Abstract
Chronic psychological stress is generally immunosuppressive and contributes to an increase in herpes simplex virus (HSV) pathogenicity. We have previously shown that mice experiencing stress at the time of intranasal HSV infection have increased levels of infectious virus in their nasal cavity, as compared to control mice that were not subjected to stress. We have extended our studies to determine the effects of stress at another clinically-relevant mucosal site by examining the immune response to and pathogenesis of vaginal HSV infection. Mice experiencing psychological stress during vaginal HSV infection exhibited an increase in both vaginal viral titers and the pathology associated with this HSV infection. We demonstrate that these observations result from the failure of both the innate and HSV-specific adaptive immune responses. At two days post-infection, NK cell numbers were significantly decreased in mice experiencing restraint stress. Studies examining the adaptive immune response revealed a decrease in the number of HSV-specific CD8+ T cells in not only the vaginal tissue itself but also the draining iliac lymph nodes (ILN). Furthermore, the number of functional cells, in terms of both their degranulation and interferon-γ production, in the ILN of stressed mice was decreased as compared to non-stressed mice. We conclude that psychological stress, through its suppression of both innate and adaptive immune responses, may be an important factor in the ability to control vaginal HSV infection.
Keywords: Psychological stress, CD8+ T lymphocytes, NK cells, HSV-1, Mucosal immunity, Vaginal infection
1. Introduction
Neuroendocrine-derived peptides and hormones are clearly recognized as immune modulators (reviewed in Avitsur et al., 2006; Godbout and Glaser, 2006; Sheridan et al., 1998; Stone and Bovbjerg, 1994). Previous studies examining the impact of psychological stress on immune function have focused on HSV-1 infection via footpad (Bonneau et al., 1993b; Bonneau et al., 1991), ocular (Freeman et al., 2007; Kip et al., 2001) and intranasal (Anglen et al., 2003; Ashcraft et al., 2008; Nair et al., 2007) routes. In one or more of these studies, stress at the time of infection has been shown to delay CD8+ T cell infiltration, limit microglial MHC class I surface expression, and increase the titer of infectious HSV at the site of infection. Although the effects of stress on the number and function of memory CTL within the vaginal epithelium has been demonstrated previously (Wonnacott and Bonneau, 2002), the impact of stress on the innate and adaptive immune response during primary vaginal HSV-1 infection has not yet been elucidated.
Exposure to infectious pathogens occurs most often via mucosal routes. These highly susceptible points of entry include the respiratory, gastrointestinal, and genitourinary tracts, and represent routes which are protected by mucosal-associated lymphoid tissues. The immune cells located within these tissues are poised to respond to infection, ideally limiting it to the mucosal site. During the first few days of a viral infection, natural killer (NK) cells non-specifically lyse virally-infected host cells, which helps limit the spread of the infection. Meanwhile, the T cell-mediated adaptive immune response beings to develop, and subsequently results in the lysis of infected cells in an antigen-specific manner.
The efficacy of mucosal-based immune responses has been shown to be compromised in individuals experiencing psychological stress. However, the majority of these studies have examined the impact of stress on the immune response within the gastrointestinal mucosa. Recently, psychological stress during gastrointestinal infection has been demonstrated to reduce the concentration of intestinal IgA (Jarillo-Luna et al., 2007), the numbers of intraepithelial lymphocytes (Jarillo-Luna et al., 2008), and the production of interferon-γ (IFN-γ) (Yang et al., 2006). Our laboratory has recently shown that psychological stress reduces the number of immune cells responsible for limiting mucosal HSV infection via the intranasal route (Ashcraft et al., 2008), resulting in increased viral replication.
The aim of the studies described herein was to determine the effects of restraint stress on the innate and CTL-based adaptive immune response during primary vaginal infection with HSV-1. We have shown that mice experiencing psychological stress during such infection exhibit increased damage to the vaginal epithelium. We present data suggesting that this observation is due to their inability to elicit a sufficiently robust anti-viral immune response. Stress-induced suppression of the innate immune response resulted in increased viral titers as early as two days post-infection. This immunosuppression extended to the adaptive immune response, as was indicated by the decreased number and function of CD8+ T cells specific for an HSV-encoded immunodominant H-2Kb-restricted CTL recognition epitope. We conclude that psychological stress at the time of primary genital HSV-1 infection leads to more severe pathophysiology through a suppression in anti-viral immunity.
2. Materials and Methods
2.1 Animals
Female C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were obtained at 5-6 weeks of age. All mice were group-housed at 4 mice per cage, maintained on a 12/12 hour light/dark cycle (lights on at 0000 and off at 1200), and were given access to food and water ad libitum. No experiments were conducted until mice were acclimated to these conditions for at least 1 week. All experimental procedures were carried out according to guidelines set by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International) and the National Institutes of Health.
2.2 Virus
HSV-1 strain McIntyre was prepared by infection of Vero cells at a multiplicity of infection of 0.01. Virus titers were assessed by plaque assay on Vero cells and viral stocks were stored at -70°C.
2.3 Intravaginal infection
Six-to-eight-week old female mice were administered a subcutaneous injection of 2 mg Depo-Provera® (Greenstone Ltd., Peapack, NJ) in a volume of 100 μL. Depo-Provera® treatment induces mice to enter a diestrous-like state (Parr et al., 1994), which allows for consistent vaginal HSV infection (Gallichan and Rosenthal, 1996). Five days later, mice were anesthetized by a 0.2 mL intraperitoneal (i.p.) injection of ketamine/xylazine (1.5 mg/kg ketamine; 0.1 mg/kg xylazine) prepared in sterile, distilled water. The vaginal lumen was gently swabbed with a sterile Dacron®-tipped polyester applicator (Puritan, Guilford, ME) moistened with phosphate-buffered saline (PBS). Mice were then infected with 5 × 106 pfu of HSV-1 McIntyre by instilling the viral inoculum (20 μL) directly into the vagina.
