Abstract
A wearable monitor that can reliably, accurately and continuously measure personal exposure levels of various toxicants would not only accelerate the current environmental and occupational health and safety studies, but also enable new studies that are not possible with the current monitoring technology. Developing such a monitor has been a difficult challenge, and requires innovative sensing science and creative engineering.
We have developed, built and tested a wearable monitor for real-time detection of toxic hydrocarbons and acids in environment. The monitor is low-cost, accurate, and user-friendly. In addition, it can communicate wirelessly with a cell phone in which the monitoring results can be processed, displayed, stored and transmitted to a designated computer. We have validated the functions and performance of the monitor, and carried out field tests with workers involving waste management, fire overhaul, and floor-cleaning activities, as well as with first- and second-hand smokers. The averaged exposure levels are in agreement with those determined by the standard NIOSH methods. The monitor provides accurate and real-time exposure assessment for the workers involving different activities. The real-time and continuous monitoring capability makes it possible to correlate the exposure levels with different activities and changes in the microenvironments. The monitor provides unprecedented real-time information that will help advance occupational safety and environmental health studies. It may also be used to better protect workers from occupational overexposure to toxic molecules.
Keywords: environmental health, occupational health, exposure assessment, real-time monitor, toxicant monitor, wireless and wearable integrated sensor
Introduction
Exposure to toxicants is a leading cause of occupational injuries (NIOSH-NORA). In addition, upon interactions with our bodies, toxicants can lead to both short and long-term diseases such as respiratory disorders (McConnell 2008; Obadia, Liss et al. 2009) and cancer (LeMasters, Genaidy et al. 2006). Accurate and timely monitoring of workers’ exposure to toxicants is the first step towards occupational safety and prevention of such diseases (Gaertner and Theriault 2002; Leong and Laortanakul 2004; Ribeiro, Santos et al. 2009). This capability is also critical for epidemiologists who study diseases and health conditions associated with occupational exposures (Jo and Yu 2001; Mustajbegovi, Zuskin et al. 2001).
The development of personal toxicant monitors is still a leading priority in environmental and occupational health and safety. Despite the significant advances made in the field, a reliable, long-operational lifetime, fast response, low cost, sensitive and wearable/hand-held monitor for toxicants represents a formidable scientific and engineering challenge. Reaction tubes, passive and active samplers, and multi-gas monitors are currently the most widely used techniques for monitoring toxicant exposure, but they have various drawbacks. For example, the reaction tubes and samplers provide one-spot- or accumulated- concentration exposure readings. The multi-gas monitors are limited to readings of inorganic gases, such as oxygen, carbon monoxide and hydrogen sulfide, and combustible levels at relatively high concentrations (part-per-million and % levels, respectively). These techniques offer also limited sensitivity and dynamic range, which often fall short in meeting the requirement to cover broad exposure levels in many practical settings. Because of these limitations, complicated NIOSH-certified laboratory-based analytical methods are still being used. These laboratory methods are labor-intensive, expensive, and more importantly, they do not provide real-time information that is critically needed to safe guard the health of workers. Finally, many of these methods require sample collection, storage and handling, which are prone to errors as volatile hazards can leak out from the sample collector (Chambers, Blount et al. 2008).
We present here a wearable toxicant monitor to overcome the limitations of the existing devices and methods. The monitor can detect total hydrocarbons and total acids in real-time, and wirelessly transmit the data instantaneously to a cell phone. The wireless wearable system has been validated with reference methods and tested in the field. Several study cases have been carried out to demonstrate its capabilities and to illustrate its potential applications. Compared to other monitors for the detection of volatile compounds, the wearable monitor is highly specific and immune from environmental interferents, such as common household, personal care products, and large changes in humidity and temperature.
Methods
The wireless wearable system is a stand-alone monitoring system developed for assessment of personal exposures to pollutants or hazards. It is an inexpensive, low power, miniaturized, and yet powerful chemical sensing system. It consists of two pieces: a wearable unit and a wirelessly connected cell phone .
