regulation of dental pulp responses to stimuli41. We have recently demonstrated that dentine-derived morphogens play an important role in the odontoblastic differentiation of SHED39. In the search for the specific molecules involved in this process, we have evaluated the role of bone morphogenetic proteins (BMP), which are known to be involved in the regulation of odontogenesis and dentine regeneration42., 43.. We observed that dentine-derived BMP-2, but not BMP-7, is required for the odontoblastic differentiation of SHED39. Collectively, these data suggest that the stimuli required for the odontoblastic differentiation of dental pulp stem cells can be recruited from the dentine itself. Under this paradigm, dentine contains ‘fossilised’ bioactive molecules ready to be engaged in processes aiming at the defence of the integrity of the dental pulp tissue. This hypothesis is largely derived from the exceptional work performed by the Smith laboratory over the last 20 years44.
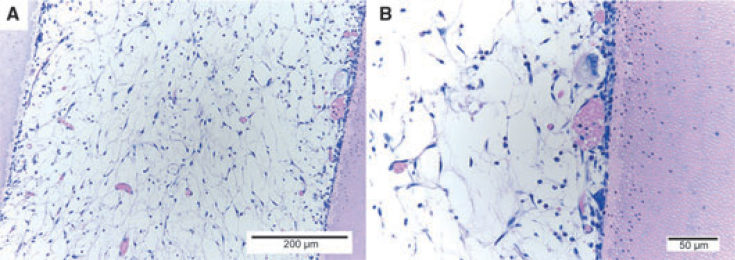
The efforts on dental pulp tissue engineering are geared towards the generation of a viable and healthy pulp throughout the entire root canal length. One study showed that SHED cells are able to attach to the dentine walls and proliferate inside root canals in vitro45. Later, it has been shown that dental pulp stem cells can regenerate a pulp-like tissue in root canals in vivo. The tissue formed had functional odontoblast-like cells able to deposit a mineralieed matrix on the root canal walls14. One recent study from our laboratory explored the use of an injectable scaffold (Puramatrix) loaded with SHED cells to engineer a dental pulp throughout the entire length of the root canal. We observed the formation of a dental pulp-like tissue (Figure 4) able to generate new dentine along the root canal walls, as confirmed by tetracycline staining (data not shown). Notably, scaffold development is an area of intense research today. The ability to inject the cells into the root canal using a biocompatible and biodegradable scaffold will be critical for the future use of stem cell-based therapies in clinical endodontics.
Figure 4.
Engineering of a dental pulp-like tissue in the root canal of a human tooth extracted for orthodontic reasons. Photomicrographs at low (A) and high (B) magnification of the tissue generated by the transplantation of SHED loaded in an injectable scaffold (Puramatrix) into the root canal of a human tooth transplanted into the subcutaneous space of an immunodeficient mouse.
FUTURE CLINICAL APPLICATIONS
The understanding of the mechanisms underlying pulp angiogenic responses is critical for the development of new, targeted therapies that aim at the conservation of dental pulp viability. For example, new therapeutic approaches could be used to regulate the expression of angiogenic factors (e.g. VEGF) to enhance the success of revascularisation of avulsed teeth46. On the other hand, inhibitors of angiogenesis and vascular permeability could be indicated as an adjuvant therapy for cases of incipient pulpitis. We believe that the area of molecularly targeted therapeutics aiming at the maintenance of pulp viability is in its infancy. However, developments in this area have the potential to revolutionise the way that we practice clinical endodontics in the future.
Perhaps one of the first indications for the translation of dental pulp tissue engineering to the clinic is in the treatment of traumatised immature permanent incisors. Dental trauma is a relatively common occurrence in children47. Avulsion, intrusion and extrusion are injuries resulted from the forceful displacement of the tooth and may lead to the rupture the apical blood and nerve bundles. As a consequence, these teeth frequently undergo pulp necrosis and interruption of dentinogenesis. This results in incomplete vertical and lateral root formation with thin and fragile dentine walls. It has been shown that these teeth are highly susceptible to root fractures upon second trauma48. However, the open apex and large pulp chamber favours regenerative cell-based therapies. This might constitute a favourable clinical scenario for the first attempts to regenerate dental pulps with stem cells49.
CONCLUSIONS
Stem cells are critical for the physiology of the dental pulp and for the response of this tissue to injury. Recent findings have unveiled dental pulp stem cells as potential therapeutic targets in cases of reversible pulpitis. Importantly, these cells may become an alternative primary strategy for the revitalisation of necrotic immature permanent teeth. Such discoveries have the potential to fundamentally change the paradigms of conservative vital pulp and root canal therapy, and perhaps allow for the treatment in the future of conditions that are presently untreatable in dentistry.
