Abstract
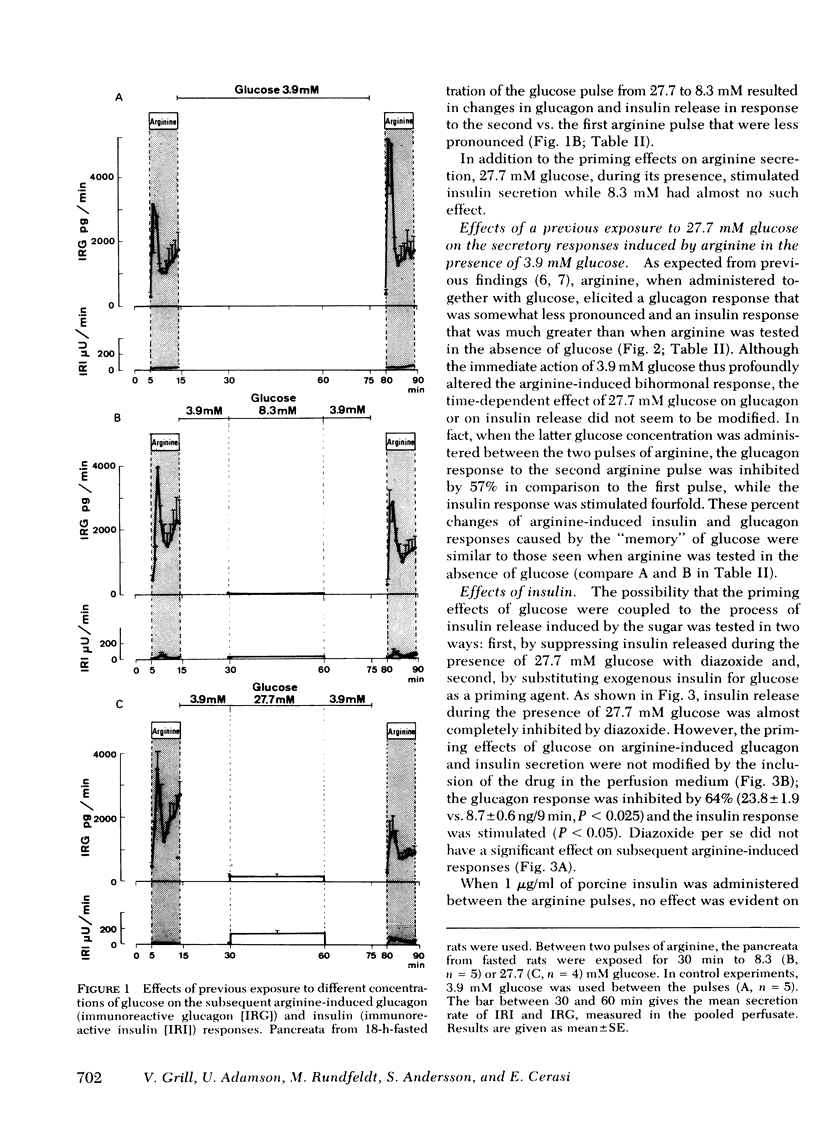
The influence of previous exposure to glucose on the subsequent B- and A2-cell secretory responses to arginine was investigated in the perfused pancreas of the rat. Arginine (8 mM) was administered in two brief (9 min) pulses separated by a period of 66 min. In pancreata from 18-h-fasted animals the two pulses of arginine elicited biphasic glucagon secretory responses, while stimulation of insulin release was barely detectable. When 27.7 mM glucose was administered for 30 min during the intervening period up to 20 min before the second pulse of arginine, the glucagon response to arginine was diminished by 55% while the insulin release was markedly increased in comparison with the first pulse. 8.3 mM glucose, when administered before the second pulse of arginine, exerted effects that were smaller but otherwise similar to those of 27.7 mM glucose.