Wearable monitor
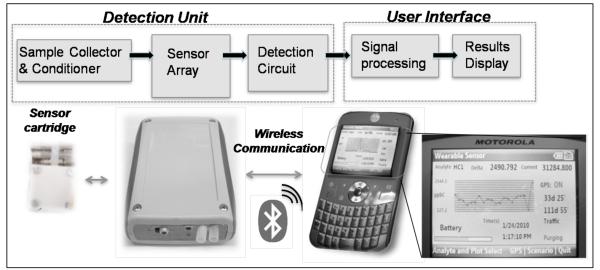
The wearable monitor unit weighs ~0.5 lbs with a size comparable to a smart cell phone, making it possible to be either handheld or wearable near the breathing zone (Fig. 1). The unit includes a sample collection, conditioning and delivery system, a sensor cartridge, a detection and control electronic circuit, operated with batteries. These components are integrated into a complete system and operate together synergistically to provide the superior performance. For example, the high sensitivity is achieved by using not only a highly sensitive microfabricated tuning fork array in the sensor cartridge, but also low noise detection circuit that allows for accurate detection of the resonant frequencies of the array. The high selectivity is a result of both the selective sensing materials and optimized sample conditioning system (supporting information).
Fig. 1.
Wearable monitor for total hydrocarbons and total acids. Top: Block diagram of functions performed by the detection unit and user interface. Bottom: Pictures of the plug-and-play sensor cartridge with a tuning fork array; the wireless hand-held unit wirelessly connected to a Motorola Q9h smart phone, which processes the data, stores and displays the detection results. Bottom right: picture of the cell phone display, showing a real-time concentration plot (ppb levels vs. time), GPS data, active displayed sensing element (hydrocarbon sensor 1: HC1), active application (traffic) and valve status (purging).
The sensor cartridge is a plug-and-play component that offers flexibility to detect different types of target analytes simultaneously. The sensor cartridge used in the present work is an array of quartz crystal tuning fork resonators optimized for selective detection of total hydrocarbons, total acids, humidity and temperature. The sensors are securely placed inside a sensor cartridge made of Teflon®. The cartridge has pin connectors that plug directly into the control circuit board. The detection circuit is based on a high resolution frequency counter (0.2 mHz) and provides an equivalent mass detection limit of ~ 1 pg/mm2. The synergic architeture of the sensing materials, smart electronics, and signal processing allows the detection of part-per-billion volume (ppb) levels of total hydrocarbons and acids. The wearable unit is powered by Li-ion polymer batteries and can be recharged by simply plugging it into a power outlet. Power distribution and hardware optimization ensure continuous operation of the wearable unit over nine hours. In addition, the detection circuit has a Bluetooth® chip for real-time data transfer to the cell phone.
Cell phone-based user interface
The cell phone receives the data from the wearable monitor, processes the information and displays the data via a graphic user interface. The data is stored in the cell phone that can be downloaded to a computer later, or emailed via the existing wireless service. In addition to reading, processing and displaying toxicant levels, the cell phone can also records the embedded GPS location. The interactive graphic user interface allows the user to access and view detailed detection information, such as real-time data for each sensing element of the array, different analytes, and operation status of the monitor (pump, valves and battery life, etc.). Another useful feature is that the user can select between different application scenarios (e.g. industrial solvent, motor vehicle emission, etc.) for hydrocarbon assessment. Each scenario has a calibration factor that best suits the chosen environment. A typical industrial or occupational activity involves exposure to a dominant hydrocarbon, which can be determined by the corresponding calibration factor. Exposures to more complex environments, such as emissions from motor vehicles, gasoline and petrochemical industries, require calibration factors that reflect the distribution of the hydrocarbons and the sensitivity of each hydrocarbon (Brown, Frankel et al. 2007). Exposure assessment in these scenarios is important for many epidemiologic studies (McConnell 2008).
