Abstract
Aim
To compare and contrast two colorimetric assays used for measurement of proliferation using two dental pulp cell types: dental pulp stem cells (DPSC) and human dental pulp fibroblasts (HDPF).
Methodology
DPSC or HDPF were seeded at 0.25 × 104 cells/well in 96-well plates. Cell proliferation was evaluated after 24-72 hours. At the end of the experimental period, the Sulforhodamine B (SRB) assay or a water-soluble tetrazolium salt (WST-1) assay was performed. Optical densities were determined in a microplate reader (Genius; TECAN). Data were analyzed by Student’s t-test (comparison between cell types), and one-way ANOVA followed by Tukey test (time point intervals). Pearson’ correlation tests were performed to compare the two assays for each cell line.
Results
Both assays showed that DPSC had higher proliferation rates than HDPF. A positive significant correlation between the two colorimetric assays tested for both cell types DPSC (Pearson’s Correlation Coefficient=0.847; p<0.05) and HDPF (Pearson’s Correlation Coefficient=0.775; p<0.05).
Conclusion
Both tests demonstrated similar trends of cell proliferation, and thus are both appropriate for the evaluation of DPSC and HDPF. The choice of assay is therefore one of practical application. SRB stained plates may be dried and stored so may have utility in laboratories where data may require review or when access to analytical equipment is limited. WST-1 assays have the benefit of both ease and speed and may have utility in laboratories requiring either high throughput or rapid analyses.
Keywords: Endodontics, Methods, Tissue Engineering, Stem Cells
Introduction
Measurement of cell proliferation has become an essential technology in the life sciences. Dental materials research has been driven by an understanding of toxicity limitations and biocompatibility of new materials with dental and other oral tissue (Schweikl et al. 2005). In particular, biocompatibility of novel endodontic materials is important for the clinical success of endodontic treatment. Clearly, toxic or irritant materials may induce or even exacerbate inflammatory reactions in the periapical tissues, which may in turn weaken the reparative materials themselves (Geurtsen 2001, Gorduysus et al. 2007). Furthermore, advances in tissue engineering are providing potential new biological therapies, especially with dental stem cells, which will allow the creation or reconstruction of dental tissue using biodegradable scaffolds and growth factor panels (Cordeiro et al. 2008, Nedel et al. 2009). Thus it is evident that effective and appropriate methods must be available to dental materials and tissue research in order assess toxicity and biocompatibility but also that with the broad spectrum of materials, reagents and cells involved, one methodology alone may perhaps be insufficient.
Modern colorimetric cell-based proliferation or toxicity assays have been optimized for the use of microtiterplates, allowing many samples to be analyzed rapidly and simultaneously using compounds which stain the cells directly or that are metabolized into colored products (Weyermann et al. 2005). A spectrophotometer is used to quantify the intensity of the color, producing numerical data (absorbance) that correlate with the number of cells present (Givens et al. 1990). Notably, significant differences may exist in the sensitivity of colorimetric methods which use different labeling systems to assay the same final growth parameters, so comparative assessment of the assay methods themselves is vital (Weyermann et al 2005).
The sulforhodamine B (SRB) assay is used in in vitro anticancer-drug screening (Papazisis et al. 1997, Lin et al. 1999, Vichai & Kirtikara 2006). Indeed the SRB assay is an American National Cancer Institute and National Institute of Health standard assay for testing novel anti-cancer drugs (http://dtp.nci.nih.gov/branches/btb/ivclsp.html). It has been widely employed for both the evaluation of cells other than cancer cells in a 96-well microplate-based assay format (Lin et al. 1999). SRB is a bright-pink aminoxanthene dye that binds to basic amino acids of cellular proteins under mild acidic conditions, and dissociates under basic conditions (Vichai & Kirtikara 2006). The binding of SRB is stoichiometric, and the amount of dye extracted from stained cells is directly proportional to the total protein mass and therefore correlated to cell number (Papazisis et al. 1997, Vichai & Kirtikara 2006).