Acknowledgements
The authors would like to thank the members of the Angiogenesis Research Laboratory at the University of Michigan School of Dentistry for their invaluable input and technical help during the execution of these studies. We would like to thank, for the support received, CAPES (Brazilian Government), the Department of Cariology, Restorative Sciences, and Endodontics for the pursuit of studies performed in our laboratory that are presented in this review.
Conflict of interest
The authors declare no conflicts of interest.
REFERENCES
- 1.Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSC) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miura M, Gronthos S, Zhao M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiraly M, Porcsalmy B, Pataki A, et al. Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochem Int. 2009;55:323–332. doi: 10.1016/j.neuint.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Koyama N, Okubo Y, Nakao K, et al. Evaluation of pluripotency in human dental pulp cells. J Oral Maxillofac Surg. 2009;67:501–506. doi: 10.1016/j.joms.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gronthos S, Brahim J, Li W, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 7.Kerkis I, Kerkis A, Dozortsev D, et al. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 2006;184:105–116. doi: 10.1159/000099617. [DOI] [PubMed] [Google Scholar]
- 8.Arthur A, Rychkov G, Shi S, et al. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26:1787–1795. doi: 10.1634/stemcells.2007-0979. [DOI] [PubMed] [Google Scholar]
- 9.Gandia C, Arminan A, Garcia-Verdugo JM, et al. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells. 2008;26:638–645. doi: 10.1634/stemcells.2007-0484. [DOI] [PubMed] [Google Scholar]
- 10.Balic A, Aguila HL, Caimano MJ, et al. Characterization of stem and progenitor cells in the dental pulp of erupted and unerupted murine molars. Bone. 2010;46:1639–1651. doi: 10.1016/j.bone.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura S, Yamada Y, Katagiri W, et al. Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J Endod. 2009;35:1536–1542. doi: 10.1016/j.joen.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Sakai VT, Zhang Z, Dong Z, et al. SHED differentiate into functional odontoblast and endothelium. J Dent Res. 2010;89:791–796. doi: 10.1177/0022034510368647. [DOI] [PubMed] [Google Scholar]
- 14.Huang G, Yamaza T, Shea LD, et al. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A. 2010;16:605–615. doi: 10.1089/ten.tea.2009.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linde A, Goldberg M. Dentinogenesis. Crit Rev Oral Biol Med. 1993;5:679–728. doi: 10.1177/10454411930040050301. [DOI] [PubMed] [Google Scholar]
- 16.Ruch JV, Lesot H, Bègue-Kirn C. Odontoblast differentiation. Int J Dev Biol. 1995;39:51–68. [PubMed] [Google Scholar]
- 17.Takahashi K. Pulpal vascular changes in inflammation. Proc Finn Dent Soc. 1992;88(Suppl 1):381–385. [PubMed] [Google Scholar]
- 18.Avery JK, Cox CF, Chiego DJ. In: Dentin and Dentinogenesis. Vol. 1. Linde A, editor. CRC Press; Boca Raton: 1984. Structural and physiologic aspects of dentin innervation; pp. 19–46. [Google Scholar]
- 19.Kramer IR. The vascular architecture of the human dental pulp. Arch Oral Biol. 1960;2:177–189. doi: 10.1016/0003-9969(60)90021-2. [DOI] [PubMed] [Google Scholar]
- 20.Linde A, Lundgren T. From serum to the mineral phase. The role of the odontoblast in calcium transport and mineral formation. Int J Dev Biol. 1995;39:213–222. [PubMed] [Google Scholar]
- 21.Smith AJ, Cassidy N, Perry H, et al. Reactionary dentinogenesis. Int J Dev Biol. 1995;39:273–280. [PubMed] [Google Scholar]
- 22.Tziafas D, Smith AJ, Lesot H. Designing new treatment strategies in vital pulp therapy. J Dent. 2000;28:77–92. doi: 10.1016/s0300-5712(99)00047-0. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi K. Vascular architecture of dog pulp using corrosion resin cast examined under a scanning electron microscope. J Dent Res. 1985;64(Spec No):579–584. doi: 10.1177/002203458506400413. [DOI] [PubMed] [Google Scholar]
- 24.Leung DW, Cachianes G, Kuang WJ, et al. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 25.Nör JE, Christensen J, Mooney DJ, et al. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154:375–384. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 27.Telles PD, Hanks CT, Machado MAAM, et al. Lipoteichoic acid upregulates VEGF expression in macrophages and pulp cells. J Dent Res. 2003;82:466–470. doi: 10.1177/154405910308200612. [DOI] [PubMed] [Google Scholar]