Analytical Validation
To examine the accuracy of the wireless wearable system, we performed intra- and inter-laboratory validations described below:
The intra-laboratory validation tested the sensitivity and selectivity of the system using gas chromatography-mass spectrometry (GC-MS) as a reference method for hydrocarbons, and recovery assays for acids. It also serves the purpose of establishing and testing the calibration factors for the different application scenarios described above. The validation for hydrocarbon detections was implemented by following a parallel sampling methodology. Air samples were collected from test locations in a 1 or 4 L Tedlar® bag while the wearable system was measuring the air at the same location. The collected air sample was then brought to an analytical lab and analyzed using a HP 5890/5972 Quadrupole GC-MS. The GC-MS method was optimized for detecting low concentration aromatic and aliphatic hydrocarbons. The hydrocarbons in the sample were preconcentrated in a 100-μm polydimethylsiloxane-coated solid phase microextraction fiber (SPME) for a period of 1 h, and then placed into a 0.75-mm diameter glass injector. The hydrocarbons adsorbed in the SPME fiber were released in the GC injector by raising the temperature to 290 °C. The separation used 30 m × 250 μm × 0.25 μm HP-5MS capillary column coated with 5% phenyl methyl siloxane. The analysis started with the temperature set at 40 °C. After 2 mins., the column temperature was raised to 100 °C at 4 °C/min and then to 295 °C at 10 °C/min. The entire sample analysis lasted ~38 mins. Identification of the analytes was performed using known standards and the mass spectrum library from NIST (AMDIS32 software). The total hydrocarbon level was obtained by adding up the individual hydrocarbons determined from the chromatogram, which was used to compare and calibrate the readings of the wearable monitor.
To calibrate the acid detection capability of the wearable monitor, standard acid gas vapors were used. After calibration, the monitor was further validated using real samples spiked with known concentrations of acid gases (e.g., different concentrations of hydrochloric acid).
Inter-laboratory validation was carried out in collaboration with the Department of Environmental Health and Safety (EHS) at Arizona State University (ASU). The wearable monitor was used to detect toxic hydrocarbons and acid vapors, and the samples were collected from the sites and shipped to a third-party laboratory (Galson Laboratories, Syracuse) for analysis using NIOSH methods. For example, NIOSH method 1005 (NIOSH1005) was used to quantify methylene chloride hydrocarbons (dominant component in the samples). The procedure included air sample collection using a solid sorbent (coconut shell charcoal tube, 100/50 mg), desorption of the sample in 1 mL of CS2, and analysis with a GC-Flame Ionization system. NIOSH method 7903 (NIOSH7903) was utilized for acid vapors. In this case, the solid sorbent was washed silica gel (400 mg/200 mg glass fiber filter plug), the desorption took place in 10 mL of 1.7 mM NaHCO3/1.8mM Na2CO3 solution, and the analysis used 50 μL of the solution in an ion chromatography system.
Results and Discussion
Intra-laboratory Validation
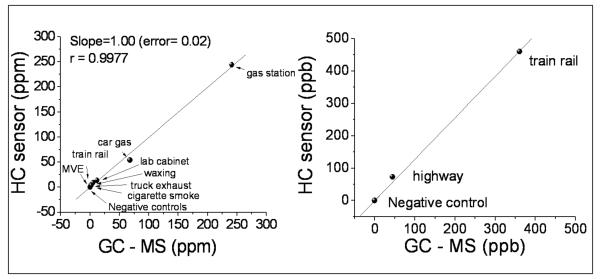
Fig. 2 compares the hydrocarbon levels determined by the monitor and by GC-MS for samples taken at different locations, including airport, gas stations, laboratory cabinets, truck exhaust exposure, cigarette smoke and roads. Because the hydrocarbon levels at these locations vary over a wide range, from a few tens of ppb to several hundred part-per-million (ppm), we present the results in two plots. The comparison shows a high degree of correlation (100%) with a relative error of 2% and a regression factor of 0.9977 over the wide dynamic range. We also performed acid detection validation and found accuracy within 95-105 % (supporting information).
Fig.2.
Intra-laboratory validation of the sensor results carried out against Gas Chromatography - Mass Spectrometry. Samples taken from different environments: gas station (near a nozzle), car gas (open tank), lab cabinet, train rail, floor waxing, truck exhaust (near the truck), cigarette smoke, motor vehicle emissions (MVE) in a highway.