2.4 Restraint stress protocol
Beginning one day prior to HSV-1 infection, mice were physically restrained (without squeezing or compression) in well-ventilated, 50 mL conical tubes containing approximately one-hundred 0.4 cm diameter holes (Anglen et al., 2003; Nair and Bonneau, 2006; Nair et al., 2007). Mice were able to move forward and backward, but were unable to turn around. This procedure induces psychological stress due to the confinement that is experienced by the animal. Mice were restrained for 16 hours (1700 to 0900) per session beginning 5 hours into the dark cycle. Since restrained mice were not able to obtain food and water during this 16-hour stress session, non-stressed control mice were deprived of food and water during the same period of time. All mice received equal access to food and water following the termination of each stress session.
2.5 Histological examination of vaginal tissue
Mice were anesthetized with sodium pentobarbital (100 mg/kg, i.p.; Nembutal®, Abbott Laboratories, North Chicago, IL) and perfused with 20 mL 10% formaldehyde (v/v). Vaginas were excised, placed in 10% formaldehyde for 48 hours at room temperature, transferred to 70% ethanol, and embedded in a paraffin block. Seven μm cross-sections were collected and stained using hematoxylin and eosin. Images were observed using an Olympus IX81 deconvolution microscope, and images were obtained using Qcapture software (Q Imaging Corp., Burnaby, BC, Canada).
2.6 Isolation of lymphoid cells from vaginas
Mice were euthanized via cervical dislocation. Vaginas were excised and placed in RPMI 1640 media (Gibco) supplemented with 10% (v/v) fetal bovine serum (FBS), 50 μM 2-mercaptoethanol, 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Vaginas from four mice were pooled in order to obtain enough lymphocytes to perform flow cytometric analysis. Vaginas were minced into pieces of approximately 1 mm3 in size, and incubated, while rocking, in 20 mL of Hank’s-buffered saline solution (HBSS) containing 0.1% (v/v) fetal bovine serum and 2 mg/mL collagenase (Roche, Indianapolis, IN) for 2 hours at 37°C. Following this incubation, the vaginal tissue was homogenized using a 60-gauge stainless steel mesh screen and the plunger of a 5 mL syringe. This homogenate was then centrifuged at 500 × g for five minutes. Red blood cells were then lysed with tris ammonium chloride (1.55 mM ammonium chloride; 34 mM tris base, pH 7.2). Cell pellets were resuspended in 5 mL of supplemented RPMI, and expressed through a 70 μm nylon cell strainer (BD Biosciences, Bedford, MA), in order to remove debris and to disrupt any remaining cell clumps. Samples were resuspended in 30% Percoll (Amersham Biosciences, Sweden), layered over an 80% Percoll cushion and centrifuged at 500 × g for 15 minutes at room temperature. The number of viable cells in each sample was determined by trypan blue dye exclusion.
2.7 Isolation of lymphoid cells from lymph nodes
Mice were euthanized via cervical dislocation. Iliac lymph nodes (ILN) were removed and placed in Iscove’s-modified Dulbecco’s media (IMDM) (Gibco) supplemented with 10% (v/v) fetal bovine serum (FBS), 50 μM 2-mercaptoethanol, 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin sulfate. Lymph nodes were then mechanically dissociated by passage through a 70 μm nylon cell strainer (BD Biosciences) and the resulting cell suspension was washed with supplemented IMDM. The number of viable cells was determined by trypan blue dye exclusion.
2.8 Analysis of cell surface markers and tetramer specificity
Flow cytometric analysis of cell surface markers was determined as described previously (Anglen et al., 2003; Nair and Bonneau, 2006; Zhang et al., 2004) with slight modifications. Briefly, CD16/CD32 Fcγ receptors on isolated mononuclear cells were blocked with antibody in a 2.4G2 hybridoma cell culture supernatant supplemented with 20% (v/v) mouse serum (Sigma) (Anglen et al., 2003). Cell surface expression of CD8 was detected using anti-CD8 APC antibody (clone 53-6.7; eBioscience) at a dilution of 1:100 in FACS buffer. For identification of CD3-, NK1.1+ NK cells, anti-CD3 PE antibody (clone 145-2C11; eBioscience) and anti-NK1.1 APC antibody (clone PK136; eBiocience) were used at a dilution of 1:100 in FACS buffer.
For the detection and quantification of gB498-505 epitope-specific T lymphocytes, cells were incubated with a PE-labeled tetramer (at a 1:1000 dilution) obtained from the NIH Tetramer Facility at Emory University. This tetramer detects the T cell receptor specific for the H-2Kb-restricted, gB498-505-specific epitope, which has been described as an HSV-1-encoded immunodominant epitope in C57BL/6 mice (Bonneau et al., 1993a; Hanke et al., 1991). Following washes with FACS buffer (PBS supplemented with 1% [v/v] FBS, 0.02% [w/v] sodium azide), cells were resuspended in 2% (w/v) paraformaldehyde (prepared in PBS) prior to analysis by flow cytometry.