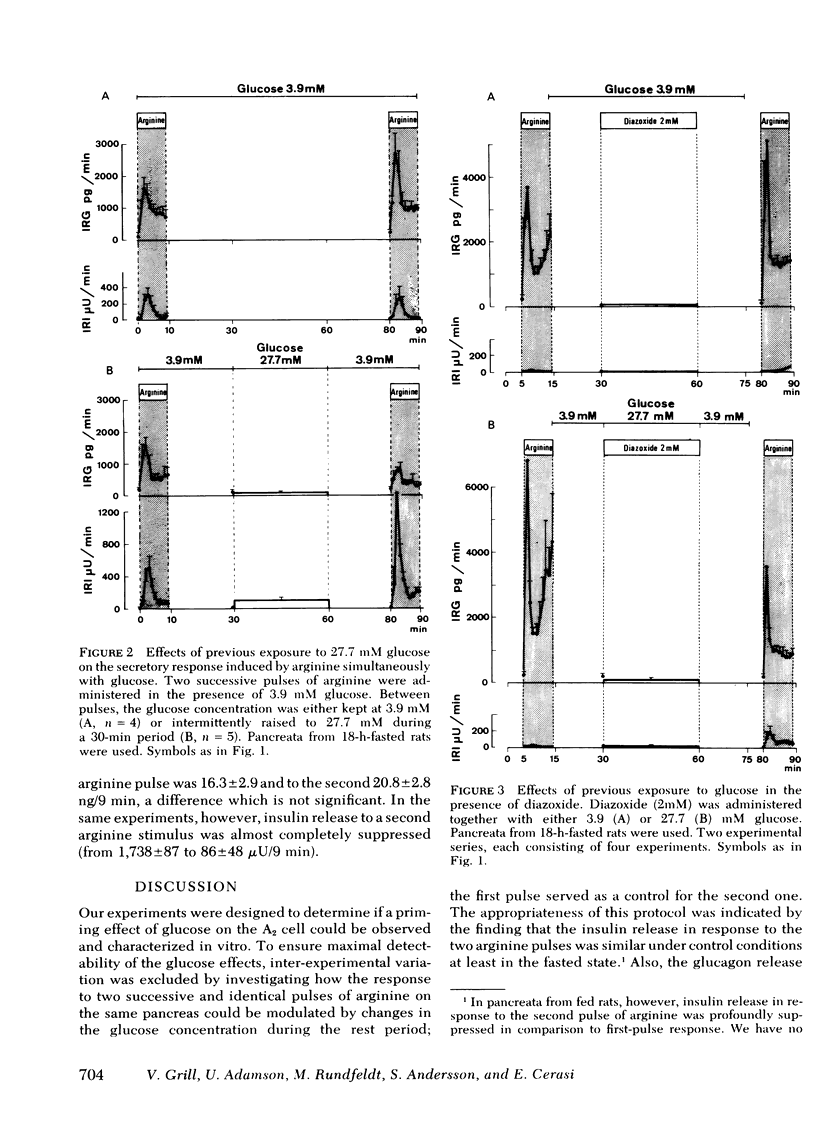
The inclusion of 3.9 mM glucose during the stimulation periods with arginine decreased the glucagon and greatly increased the insulin secretory response. Under these conditions, previous exposure to 27.7 mM glucose inhibited the glucagon and enhanced the insulin response to the second stimulatory pulse of arginine to the same relative degree as when arginine was administered alone.
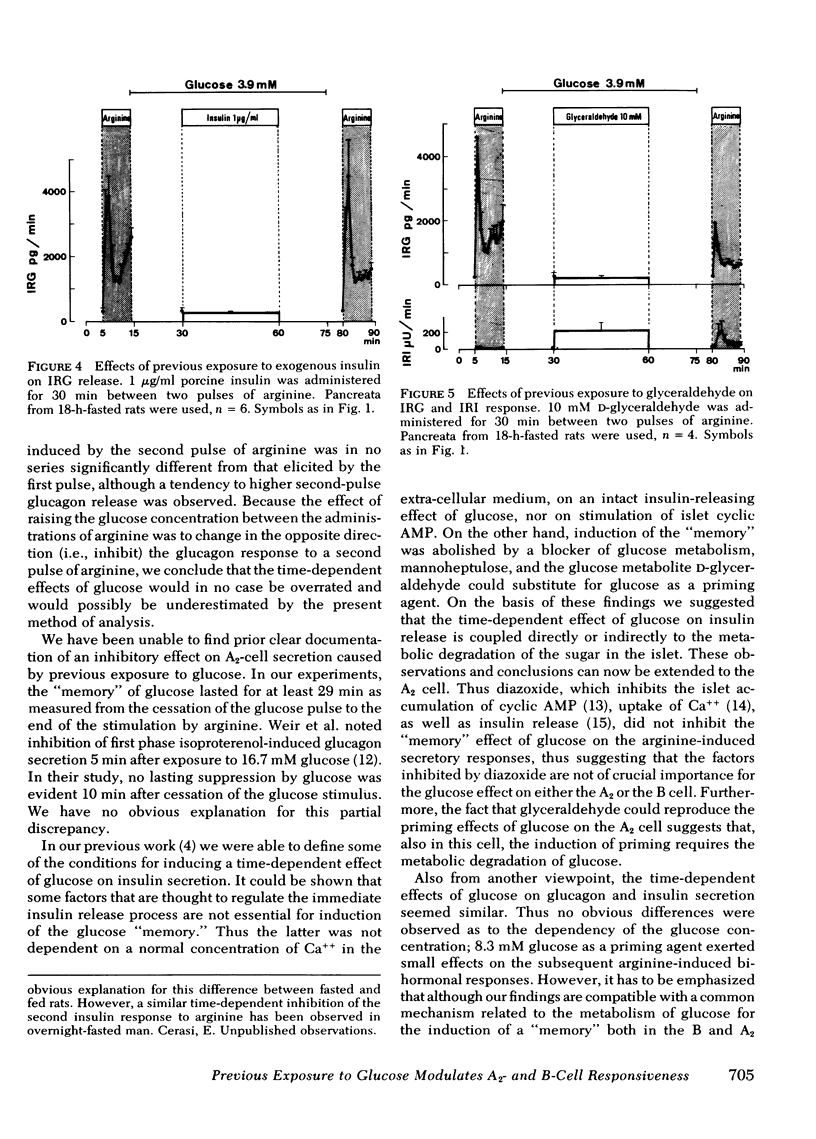
Diazoxide (2 mM), when administered together with 27.7 mM glucose, almost completely inhibited insulin release induced by the presence of glucose, yet did not influence the modulation exerted by glucose on the subsequent insulin and glucagon secretory response to arginine. Conversely, these effects of the glucose pulse could not be reproduced by 1 μg/ml of porcine insulin. Previous exposure to glyceraldehyde (10 mM) mimicked the glucose effects.