Inter-laboratory Validation
The test was carried out with the help of industrial hygienists in EHS, ASU, during dumping of organic and acid hazardous wastes. The waste disposal involved mostly methylene chloride and low percentages of chloroform and toluene. The concentration of methylene chloride determined by Galson Laboratories was 2.2 ppm, while the average concentration detected by the wearable monitor during the same sampling period was 2.6 ppm. Considering that the wearable monitor measured not only methylene chloride, but also components, such as chloroform and toluene, the agreement is reasonable. The acid levels determined by the NIOSH method were below the detection limit, which ranges between 0.06 - 0.3 ppm depending on the type of acid. The average acid level measured by the wearable monitor in the same testing period was 0.012 ppm, which is consistent with the results by the NIOSH method.
Field Testing
Several field tests under different scenarios were carried out and the findings are summarized below.
Case study 1: Hazardous waste exposure at the ASU Waste Management Facility
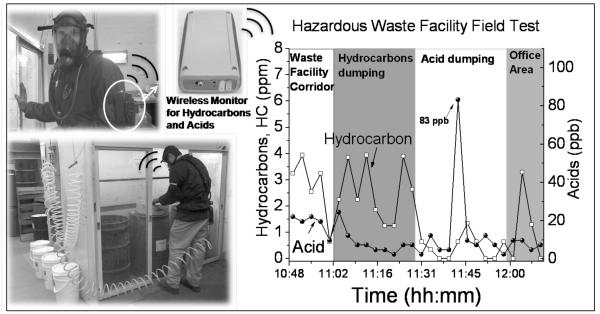
Waste management facility and chemical laboratories are potential sources of concern for health and safety of workers (Xu and McGlotin 2003). Poor ventilation and air quality inside a waste management facility are leading causes of serious illness and loss of productivity in these workplaces. Continuous monitoring of hazardous toxicants is therefore an essential part of health and safety that could make a significant impact (Je, Stone et al. 2007). We demonstrated that the wearable monitor could provide effective monitoring of hazardous toxic exposures at these sites. As an example, Fig. 3 shows the levels of hydrocarbons and acids monitored by our wearable monitor worn on the arm of a staff at the ASU Hazardous Waste Facility. The levels of hydrocarbons and acids increase sharply every time when he handled solvent and acid dumping in a well-ventilated hood. Significant levels of hydrocarbons were also detected in the corridor and office area of the facility.
Fig.3.
Test performed at the ASU Hazardous Waste Management Facility to check the exposure level of a worker involved in the disposal activity. The sensor was able to detect real-time short-term exposure levels (in 1 minute intervals). The highest acid level was detected during acid dumping, while the highest solvent exposure levels occurred during solvent dumping (ventilated) and in other places of the facility. The detection process included 1 minute sampling and 2 minutes purging.
The real-time monitoring capability of the wearable monitor also revealed interesting details of the chemical exposure. As shown in Fig. 3, the hydrocarbon level reached nearly 4 ppm at three different occasions during organic solvent dumping activity, while the average exposure level of the entire activity was only 2.6 ppm. This important short-term exposure information was possible only by using the real-time monitor with adequate time resolution. Similar real-time detection of acid exposure detected peak values of ~0.083 ppm. This level of acid cannot be detected using the current NIOSH methods, demonstrating the superior sensitivity of our wearable monitor.
Case study 2: First and second hand cigarette smoke exposure
Cigarette smoke exposure has been identified as one of the major sources of unintentional exposure to carcinogens. A recent study by Carrieri et al. (Carrieri, Tranfo et al.) indicates that smokers are exposed to more benzene than non-smokers working at petrochemical industries. This finding has motivated epidemiologists, toxicologists and air-quality researchers to study health consequences of general public exposure at smoking places (Sleiman, Gundel et al. 2010). Specific components of cigarette smoke were first characterized by GC-MS, which identified hydrocarbon components detected by our wearable monitor. The study showed that although cigarette smoke is a complex mixture of gases, only aromatic hydrocarbons, such as toluene, and benzene were detected (supplementary information).