2.9 Degranulation assay for T cell lytic function
CD107a (LAMP-1) expression was determined as described previously (Betts and Koup, 2004), with slight modifications. Lymphocytes were resuspended in supplemented IMDM and incubated with 1 μM gB498-505 (SSIEFARL) peptide and a 1:100 dilution of anti-CD107a FITC antibody (clone 1D4B; BD Pharmingen) for 1 hour at 37°C. All cells were then treated with 10 mM of ammonium chloride for three hours at 37°C to prevent acidification of endosomes and the subsequent loss of the FITC signal (Henderson et al., 2002; Hoppe et al., 2004). Following this incubation, cells were washed twice with FACS buffer and the CD16/CD32 Fcγ receptors were blocked with 2.4G2 cell culture supernatant supplemented with 20% (v/v) mouse serum. Cells were then incubated with anti-CD8 APC antibody (at a 1:100 dilution), resuspended in 2% paraformaldehyde, and analyzed by flow cytometry.
2.10 Intracellular cytokine staining for IFN-γ
Lymph node-derived lymphocytes were isolated as described above. These cells were resuspended in supplemented IMDM and incubated with 1 μM gB498-505 peptide (synthesized at The Pennsylvania State University College of Medicine Macromolecular Core Facility) for 2 hours at 37°C. Cells were treated with brefeldin A (Sigma) (final concentration of 5 μg/mL) to prevent the secretion of cytokines and incubated for an additional 4 hours at 37°C. Cells were then washed twice with FACS buffer and the CD16/CD32 Fcγ receptors blocked with 2.4G2 cell culture supernatant supplemented with mouse serum as described above. To identify CD8+ T lymphocytes, cells were incubated with anti-CD8 APC antibody at a 1:100 dilution. Following staining for CD8, cells were fixed in 2% paraformaldehyde and incubated at room temperature for 20 minutes in the dark. Cells were then washed twice with FACS buffer and incubated with anti-IFN-γ FITC antibody (clone XMG1.2; eBioscience) diluted 1:100 in 0.5% (w/v) saponin (Sigma) prepared in FACS buffer. Subsequently, cells were washed twice in 0.5% saponin, resuspended in 2% paraformaldehyde, and analyzed by flow cytometry.
2.11 Viral plaque assays from vaginal washes
Mice were euthanized via cervical dislocation. Vaginal washes using PBS/1% FBS (v/v) were collected as described previously (Gallichan and Rosenthal, 1996). The recovered PBS/1% FBS was stored at -70°C until plaque assays were performed. The PBS washes underwent three freeze/thaw cycles, and were sonicated for three minutes in a water bath sonicator (Sinosonic Industrial Co., Taiwan). These sonicates were centrifuged at 16,000 × g for 10 minutes to remove cell debris, and virus levels were determined by standard plaque assay on Vero cells. Cells were fixed and stained with 5% (v/v) formaldehyde/0.5% (w/v) crystal violet.
2.12 Flow cytometry analysis
Flow cytometric analysis was conducted using a FACSCalibur flow cytometer (Becton Dickinson, San Diego, CA). Using forward-angle light scatter and 90° light scatter profiles, electronic gates were set around the live cells and at least 50,000 events were collected per sample. Dot plots and histograms were analyzed using FlowJo Software (TreeStar, Inc.; Ashland, OR). The total number of cells per sample was determined as follows: [percentage of specific cell type in sample] × [number of viable cells in sample].
2.13 Statistical analysis
Statistical significance was determined by unpaired t-test using StatView 5.0.1 software (SAS Institute Inc, Cary, NC). Comparisons between groups were performed and p values < 0.05 were considered significant.
3. Results
3.1 Psychological stress increases HSV-1-induced pathology in the vagina
To determine the effects of stress on HSV-1-mediated pathology in the vaginal epithelium, we examined vaginal tissue via light microscopy. Mice were either restrained or food-and-water-deprived for one 16-hour session prior to intravaginal infection with 5 × 106 pfu HSV-1. Restraint stress sessions continued daily thereafter, and mice were euthanized seven days post-infection. Vaginal tissue was excised, and prepared as described in the Materials and Methods. As was expected, vaginal tissue from non-infected, food-and-water deprived control mice displayed no HSV lesions and the columnar mucosa lining the vaginal epithelium was organized and intact. There was very little lymphocyte infiltration, and vaginal secretions did not contain a significant number of immune cells (Figure 1A, 1B). HSV-1 infected, food-and-water deprived mice did not exhibit damage in the columnar mucosa, however, increased secretions within the vaginal lumen were observed (Figure 1C, 1D). Lymphocyte infiltration of the mucosa was also observed (Figure 1D, indicated by arrows). In contrast, vaginal tissue from infected, restrained mice displayed areas of extensive damage to the columnar mucosa (Figure 1E, 1F). Lymphocyte presence was increased, as compared to non-infected mice. In addition, a number of HSV-induced lesions were observed (Figure 1F, indicated by arrow). Thus, histological examination alone indicated that restraint stress exacerbated the severity of vaginal HSV infection.
Figure 1.