Metabolism of tetrazolium salts, such as 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), sodium 3′-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis (4-methoxy-6-nitro) benzene sulfonic acid hydrate (XTT), and 4-[3-(4-Iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate (WST-1), form the basis of other colorimetric assays. In contrast to the SRB, tetrazolium salt assays are based on the metabolic reduction of the salt to highly coloured formozan end-products (Berridge et al. 1996). Despite several studies examining methods for measuring reductase enzymes, the precise nature of the bioreduction of tetrazolium salts is still being investigated (Berridge et al. 1996, Tan & Berridge 2000). These colorimetric assays are used extensively in cell proliferation and cytotoxicity analysis, enzyme analysis and bacteriological screening (Berridge & Tan 1993, Berridge et al. 1996, Ngamwongsatit et al. 2008). More specifically WST-1 has been used in precursor cells such as human embryonic stem cell (Dvorak et al. 2005) and mesenchymal stem cells (Dang et al. 2006).
A review of the literature revealed that no report has directly compared these two colorimetric assays for evaluation of the proliferation rates of human dental pulp stem cells (DPSC). Such data are critically important for determination of dental pulp cell function in biocompatibility studies and studies of novel dental tissue engineering methodologies, particularly where one assay relies on metabolic function which may differ from cell type to cell type. The aim of this study was to perform a comparative analysis of SRB and WST-1 for measurement of the proliferation of DPSC and human dental pulp fibroblasts (HDPF).
Material and Methods
Cells
Human dental pulp stem cells (DPSC) were obtained from Dr. Songtao Shi (University of Southern California, USA), and human dental pulp fibroblasts (HDPF) were retrieved from permanent teeth extracted in the University of Michigan Oral Surgery department. The research protocol was approved by the Research Ethics Committee of the Federal University of Pelotas, Brazil. Both cell types were cultivated in Dulbecco’s Modified Eagle Medium (DMEM) low glucose (DPSC) or high glucose (HDPF) (Invitrogen, Grand Island, NY, USA) containing 10% fetal bovine serum (Invitrogen) and 1% penicillin/streptomycin solution (Invitrogen) at 37°C in 5% CO2. Cells from passage 4-6 were used for these experiments. Subconfluent (80%) DPSC and HDPF were detached with 0.25% trypsin-EDTA ( Invitrogen). Each cell lineage was seeded at 0.25 × 104 cells/well in 96-well plates. Immediately after seeding, samples were placed in an incubator (37°C in 5% CO2). Absorbance was then determined after 24, 48 and 72 hours using the WST-1 or the SRB assay.
Sulforhodamine B assay (SRB)
At the end of the experimental period, cells were washed with PBS and fixed with 10% trichloroacetic acid (TCA) for 1 hour at 4°C. Then, plates were washed 5 times under running water and dried at room temperature. To stain cells, 50 μL of the SRB pre-mixed solution (0.4% SRB dissolved in 0.1% acetic acid) were added to each well and incubated for 30 min at room temperature. Cells were washed 4 times with 1% acetic acid to remove unbound excess dye, and then dried at room temperature. 150 μL of 10 mM unbuffered trizma-base were added to solubilize the bound dye, followed by incubation at room temperature for 1 hour. Finally, the plates were placed in a shaker for 1 minute. The absorbance was determined in a microplate reader (TECAN, Genius, Männedorf, Switzerland) at 565 nm (Lin et al. 1999, Houghton et al. 2007).
WST-1 assay
At indicated time points, 20 μL of the WST-1 pre-mixed reagent (Cell Proliferation Reagent WST-1 (Roche Molecular Biochemicals, Basel, Switzerland) were added to each well and the plates were incubated at 37°C for 1 hour, as recommended by the manufacturer. Then, the plates were placed on a shaker for 1 min and the absorbance was determined in a microplate reader at 450 nm.
Statistical analysis
Data were analyzed by Student’s t-test (comparison between cell types), and one-way ANOVA followed by Tukey test (time point intervals). Pearson’ correlation tests were performed to compare the two assays for each cell line. Statistical analyses were carried out using the SigmaStat 2.0 software package (SSPS, Chicago, IL, USA). The significance level was set at P<0.05. Triplicate wells were analyzed for each experimental data point, and the experiments were repeated at least three times to verify reproducibility of results.