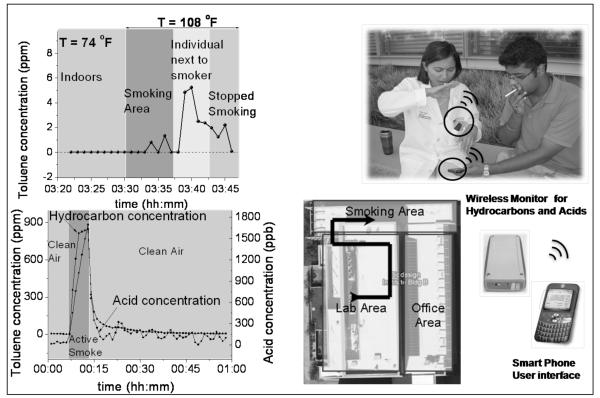
As an example, Fig. 4 shows the exposure of a non-smoker wearing the monitor in the front pocket located near the breathing zone. When the non-smoker passed a smoking area, the second hand exposure to hydrocarbons increased from the background noise (a few ppb) to 1.5 ppm. The exposure level reached as high as 5.2 ppm when the non-smoker sat next to an active smoker. The exposure level of the active smoker was also monitored with the wearable monitor, which measured 2 orders of magnitude higher hydrocarbon levels than the second-hand smoker. The exposure hydrocarbon levels of the first-hand smoker determined here are in good agreement with the previously reported values in literature (Hatzinikolaou, Lagesson et al. 2006). Note that the wearable monitor also measured the acid levels, which were in the range of several hundred ppb. The sources of the acid levels are likely due to hydrochloric acid, hydrogen cyanide and hydrogen sulfide (Bolstad-Johnson, Burgess et al. 2000; Parrish, Lyons-Hart et al. 2001; Hatzinikolaou, Lagesson et al. 2006). Note also that the test was carried out during summer in Phoenix, with outdoor temperature as high as 108 °F (42.2 °C), which demonstrates the robustness of the monitor.
Fig.4.
Top: Test performed to assess the exposure to cigarette smoke by a passive smoker in indoor (lab area), smoking area, and next to a smoker (see also picture and map). Bottom: Test performed to evaluate active smoker’s exposure to cigarette smoke. The detection process included10 seconds sampling and 50 seconds purging.
Case study 3: Exposure of cleaning workers
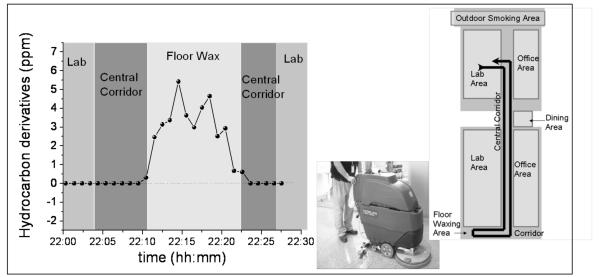
Higher work-related asthma risk has been reported for cleaning workers (Obadia, Liss et al. 2009). The activities of these workers include waxing floors, cleaning carpets, tiles and grout. We monitored the exposure levels of hydrocarbons during floor waxing activities with the wearable monitor. We programmed the monitor to sample and detect for 10 seconds and then followed by a 50 second purging period. A person wearing our monitor walked by a floor waxing area and recorded the hydrocarbon exposure level (Fig. 5). The hydrocarbon level increased above 6 ppm when the person approached the floor waxing area, and the reading returned to nearly zero (< a few ppb) when the person left the waxing area. The test demonstrates again the capability of the wearable monitor for real-time monitoring of toxicant levels in a microenvironment.
Fig.5.
Test performed during floor waxing activity at the Biodesign Institute, ASU. High concentrations of hydrocarbons were obvious in the area where floor waxing was taking place. The map on the right displays the path followed during the test by the worker wearing the monitor. The detection process included10 seconds sampling and 50 seconds purging.