- 28.Botero TM, Mantellini MG, Song W, et al. Effect of lipopolysaccharides on vascular endothelial growth factor expression in mouse pulp cells and macrophages. Eur J Oral Sci. 2003;111:228–234. doi: 10.1034/j.1600-0722.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 29.Botero TM, Son JS, Vodopyanov D, et al. MAPK signaling is required for LPS-induced VEGF in pulp stem cells. J Dent Res. 2010;89:264–269. doi: 10.1177/0022034509357556. [DOI] [PubMed] [Google Scholar]
- 30.Güven G, Altun C, Günhan O, et al. Co-expression of cyclooxygenase-2 and vascular endothelial growth factor in inflamed human pulp: an immunohistochemical study. J Endod. 2007;33:18–20. doi: 10.1016/j.joen.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Gille H, Kowalski J, Li B, et al. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor specific vascular endothelial growth factor mutants. J Biol Chem. 2001;276:3222–3230. doi: 10.1074/jbc.M002016200. [DOI] [PubMed] [Google Scholar]
- 32.Mattuella GL, Figueiredo JA, Nör JE, et al. Vascular endothelial growth factor receptor-2 expression in the pulp of human primary and young permanent teeth. J Endod. 2007;33:1408–1412. doi: 10.1016/j.joen.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 33.Hahn CL, Liewehr FR. Relationships between caries bacteria, host responses, and clinical signs and symptoms of pulpitis. J Endod. 2007;33:213–219. doi: 10.1016/j.joen.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Mutoh N, Tani-Ishii N, Tsukinoki K, et al. Expression of toll-like receptor 2 and 4 in dental pulp. J Endod. 2007;33:1183–1186. doi: 10.1016/j.joen.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 35.Wadachi R, Hargreaves KM. Trigeminal nociceptors express TLR-4 and CD14: a mechanism for pain due to infection. J Dent Res. 2006;85:49–53. doi: 10.1177/154405910608500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soden RI, Botero TM, Hanks CT, et al. Angiogenic signaling triggered by cariogenic bacteria in pulp cells. J Dent Res. 2009;88:835–840. doi: 10.1177/0022034509341946. [DOI] [PubMed] [Google Scholar]
- 37.Botero TM, Shelburne CE, Holland GR, et al. TLR4 mediates LPS-induced VEGF expression in odontoblasts. J Endod. 2006;32:951–955. doi: 10.1016/j.joen.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Cordeiro MM, Dong Z, Kaneko T, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34:962–969. doi: 10.1016/j.joen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Casagrande L, Demarco FF, Zhang Z, et al. Dentin-derived BMP-2 and odontoblastic differentiation of SHED. J Dent Res. 2010;89:603–608. doi: 10.1177/0022034510364487. [DOI] [PubMed] [Google Scholar]
- 40.Gonçalves SB, Dong Z, Bramante CM, et al. Tooth slice-based models for the study of human dental pulp angiogenesis. J Endod. 2007;33:811–814. doi: 10.1016/j.joen.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Graham L, Cooper PR, Cassidy N, et al. The effect of calcium hydroxide on solubilisation of bioactive dentine matrix components. Biomaterials. 2006;14:2865–2873. doi: 10.1016/j.biomaterials.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 42.Nakashima M, Reddi AH. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. 2003;21:1025–1032. doi: 10.1038/nbt864. [DOI] [PubMed] [Google Scholar]
- 43.Yamashiro T, Tummers M, Thesleff I. Expression of bone morphogenetic proteins and Msx genes during root formation. J Dent Res. 2003;82:172–176. doi: 10.1177/154405910308200305. [DOI] [PubMed] [Google Scholar]
- 44.Smith AJ, Tobias RS, Plant CG, et al. In vivo morphogenetic activity of dentine matrix proteins. J Biol Buccale. 1990;18:123–129. [PubMed] [Google Scholar]
- 45.Gotlieb EL, Murray PE, Namerow KN, et al. An ultrastructural investigation of tissue-engineered pulp constructs implanted within endodontically treated teeth. J Am Dent Assoc. 2008;139:457–465. doi: 10.14219/jada.archive.2008.0189. [DOI] [PubMed] [Google Scholar]
- 46.Mullane EM, Dong Z, Sedgley CM, et al. Effects of VEGF and FGF2 on the revascularization of severed human dental pulps. J Dent Res. 2008;87:1144–1148. doi: 10.1177/154405910808701204. [DOI] [PubMed] [Google Scholar]
- 47.Andreasen JO, Ravn JJ. Epidemiology of traumatic dental injuries to primary and permanent teeth in a Danish population sample. Int J Oral Surg. 1972;1:235–239. doi: 10.1016/s0300-9785(72)80042-5. [DOI] [PubMed] [Google Scholar]
- 48.Cvek M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Endod Dent Traumatol. 1992;8:45–55. doi: 10.1111/j.1600-9657.1992.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 49.Nör JE. Tooth regeneration in operative dentistry. Oper Dent. 2006;31:633–642. doi: 10.2341/06-000. [DOI] [PubMed] [Google Scholar]