Also, in pancreata from fed rats, previous exposure to 27.7 mM glucose markedly inhibited subsequent arginine-induced glucagon secretion while the concomittant insulin response was enhanced.
It is concluded that: (a) both A2- and B-cell responsiveness is modulated by a previous exposure to glucose which produces opposite effects in the two cell types, (b) this action of glucose does not depend on its insulin-releasing capacity, and (c) instead, a “memory” of glucose is induced as a consequence of the metabolism of the sugar in the A2 and B cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J., Weerasinghe L. C., Randle P. J. Interrelationship of islet metabolism, adenosine triphosphate content and insulin release. Biochem J. 1973 Feb;132(2):223–231. doi: 10.1042/bj1320223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerasi E. Potentiation of insulin release by glucose in man. I. Quantitative analysis of the enhancement of glucose-induced insulin secretion by pretreatment with glucose in normal subjects. Acta Endocrinol (Copenh) 1975 Jul;79(3):483–501. [PubMed] [Google Scholar]
- Cerasi E. Potentiation of insulin release by glucose in man. II. Role of the insulin response, and enhancement of stimuli other than glucose. Acta Endocrinol (Copenh) 1975 Jul;79(3):502–510. [PubMed] [Google Scholar]
- Gerich J. E., Charles M. A., Grodsky G. M. Characterization of the effects of arginine and glucose on glucagon and insulin release from the perfused rat pancreas. J Clin Invest. 1974 Oct;54(4):833–841. doi: 10.1172/JCI107823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich J. E., Charles M. A., Grodsky G. M. Regulation of pancreatic insulin and glucagon secretion. Annu Rev Physiol. 1976;38:353–388. doi: 10.1146/annurev.ph.38.030176.002033. [DOI] [PubMed] [Google Scholar]
- Grill V., Adamson U., Cerasi E. Immediate and time-dependent effects of glucose on insulin release from rat pancreatic tissue. Evidence for different mechanisms of action. J Clin Invest. 1978 Apr;61(4):1034–1043. doi: 10.1172/JCI109002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodsky G. M., Curry D., Landahl H., Bennett L. [Further studies on the dynamic aspects of insulin release in vitro with evidence for a two-compartmental storage system]. Acta Diabetol Lat. 1969 Sep;6 (Suppl 1):554–578. [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. Calcium and secretion: distinction between two pools of glucose-sensitive calcium in pancreatic islets. Science. 1976 Dec 24;194(4272):1421–1423. doi: 10.1126/science.795030. [DOI] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. Effects of glucose and other modifiers of insulin release on the oxidative metabolism of amino acids in micro-dissected pancreatic islets. Biochem J. 1971 Jul;123(4):513–521. doi: 10.1042/bj1230513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Howell S. L., Taylor K. W. The secretion of newly synthesized insulin in vitro. Biochem J. 1967 Mar;102(3):922–927. doi: 10.1042/bj1020922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loubatières A., Mariani M. M., Ribes G., de Malbosc H., Chapal J. Etude expérimentale d'un nouveau sulfamide hypoglycémiant particulièrement actif, le HB 419 ou glibenclamide. Diabetologia. 1969 Feb;5(1):1–10. doi: 10.1007/BF01212212. [DOI] [PubMed] [Google Scholar]
- Montague W., Cook J. R. The role of adenosine 3':5'-cyclic monophosphate in the regulation of insulin release by isolated rat islets of Langerhans. Biochem J. 1971 Mar;122(1):115–120. doi: 10.1042/bj1220115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W. A., Faloona G. R., Unger R. H. The influence of the antecedent diet upon glucagon and insulin secretion. N Engl J Med. 1971 Dec 23;285(26):1450–1454. doi: 10.1056/NEJM197112232852603. [DOI] [PubMed] [Google Scholar]
- Pagliara A. S., Stillings S. N., Hover B., Martin D. M., Matschinsky F. M. Glucose modulation of amino acid-induced glucagon and insulin release in the isolated perfused rat pancreas. J Clin Invest. 1974 Oct;54(4):819–832. doi: 10.1172/JCI107822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando H., Grodsky G. M. Dynamic synthesis and release of insulin and proinsulin from perifused islets. Diabetes. 1973 May;22(5):354–360. doi: 10.2337/diab.22.5.354. [DOI] [PubMed] [Google Scholar]
- Weir G. C., Knowlton S. D., Martin D. B. Glucagon secretion from the perfused rat pancreas. Studies with glucose and catecholamines. J Clin Invest. 1974 Dec;54(6):1403–1412. doi: 10.1172/JCI107887. [DOI] [PMC free article] [PubMed] [Google Scholar]