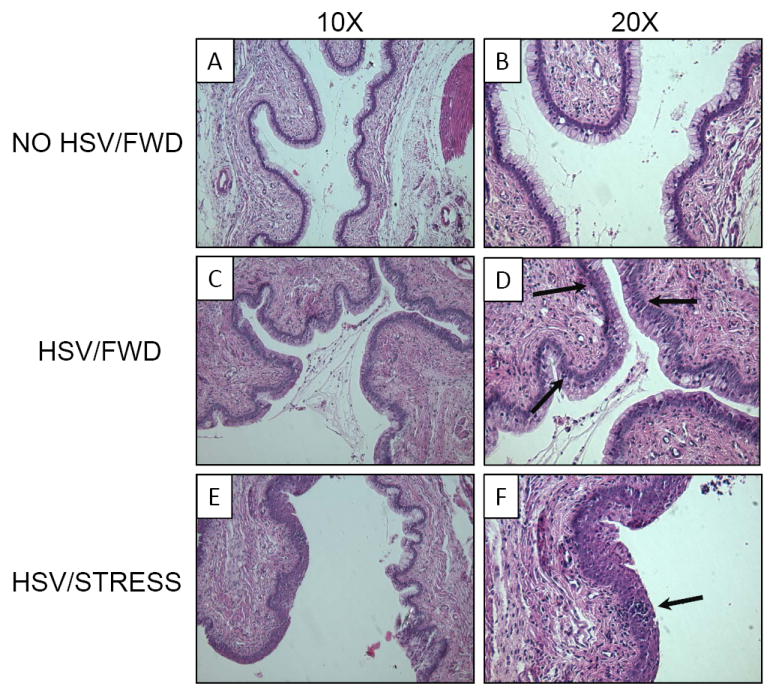
Effect of restraint stress on HSV-1-induced vaginal pathology. Mice were infected intravaginally with 5 × 106 pfu HSV-1. Control mice were mock-infected with phosphate-buffered saline (PBS) (A, B). Food-and-water deprivation (C, D) or restraint stress (E, F) began one day prior to infection and continued daily thereafter. Cross-sections (7 μm) were observed at 10× (A, C, E) and 20× (B, D, F) using an Olympus IX81 deconvolution microscope and QCapture software. A representative field is presented for each condition.
3.2 Psychological stress limits immediate control of local infection
We hypothesized that one reason for the increased vaginal pathology in mice experiencing restraint stress was due to increased levels of infectious virus within the vaginal lumen. To address this hypothesis, mice were either restrained or food-and-water-deprived for one 16-hour session prior to intravaginal infection with 5 × 106 pfu HSV-1. Mice underwent daily restraint or food-and-water deprivation sessions thereafter. At two, four, and six days post-infection, vaginal washes from different cohorts of mice were collected and titered for infectious HSV-1 as described in the Materials and Methods. Mice that were subjected to restraint stress had significantly greater levels of infectious virus within the vaginal lumen compared to non-stressed mice on days 2 and 6 post-infection (Figure 2). We were unable to detect infectious virus in any of the food-and-water-deprived mice at 6 days post-infection.
Figure 2.
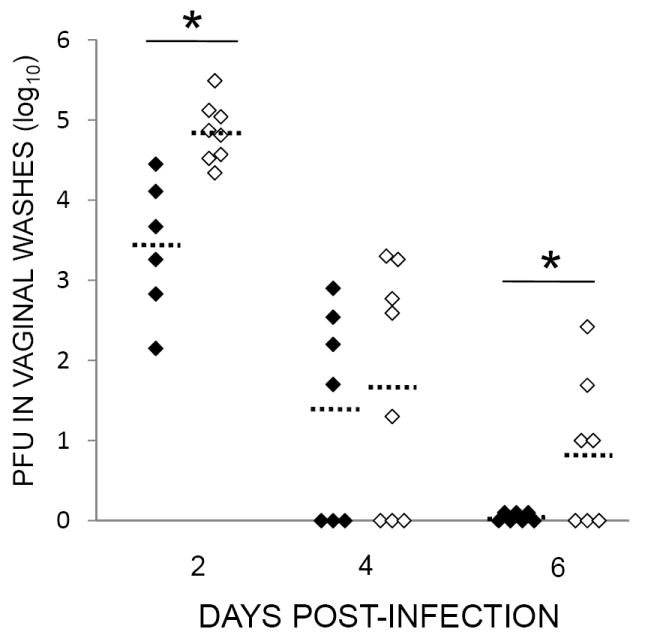
Effect of stress on the local control of vaginal HSV-1 infection. Mice were infected with 5 × 106 pfu HSV-1. Restraint stress (open diamonds) began one day prior to infection and continued daily thereafter; control mice were deprived of food and water (filled diamonds). At two, four, and six days post-infection, mice were euthanized and vaginal washes were collected and titered as described in the Materials and Methods. Means for each group are indicated by dashed bars. n = 6-8 for all groups. * Significant difference (p < 0.05) between indicated the groups.
3.3 Psychological stress reduces the number of NK cells in the vaginal tissue
The increased level of infectious virus at two days post-infection suggested that early, innate components of the immune response are impaired in stressed mice. Because natural killer (NK) cells limit initial spread of infection by non-specifically lysing virally-infected cells, we determined the number of NK cells at the local site of infection at this timepoint. Mice were either restrained or food-and-water-deprived for one 16-hour session prior to intravaginal infection with 5 × 106 pfu HSV-1. Mice underwent daily restraint sessions thereafter. At two days post-infection, vaginal tissue was excised and lymphoid cells were isolated as described in the Materials and Methods. As was expected, HSV-infection was associated with a significant increase in both the percentage and number of lymphoid cells with the CD3-NK1.1+ phenotype as compared to uninfected control mice (Figure 3). However, the vaginal tissue from HSV-1 infected mice experiencing restraint stress exhibited a significant decrease in the number of NK cells (Figure 3). The percentage of lymphoid cells expressing the NK cell phenotype was slightly decreased in restrained mice (13.1% vs. 11.1%), however, this difference was not significant.
Figure 3.