Case study 4: Exposure assessment of fire overhauls activities
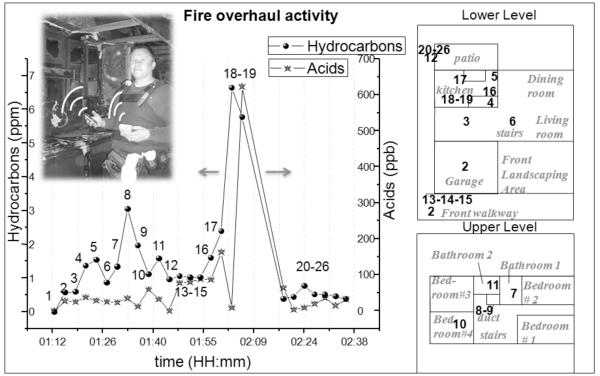
Fire overhaul is the phase after a fire has been extinguished. This is the time period when firefighters seek for potential re-ignition spots and arson investigators explore the potential source of the fire. Exposure of fire workers during overhaul activities has been studied by Burgess et al (Bolstad-Johnson, Burgess et al. 2000; Burgess, Nanson et al. 2001). Several toxicants, such as aromatic hydrocarbons (benzene), acids (hydrochloric acid), and aldehydes (formaldehyde) have been found to be present in these environments (Bolstad-Johnson, Burgess et al. 2000). Another important point is the way the monitor can aid arson investigators tasks (Burgess and Crittenden 1995). The current method used by the fire investigation team for this activity commonly involves the collection of the air sample on a sorbent tube for a long duration and its analysis by a certified laboratory later, which only provides averaged concentration. In collaboration with Phoenix Fire Department, the wearable monitor was used to map toxicant levels in fire overhauls.
Fig. 6 shows as an example of real-time exposure data collected in a fire overhaul by the wearable monitor carried by an arson investigator. The monitor allowed the investigator to map the concentrations of toxicants. Before entering the burnt down house, the hydrocarbon and acid levels were nearly zero (1). The toxicant levels increased as soon as the arson investigator entered the front walkway (2) of the house. A point of interest in this house was the air conditioning duct where ~3.3 ppm level of hydrocarbons was detected (8). The monitor detected the highest concentrations of hydrocarbons (~ 7 ppm) and acids (~600 ppb) in an area pointed out by the arson investigator as the origin of the fire (18-19). One interesting observation was that toxicant levels showed strong correlations with the location and distance from burnt objects. Another interesting observation was that burnt places containing furniture, decorative ornaments, carpets and other objects showed high levels of toxicants and thus represented greater exposure risks to firefighters and arson investigators.
Fig.6.
Test performed during a fire overhaul test in Phoenix to assess the exposure level of the fire workers during overhaul activities. Highest exposure levels were detected in the duct of the house, a source of the toxic gases (8), and in the place where the fire was suspected to have started (18-19). The detection process included 1 minute sampling and 2 minutes purging.
Comparison of the wearable monitor to existing technologies
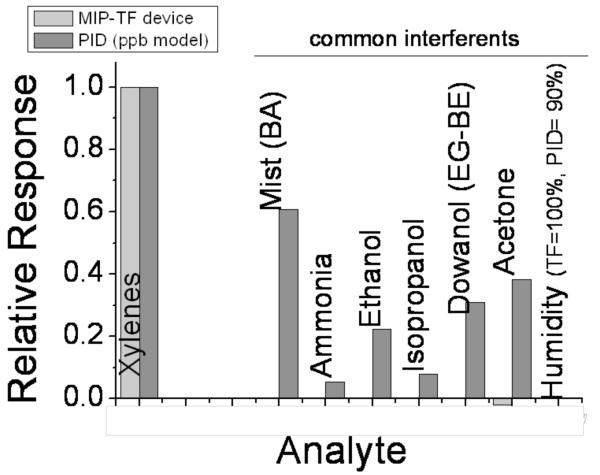
The performance of our wearable monitor was compared with a commercial photoionization detector (PID) using a 10.6 eV UV lamp to detect ppb levels of volatiles compounds. This type of PID-based monitors is capable of ionizing volatile compounds from different families, including alcohols, ketones and ammonia, but it cannot ionize some hydrocarbons, such as short alkyl hydrocarbons that are constituents of diesel and gasoline. Unlike the PID detectors, our wearable monitor is more selective for the detection of toxic hydrocarbon derivatives from the petroleum products and immune to interferents, such as alcohol, ketones, and ammonia. Fig. 7 shows a comparison of the selectivity of our monitor with the PID detector. The PID detector detects total volatile compounds exposure, including the interferents, and our wearable monitor targets specifically hydrocarbon compounds from petroleum including benzene, toluene, xylenes, and short and long alkyl hydrocarbons. These hydrocarbons are ozone precursors, which are important to respiratory health (EPA).