Results
The SRB assay showed that DPSC displayed significantly greater dye incorporation than HPDF (p<0.05) for each time point evaluated (p<0.05) (Figure 1). Similar results were observed when cells were analyzed with the WST-1 assay (p<0.05) in each time point (p<0.05), which revealed that DPSC produced significantly more dye product than HPDF (Figure 1). The overall trends of cell proliferation were similar using both methods. To test whether both colorimetric methods displayed the same proliferation trends using both cell lines a Pearson’s correlation test was used; this demonstrated a strong correlation between the two assays for DPSC (Correlation Coefficient = 0.847, p<0.05) (Figure 2). For HDPF cells, a positive correlation between the two assays was also observed (Correlation Coefficient = 0.775, p<0.05) (Figure 2).
Figure 1.
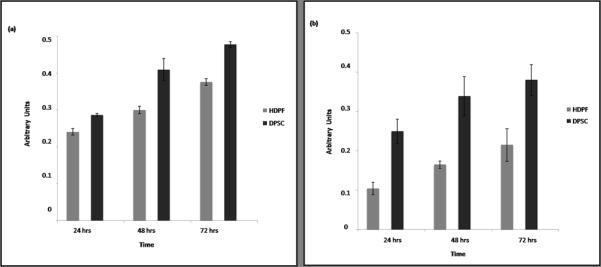
Proliferation of HDPF and DPSC cells over time. Cells (DPSC or HDPF) were seeded in 96-well plates at 0.25×104 cells/well and grown for indicated time periods. (A) For SRB assay, cells were fixed with TCA, stained and evaluated at 565 nm in a microplate reader. (B) For WST-1 assay, cells were incubated 1 hour in the pre-mixed solution of WST-1. Dye intensity was measured at 450 nm using a microplate reader. Data was analyzed from triplicate wells per experimental point and cell line, and reflect the results of 3 independent experiments.
Figure 2.
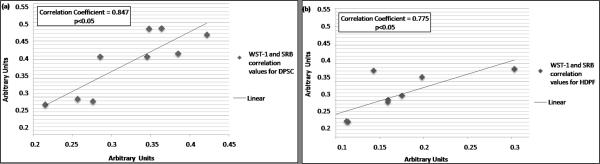
Correlation between the results obtained for WST-1 and SRB assays for DPSC and HDPF. (A) Graph depicting the correlation between WST-1 and SRB for DPSC cells (Correlation Coefficient = 0.847, p<0.05). (B) Graph depicting the correlation between WST-1 and SRB for HDPF cells (Correlation Coefficient = 0.847, p<0.05).
Discussion
Tissue engineering is an emerging and multidisciplinary field with potential for designing and constructing tissues or organs in order to restore their function or even completely replace them. One of the main components of tissue engineering are stem cells that respond to morphogenic signals, proliferating and differentiating to give rise to tissue and organs (Nakashima & Akamine 2005, Nedel et al. 2009). Therefore, the certainty of effective and simple methods for the evaluation of stem cell proliferation is critical to expedite the progress of research in the field of dental pulp tissue engineering.
SRB is a technique that requires several steps with one very critical step, subject to error, being the initial addition of trichloroacetic acid (TCA). The acid must be added gently so that the cells are not dislodged before they become fixed. Once fixation occurs, the cells become fairly resistant to damage (Papazisis et al. 1997). In addition to mechanical error, exogenous proteins present in fetal bovine serum can potentially be fixed along with the cells and this may affect the results causing high background values and high coefficients of variation (Papazisis et al. 1997). In contrast, the WST-1 assay is less technique sensitive. Specifically, WST-1 is available as a ready-to-use solution and, once applied, the plates can be read generally without further manipulation.
Notably timing is a major factor separating the methods. The SRB assay takes approximately 5 or more hours to perform, irrespective of cell type, whilst the WST-1 assay requires between 0.5 to 4 hours, depending on cell type. For DPSC, the WST-1 assay was typically performed in approximately 1 hour. Therefore, the WST-1 assay is a more rapid method for evaluation of dental pulp stem cell proliferation. However, an important benefit of the SRB assay is that at any point after TCA-fixation, plates can be dried and stored under dark and cool conditions (Vichai & Kirtikara 2006).This may be of benefit, for example, in laboratories that require review, or reanalysis, of original data after the end of experimental studies. Alternatively, in laboratories with limited equipment the plates may be stored until such time as equipment becomes available or, for example, where absorbance analysis must be performed at another physical site.