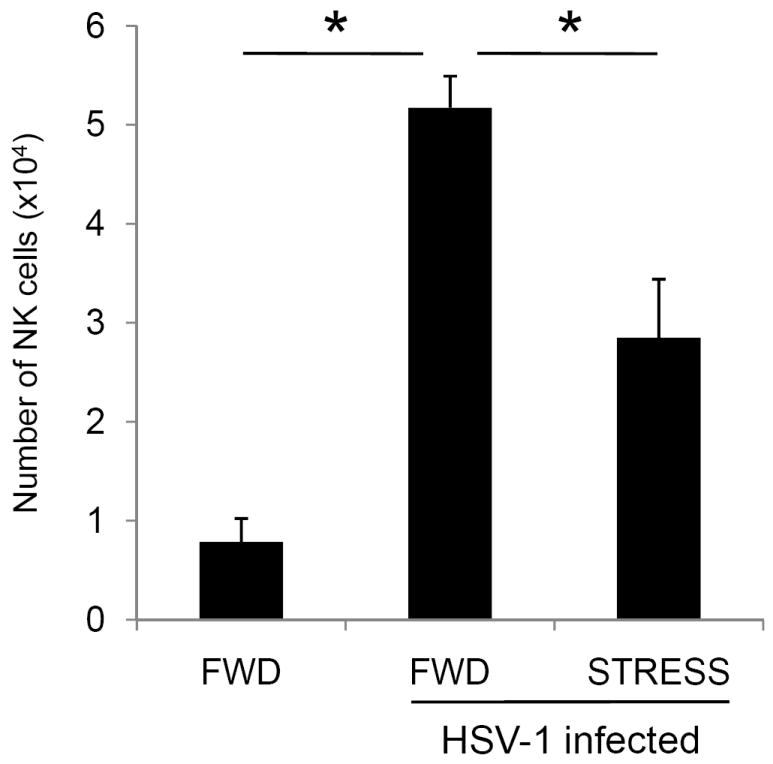
Effect of restraint stress on NK cell numbers. Mice were subjected to restraint stress (STRESS) or food-and-water deprivation (FWD) and infected with 5 × 106 pfu HSV-1. Control mice were mock-infected with PBS. At two days post-infection, mice were euthanized, and lymphocytes from the vaginal tissue were collected. The percentage of CD3-NK1.1+ NK cells was determined via flow cytometry, and total numbers of NK cells were quantified as described in the Materials and Methods. n = 4 for all groups. * Significant difference (p < 0.05) between the indicated groups.
3.4 Psychological stress decreases the number of HSV-specific CD8+ T cells in the draining iliac lymph nodes (ILN)
We assessed the magnitude of the immune response in the iliac lymph nodes (ILN), which are the lymph nodes that drain the genital region (King et al., 1998). Mice were either restrained or food-and-water-deprived for one 16-hour session prior to vaginal infection with 5 × 106 pfu HSV-1. Mice underwent daily restraint sessions thereafter. Seven days post-infection, mice were euthanized and the iliac lymph nodes were removed and assessed for both total CD8+ T cells and gB498-505-specific CD8+ T cells. Viability was assessed via trypan blue dye exclusion. HSV-infection resulted in increased numbers of viable cells in the ILN (Table 1). This increase was slightly hindered in mice experiencing restraint stress during infection, however the difference was not statistically significant between the groups. The population of infiltrating cells at this timepoint likely included CD8+ T cells, as well as other lymphocytes, and antigen presenting cells. Indeed, when we examined cells for CD8+ expression, there was a significant increase in both the number of total CD8+ T cells (Figure 4A) and gB498-505-specific CD8+ cells (Figure 4B) in the ILN of infected mice, as compared to mice that were not infected. However, the ILN from mice that underwent stress during the infection had significantly fewer numbers of both total (Figure 4A) and gB498-505-specific CD8+ T cells (Figure 4B), as compared to food-and-water-deprived, HSV-1 infected mice.
TABLE 1.
The effects of restraint stress on viable cell numbers at day 7 post-infection
Number of cells × 104 SEM. n = 8-12 for all groups
Number of cells × 104 SEM. n = 3-6 for all groups
Figure 4.
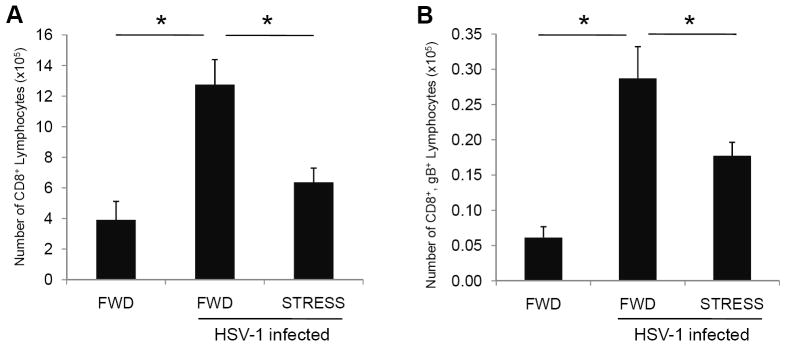
Effect of restraint stress on total and gB498-505-specific CD8+ T lymphocytes within the iliac lymph nodes (ILN). Mice were subjected to restraint stress (STRESS) or food-and-water deprivation (FWD) and infected with 5 × 106 pfu HSV-1. At seven days post-infection, mice were euthanized and lymphocytes from the ILN were collected and quantified for CD8+ T cells (A) and assessed for gB498-505-specificity (B). n = 8-12 for all groups. * Significant difference (p < 0.05) between the indicated groups.
3.5 Psychological stress decreases the number of functional HSV-specific CD8+ T cells in the draining iliac lymph nodes
To assess the effect of stress on the function of gB498-505-specific T cells in the iliac lymph nodes, we tested the CD8+ T cells from these lymph nodes for their ability to express the degranulation marker CD107a and to produce intracellular interferon-γ (IFN-γ) in response to gB498-505-specific peptide stimulation. Mice were infected intravaginally with 5 × 106 pfu HSV-1, and subjected to restraint stress beginning one day prior to infection and continuing daily. Seven days post-infection, lymphoid cells from the ILN were isolated and assessed for their ability to degranulate and produce IFN-γ.