Fig. 7.
Selectivity comparison of the wearable monitor (light grey bars) with a PID detector for the detection of ppb levels of volatile compounds (dark grey bars). The interferents are mist or its equivalent fragrance molecule - benzyl acetate (BA), ammonia, ethanol, isopropanol, dowanol or its parent molecules - ethyleneglycol (EG) or butyleneglycol (BE), acetone, and humidity. Note that the wearable monitor is immune even to 100 % relative humidity, while the manufacturer of the PID specifies a maximum of 90% relative humidity.
Conclusion
We have developed a wearable wireless monitor that can detect local chemical exposures in real time. It is a small, light and low-cost device with user-friendly interface, which is suitable for industrial hygienists and workers as well as for personal exposure studies. The monitor can reliably detect low ppb concentrations of total hydrocarbons and total acids simultaneously, and it is immune to environmental changes such as humidity, temperature and personal care products. Using a smart cell phone as a user interface, the monitor allows the user to obtain concentration profiles as a function of time and position.
The toxicant monitor was validated and calibrated with the standard GC-MS and NIOSH methods. The monitor was shown to provide superior performance in terms of both sensitivity and selectivity when compared to the conventional methods and technologies. Several field studies under different application scenarios were carried out to demonstrate the monitor’s capabilities for occupational and environmental health studies. We anticipate that the wearable sensor will be used as a new enabling tool for monitoring personal exposure and for detecting potential toxic exposure risks remotely, which will lead to a better understanding of the nature of toxicant exposures, and its links to human health.
Supplementary Material
Acknowledgements
This project was supported by NIEH/NIH (#5U01ES016064) via the Genes, Environment and Health Initiative (GEI) program. The authors are deeply thankful to collaborators Jay A. Gandolfi and Jeff Burgess at University of Arizona, Dawn Bolstad-Johnson, Willie Nelson and Jack Ballentine at Phoenix Fire Department, Christopher Knobbe at Arizona OSHA, who have contributed to this work with suggestions and ideas about the different scenarios to be tested and/or actual field-testing. The authors also appreciate help from Tom Colella at Goldwater Environmental Laboratory, ASU, and John Lemanski, Michael Ochs, and Henry Walsh at Department of EHS, ASU for validation studies of the monitor, and the cooperation from Jeffrey Bender, Stephen Scheufler, Jason Neal and John Rodriguez at the Hazardous Waste Management Facility, ASU.
Footnotes
Supplemental Material is available online
REFERENCES
- Bolstad-Johnson DM, Burgess JL, et al. Characterization of firefighter exposures during fire overhaul. Aihaj. 2000;61(5):636–641. doi: 10.1080/15298660008984572. [DOI] [PubMed] [Google Scholar]
- Brown SG, Frankel A, et al. Source apportionment of VOCs in the Los Angeles area using positive matrix factorization. Atmospheric Environment. 2007;41(2):227–237. [Google Scholar]
- Burgess JL, Crittenden JC. Tucson firefighter exposure to products of combustion; A risk assessment. Appl Occup Environ Hyg. 1995;10:37–42. [Google Scholar]
- Burgess JL, Nanson CJ, et al. Adverse respiratory effects following overhaul in firefighters. Journal Of Occupational And Environmental Medicine. 2001;43(5):467–473. doi: 10.1097/00043764-200105000-00007. [DOI] [PubMed] [Google Scholar]
- Carrieri M, Tranfo G, et al. Correlation between environmental and biological monitoring of exposure to benzene in petrochemical industry operators. Toxicology Letters. 192(1):17–21. doi: 10.1016/j.toxlet.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Chambers DM, Blount BC, et al. Picogram measurement of volatile n-alkanes (n-hexane through n-dodecane) in blood using solid-phase microextraction to assess nonoccupational petroleum-based fuel exposure. Analytical Chemistry. 2008;80(12):4666–4674. doi: 10.1021/ac800065d. [DOI] [PubMed] [Google Scholar]
- EPA http://www.epa.gov/appcdwww/apb/mobile.htm.