It has been observed that the SRB assay displays lower variation between different cell-lines when compared to colorimetric assays relying on cell metabolism such as MTT, a dye similar to WST-1 (Keepers et al. 1991). This is due to the different reduction capacity of each cell type, resulting in varying formozan product levels and thus in varying measured optical density (Keepers et al. 1991). The reduction of WST-1 has been associated with superoxide, and it seems to occur in the extracellular environment or associated with the plasma membrane (Berridge et al. 1996, Tan and Berridge 2000). Therefore depletion of NADH or some other mechanism that involves superoxide may potentially interfere with the reduction process of WST-1 (Berridge et al. 1996). Also, studies have indicated that different human cell types have varied extracellular superoxide content (Marklund 1984, Fattman et al. 2003). This variation could interfere in the reduction capacity of each cell type to different degrees. Since the discovery of stem cells in the dental pulp, many studies have been carried out using these cells (Gronthos et al. 2000, Cordeiro et al. 2008, Yang et al. 2009). Therefore it is important to test the response of DPSC towards colorimetric assays that are being used, to ensure the quality of results. In the present study a positive correlation was demonstrated between both colorimetric assays (WST-1 and SRB) in the two cell lines tested. This suggests that possible variations in DPSC metabolism did not interfere significantly in the linearity of WST-1 results compared to the more robust SRB assay.
Conclusion
Both tests demonstrated similar trends, with increasing absorbance apparently relating to increasing cell densities over time. Both assays are therefore suitable for analysis of dental pulp stem cells. Therefore the choice between one assay or another (WST-1 or SRB) should be based on the practical advantages or disadvantages of each method. The SRB may be more useful in laboratories where results may require reexamination at a later date whereas WST-1 could be used in daily laboratory routine where a more rapid and easier method is required.
Acknowledgements
This study was supported by a scholarship (BEX 0234-1) to the first author and a grant (484329/2007-3) provided by two Brazilian Government Agencies (CAPES and CNPq).
References
- Berridge MV, Tan AS. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Archives of Biochemistry and Biophysics. 1993;303:474–82. doi: 10.1006/abbi.1993.1311. [DOI] [PubMed] [Google Scholar]
- Berridge MV, Tan AS, McCoy KD, Wang R. The biochemical and cellular basis of cell proliferation assays that use tetrazolium salts. Biochemica. 1996;4:14–9. [Google Scholar]
- Cordeiro MM, Dong Z, Kaneko T, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. Journal of Endodontics. 2008;34:962–9. doi: 10.1016/j.joen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Dang JM, Sun DD, Shin-Ya Y, Sieber AN, Kostuik JP, Leong KW. Temperature-responsive hydroxybutyl chitosan for the culture of mesenchymal stem cells and intervertebral disk cells. Biomaterials. 2006;27:406–18. doi: 10.1016/j.biomaterials.2005.07.033. [DOI] [PubMed] [Google Scholar]
- Dvorak P, Dvorakova D, Koskova S, et al. Expression and potential role of fibroblast growth factor 2 and its receptors in human embryonic stem cells. Stem Cells. 2005;23:1200–11. doi: 10.1634/stemcells.2004-0303. [DOI] [PubMed] [Google Scholar]
- Fattman CL, Schaefer LM, Oury TD. Extracellular superoxide dismutase in biology and medicine. Free radical biology & medicine. 2003;1:236–56. doi: 10.1016/s0891-5849(03)00275-2. [DOI] [PubMed] [Google Scholar]
- Geurtsen W. Biocompatibility of root canal filling materials. Australian Endodontic Journal. 2001;27:12–21. doi: 10.1111/j.1747-4477.2001.tb00445.x. [DOI] [PubMed] [Google Scholar]