As was expected, relatively few CD8+ T cells in the ILN of non-infected, food-and-water-deprived control mice expressed CD107 (Figure 5A) or produced IFN-γ (Figure 5B). In HSV-1 infected mice, we detected significantly greater numbers of cells expressing CD107a or producing IFN-γ. However, in mice experiencing restraint stress at the time of infection, there were significantly fewer cells expressing CD107a or producing IFN-γ. This reduction was primarily due to a decrease in the number of CD8+ T cells in the lymph nodes; however a slight reduction in the percentage of CD8+ T cells responding to peptide was also observed (data not shown).
Figure 5.
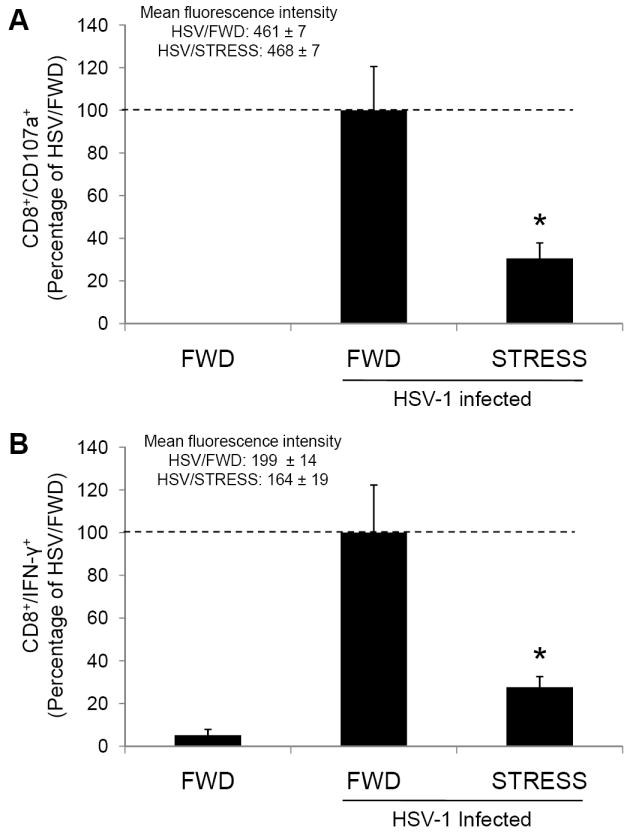
Effect of stress on CD8+ T cell function within the ILN. Mice were subjected to restraint stress (STRESS) or food-and-water deprivation (FWD) and infected with 5 × 106 pfu HSV-1. Seven days post-infection, mice were euthanized and lymphocytes from the ILN were assessed for degranulation (A) or interferon-γ production (B) in response to stimulation with gB498-505 peptide. Results are normalized within individual experiments, and are expressed as the percentage of functioning cells in the HSV-1-infected, food-and-water-deprived mice. n = 4-12 for all groups. * Significant difference (p < 0.01) between the indicated groups.
To assess the level of function of individual cells we also examined the mean fluorescence intensity (MFI) of those CD8+ cells that expressed CD107a or produced IFN-γ. There was no significant difference in the MFI of either CD107a+ or IFN-γ+ cells between HSV-infected mice that were subjected to food-and-water-deprivation or restraint stress. These findings indicate that psychological stress does not impair the ability of gB498-505-specific cells to respond on a per-cell basis.
3.6 Psychological stress decreases the number of lymphocytes that infiltrate the vaginal tissue during HSV-1 infection
Having shown that psychological stress at the time of infection results in a decreased immune response in the ILN we hypothesized that the immune response in vaginal tissue of stressed mice would also be less robust than in food-and-water deprived mice. To evaluate the local immune response to vaginal infection we measured the number of HSV-1 gB498-505-specific T cells in the vaginal tissue. Mice were subjected to one 16-hour restraint session prior to intravaginal infection with 5 × 106 pfu HSV-1. Restraint stress continued daily thereafter, and mice were euthanized seven days post-infection. Vaginal tissue was excised and lymphoid cells were isolated as described in the Materials and Methods. We first assessed the number of viable cells using trypan blue dye exclusion (Table 1) and found an overall increase in cell numbers in the vaginal tissue in mice that were infected with HSV. Restraint during infection slightly inhibited this increase in cellularity, however this inhibition was not significantly different than the food-and-water deprived controls. As was expected, non-infected mice had relatively low numbers of CD8+ T cells in the vaginal epithelium (Figure 6A, 6D). We observed a significant increase in the number of CD8+ T cells in mice infected with HSV-1 (Figure 6B, 6D). However, mice subjected to restraint stress exhibited less of an increase than did the food-and-water-deprived control mice. Although the numbers of CD8+ T cells in the vaginal tissue was lower in infected, restrained mice than in infected, food-and-water deprived mice, there was not a statistical difference between these two groups (p < 0.08) (Figure 6C, 6D).
Figure 6.