- Gaertner RRW, Theriault GP. Risk of bladder cancer in foundry workers: a meta-analysis. Occupational And Environmental Medicine. 2002;59(10):655–663. doi: 10.1136/oem.59.10.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzinikolaou DG, Lagesson V, et al. Analysis of the gas phase of cigarette smoke by gas chromatography coupled with UV-diode array detection. Analytical Chemistry. 2006;78(13):4509–4516. doi: 10.1021/ac052004y. [DOI] [PubMed] [Google Scholar]
- Je CH, Stone R, et al. Development and application of a multi-channel monitoring system for near real-time VOC measurement in a hazardous waste management facility. Science Of The Total Environment. 2007;382(2-3):364–374. doi: 10.1016/j.scitotenv.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Jo WK, Yu CH. Public bus and taxicab drivers’ exposure to aromatic work-time volatile organic compounds. Environmental Research. 2001;86(1):66–72. doi: 10.1006/enrs.2001.4257. [DOI] [PubMed] [Google Scholar]
- LeMasters GK, Genaidy AM, et al. Cancer risk among firefighters: A review and meta-analysis of 32 studies. Journal Of Occupational And Environmental Medicine. 2006;48(11):1189–1202. doi: 10.1097/01.jom.0000246229.68697.90. [DOI] [PubMed] [Google Scholar]
- Leong ST, Laortanakul P. Benzene and lead exposure assessment among occupational bus drivers in Bangkok traffic. Journal Of Environmental Sciences-China. 2004;16(1):61–66. [PubMed] [Google Scholar]
- McConnell R. Asthma and Traffic-Related Exposure at Schools, Homes and on Roadways. Epidemiology. 2008;19(6):S58–S58. [Google Scholar]
- Mustajbegovi J,E, Zuskin, et al. Respiratory function in active firefighters. American Journal Of Industrial Medicine. 2001;40(1):55–62. doi: 10.1002/ajim.1071. [DOI] [PubMed] [Google Scholar]
- NIOSH1005 http://www.cdc.gov/niosh/docs/2003-154/pdfs/1005.pdf.
- NIOSH7903 http://cdc.gov/niosh/docs/2003-154/pdfs/7903.pdf.
- NIOSH-NORA http://www.cdc.gov/niosh/NORA/about.html.
- Obadia M, Liss GM, et al. Relationships Between Asthma and Work Exposures Among Non-Domestic Cleaners in Ontario. American Journal Of Industrial Medicine. 2009;52(9):716–723. doi: 10.1002/ajim.20730. [DOI] [PubMed] [Google Scholar]
- Parrish ME, Lyons-Hart JL, et al. Puff-by-puff and intrapuff analysis of cigarette smoke using infrared spectroscopy. Vibrational Spectroscopy. 2001;27(1):29–42. [Google Scholar]
- Ribeiro M, Santos UD, et al. Prevalence and Risk of Asthma Symptoms Among Firefighters in Sao Paulo, Brazil: A Population-Based Study. American Journal Of Industrial Medicine. 2009;52(3):261–269. doi: 10.1002/ajim.20669. [DOI] [PubMed] [Google Scholar]
- Sleiman M, Gundel LA, et al. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. PNAS asap. 2010 doi: 10.1073/pnas.0912820107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, McGlotin J. Video exposure assessments of solvent exposures in university pharmaceutical laboratories—a pilot study. Chem. Heal. Saf. 2003;10:23–28. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.