- Givens KT, Kitada S, Chen AK, Rothschiller J, Lee DA. Proliferation of human ocular fibroblasts. An assessment of in vitro colorimetric assays. Investigative Ophthalmology & Visual Science. 1990;31:1856–62. [PubMed] [Google Scholar]
- Gorduysus M, Avcu N, Gorduysus O, Pekel A, Baran Y, Avcu F, Ural AU. Cytotoxic effects of four different endodontic materials in human periodontal ligament fibroblasts. Journal of Endodontics. 2007;33:1450–4. doi: 10.1016/j.joen.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences of the U S A. 2000;97:13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton P, Fang R, Techatanawat I, Steventon G, Hylands PJ, Lee CC. The sulphorhodamine (SRB) assay and other approaches to testing plant extracts and derived compounds for activities related to reputed anticancer activity. Methods. 2007;42:377–87. doi: 10.1016/j.ymeth.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Keepers YP, Pizao PE, Peters GJ, van Ark-Otte J, Winograd B, Pinedo HM. Comparison of the sulforhodamine B protein and tetrazolium (MTT) assays for in vitro chemosensitivity testing. European Journal of Cancer. 1991;27:897–900. doi: 10.1016/0277-5379(91)90142-z. [DOI] [PubMed] [Google Scholar]
- Killough SA, Lundy FT, Irwin CR. Substance P expression by human dental pulp fibroblasts: a potential role in neurogenic inflammation. Journal of Endodontics. 2009;35:73–7. doi: 10.1016/j.joen.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Lin ZX, Hoult JR, Raman A. Sulphorhodamine B assay for measuring proliferation of a pigmented melanocyte cell line and its application to the evaluation of crude drugs used in the treatment of vitiligo. Journal of Ethnopharmacology. 1999;66:141–50. doi: 10.1016/s0378-8741(98)00199-8. [DOI] [PubMed] [Google Scholar]
- Marklund SL. Extracellular superoxide dismutase in human tissues and human cell lines. The Journal of Clinical Investigation. 1984;74:1398–403. doi: 10.1172/JCI111550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. Journal of Endodontics. 2005;31:711–8. doi: 10.1097/01.don.0000164138.49923.e5. [DOI] [PubMed] [Google Scholar]
- Nedel F, André DA, de Oliveira IO, et al. Stem-cells: therapeutic potential in Dentistry. The Journal of Contemporary Dental Practice. 2009;10:90–6. [PubMed] [Google Scholar]
- Ngamwongsatit P, Banada PP, Panbangred W, Bhunia AK. WST-1-based cell cytotoxicity assay as a substitute for MTT-based assay for rapid detection of toxigenic Bacillus species using CHO cell line. Journal of Microbiological Methods. 2008;73:211–5. doi: 10.1016/j.mimet.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Papazisis KT, Geromichalos GD, Dimitriadis KA, Kortsaris AH. Optimization of the sulforhodamine B colorimetric assay. Journal of Immunological Methods. 1997;208:151–8. doi: 10.1016/s0022-1759(97)00137-3. [DOI] [PubMed] [Google Scholar]
- Schweikl H, Hiller KA, Bolay C, Kreissl M, Kreismann W, Nusser A, Steinhauser S, Wieczorek J, Vasold R, Schmalz G. Cytotoxic and mutagenic effects of dental composite materials. Biomaterials. 2005;26:1713–9. doi: 10.1016/j.biomaterials.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Tan AS, Berridge MV. Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt, WST-1 to produce a soluble formazan: a simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. Journal of Immunological Methods. 2000;238:59–68. doi: 10.1016/s0022-1759(00)00156-3. [DOI] [PubMed] [Google Scholar]
- Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nature Protocols. 2006;1:1112–6. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- Weyermann J, Lochmann D, Zimmer A. A practical note on the use of cytotoxicity assays. International Journal of Pharmaceutics. 2005;288:369–76. doi: 10.1016/j.ijpharm.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Yang X, Yang F, Walboomers XF, Bian Z, Fan M, Jansen JA. The performance of dental pulp stem cells on nanofibrous PCL/gelatin/nHA scaffolds. Journal of Biomedical Materials Research. Part A. 2009;25 doi: 10.1002/jbm.a.32535. [DOI] [PubMed] [Google Scholar]