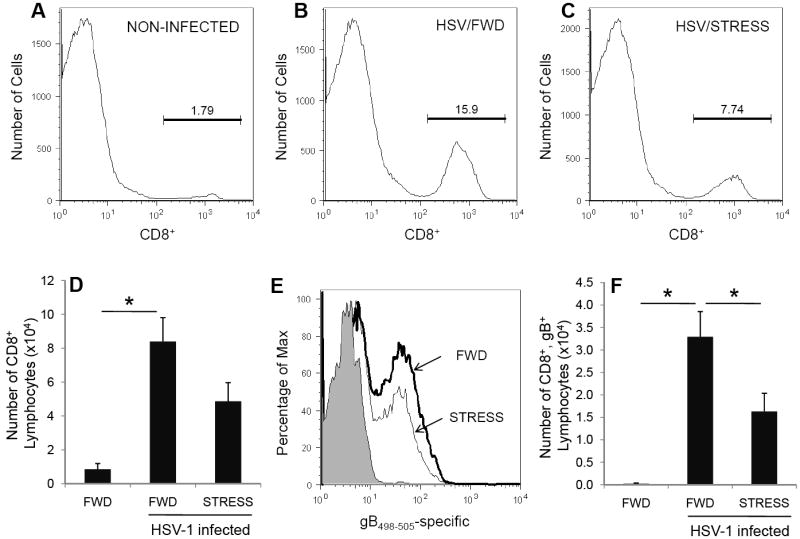
Effect of stress on total and gB498-505-specific CD8+ T lymphocytes within vaginal tissue. Mice were subjected to restraint stress (STRESS) or food-and-water deprivation (FWD) and infected with 5 × 106 pfu HSV-1. Seven days post-infection, mice were euthanized and lymphocytes from the vaginal tissue were collected and quantified for CD8+ T cells (A-D) and assessed for gB498-505-specificity (E, F). In panel (E), the number of gB498-505-specific CD8+ T cells is indicated as follows: PBS/FWD (shaded region) HSV/FWD (bold line) and HSV/STRESS (thin line.) Vaginas from four mice were pooled. n = 3-6 pools for all groups (12-24 mice total for each group). Results are normalized within individual experiments to the number of functioning cells in HSV-1 infected, food-and-water deprived mice. * Significant difference (p < 0.05) between the indicated groups.
We also used tetramer-based flow cytometric analysis to quantify those CD8+ T cells whose receptors are specific for the H-2Kb-restricted immunodominant epitope gB498-505. As was expected, infected, food-and-water-deprived mice had a significantly greater number of gB498-505-specific CD8+ T cells as compared to non-infected mice (Figure 6E, 6F). However, mice that were undergoing stress throughout the infection had significantly fewer gB498-505-specific CD8+ T cells as compared to non-stressed controls.
4. Discussion
Many studies have examined the impact of psychological stress on the immune response to viral infection (Brenner and Moynihan, 1997; Campbell et al., 2001; Dobbs et al., 1996; Engler et al., 2005; Feng et al., 1991; Hermann et al., 1995; Hunzeker et al., 2004; Konstantinos and Sheridan, 2001; Mi et al., 2006; Ortiz et al., 2003; Tseng et al., 2005). Such studies have indicated that psychological stress and associated neuroendocrine peptides and hormones are generally immunosuppressive. Despite the multitude of studies on restraint stress during primary HSV-1 infection (Anglen et al., 2003; Ashcraft et al., 2008; Bonneau et al., 1993b; Bonneau et al., 1991; Brenner and Moynihan, 1997; DeLano and Mallery, 1998; Nair et al., 2007; Ortiz et al., 2003), there have been no publications on the effect of stress on primary vaginal HSV-1 infection. Therefore, the studies presented here aimed to determine the impact of restraint stress during primary vaginal HSV-1 infection on the number and function of immune cells governing both the innate and adaptive immune response. Using a clinically-relevant mucosal infection model, our studies examined the effects of stress on the immune response to a vaginal HSV-1 infection.
4.1 The model
In the United States, 58% and 17% of patients are seropositive for HSV-1 and HSV-2, respectively (Xu et al., 2006). Although genital herpes infections were once caused primarily by HSV-2, there has been a substantial increase in the number of infections in which HSV-1 is the causative agent (Malkin, 2004; Samra et al., 2003). For example, HSV-1 isolates accounted for 78% of genital infections in 2001, as compared to only 31% in 1993 (Roberts et al., 2003). Given this changing trend, we chose to determine the impact of psychological stress on the immune response to vaginally-acquired HSV-1 infection. Our infection model included a gentle swabbing of the vaginal epithelium prior to instilling the viral inoculum into the lumen. This approach not only mimicked the mild abrasion that may occur during the acquisition of a vaginal infection, but also resulted in a more consistent infection than when virus was instilled into the vagina without swabbing (data not shown).
Vaginal infections in experimental settings pose a unique challenge due to the cycling hormones. As has been reported previously, the various stages of the estrous cycle result in changes in infectivity in mice infected with either HSV-1 or HSV-2 (Gallichan and Rosenthal, 1996; Gillgrass et al., 2003; Gillgrass et al., 2005; Kaushic et al., 2003; Linehan et al., 2004; Parr et al., 1994). These differences in infectivity result from changes in antibody levels within vaginal washes (Gallichan and Rosenthal, 1996), and altered expression of nectin-1, which serves as an HSV-receptor (Linehan et al., 2004). These and other studies have demonstrated the importance using synthetic hormones to induce mice to enter a diestrous-like state prior to vaginal infection. Therefore, all mice were treated with Depo-Provera® five days prior to vaginal infection, as has become standard in studies of this nature.
4.2 Glucocorticoids increase the severity of vaginal infection
Given the immunosuppressive nature of glucocorticoids, we hypothesized that restraint stress would result in a more severe vaginal infection that would cause more extensive damage to the vaginal epithelium. We have previously reported that our model of restraint stress increases serum corticosterone levels to approximately 300 ng/mL, whereas food-and-water-deprived control mice have serum corticosterone levels of less than 100 ng/mL (Ashcraft et al., 2008). Consistent with our hypothesis, mice that experienced restraint stress displayed more extensive vaginal pathology than did mice that were food-and-water deprived throughout infection (Figure 1). Disrupted areas of the columnar mucosa, as well as the presence of HSV lesions, were observed in mice experiencing restraint stress. Because mice were observed at only seven days post-infection, we were unable to address the question of whether restraint stress resulted in lesions only in mice that were restrained, or if lesion healing in restrained mice was simply delayed. Given the multitude of studies reporting that stress delays wound healing (reviewed in Christian et al., 2006), this is certainly a possibility, although not one that we chose to pursue in this study.
Cervicovaginal secretions from HSV-2-infected humans contain not only neutrophils (John et al., 2005) but also HSV-specific IgG and IgA (Mbopi-Keou et al., 2003) with neutralizing activity. Vaginal secretions were observed in infected, food-and-water-deprived mice but not in restrained mice. This absence of secretions and associated immune factors in restrained mice suggests that their immune response may be less able to control the levels of infectious virus. To address this possibility, we measured viral titers in vaginal washes two, four, and six days post-infection (Figure 2). Consistent with our hypothesis, restrained mice had increased viral titers in their vaginal washes. Our method of collecting vaginal washes included a swabbing of the vaginal epithelium, which allowed us to collect HSV-infected epithelial cells. Therefore, the virus titers presented include not only virus that was released into the vagina from lysed cells, but also virus that was released from these collected epithelial cells upon in vitro freeze/thaw and sonication. From a clinical standpoint, this increase could result in a greater chance of spreading the disease to non-infected partners.
4.3 Psychological stress impairs the early innate immune response to vaginal HSV-1 infection
As T cell-mediated, adaptive immune responses typically take 5-7 days to develop, the significant increase in infectious viral titers at two days post-infection suggested that restraint stress caused a failure in the ability of the innate immune response to control the infection. To address this hypothesis, we focused on NK cells, given their anti-viral functions and their presence early in the course of intranasal HSV-1 infection. On days 1 and 3 post-intranasal HSV-1 infection, NK cells are the dominant lymphocyte present in bronchoalveolar lavage fluid (Reading et al., 2006). Although macrophages (Inagaki-Ohara et al., 2000; Morahan et al., 1978) and plasmacytoid dendritic cells (Lund et al., 2006) are also present at this time during vaginal HSV-2 infection, these cells do not directly lyse infected cells. Therefore, we felt that NK cell numbers would be a more relevant measure of the innate anti-viral immune response to vaginal HSV infection. As was expected based on our HSV titer data (Figure 2), we found that restraint stress significantly reduced the number of NK cells in the vaginal tissue of infected mice (Figure 3).
Since only 10% of NK cells proliferate at peripheral sites (Vitale et al., 2004), the influx of NK cells into an infected area is highly dependent on trafficking to these sites. Chronic psychological stress has been shown to impair trafficking of other lymphoid cells, and may be detrimental to NK cell trafficking as well. Another possibility is that impaired cytokine production is limiting NK cell activation and recruitment. Specifically, IL-2, IL-12, and IL-15 are required for NK cell responses (Ferlazzo et al., 2004; Moretta et al., 2006; Puzanov et al., 1996), and the production of each of these cytokines has been shown to be suppressed during chronic stress (Hunzeker et al., 2004; Sheridan et al., 1991).
A reduction in the number of NK cells may impair aspects of not only the innate immune response but also the adaptive immune response, since NK cells can modulate the developing dendritic cell (DC)-mediated immune response (Moretta et al., 2006). For example, activated NK cells induce DC maturation (Vitale et al., 2005), increase IFN-α and IL-6 production by plasmacytoid DC (Della Chiesa et al., 2006), and increase CD80 expression by myeloid-derived DC (Gerosa et al., 2005). Therefore, reduced NK cell numbers may impair DC-priming of naive T cells, thus indirectly contributing to an impaired cell-mediated immune response.
Previous studies have shown that NK cell cytotoxicity is suppressed in mice experiencing restraint stress. These findings were shown using popliteal lymph node-derived NK cells during HSV footpad infection (Bonneau et al., 1991). Other viral infection models have shown that restraint stress decreases NK cell activity in the spleen (Tseng et al., 2005; Welsh et al., 2004) and at the site of infection (Hunzeker et al., 2004). Due to the technical difficulties in obtaining enough vaginal-derived NK cells to conduct cytotoxicity assays, we could not examine these cells in our model. However, we would expect that their function was impaired in our model as well.
4.4 Restraint stress impairs ILN-derived T cell number and function
4.4.1 Restraint stress decreases ILN-derived HSV-specific T cell numbers
The significantly decreased numbers of both total CD8+ T cells (Figure 4A) and CD8+ T cells specific for gB498-505 peptide (Figure 4B) in the ILN could represent a failure of a myriad of aspects of the immune response. During viral infection, antigen presenting cells traffick to the nearest lymph node, where they activate naive T cells whose receptors recognize the epitope being presented. Following this activation, antigen-specific T cells greatly expand in number and migrate to the site of infection. As is explained above, decreased numbers of NK cells may inhibit antigen presentation by DC, resulting in fewer naive CD8+ T cells being activated. Impaired lymphocyte proliferation may also be responsible for the decreased number of CD8+ T cells. Future studies will pinpoint which one or more of these events are hindered in our stress model. In addition, it has long been recognized that stress and associated increases in corticosterone lead to increased lymphocyte apoptosis (Blewitt et al., 1983; Caron-Leslie et al., 1991; Cohen and Duke, 1984; Wyllie, 1980), an event which may also contribute to the reduced numbers of CD8+ T cells.
4.4.2 Restraint stress decreases ILN-derived HSV-specific T cell function
Although the number of CD8+ T cells is obviously important, the ability of these cells to function is also necessary to efficiently clear an infection. We have determined that the peak of the adaptive immune response in this model occurs at seven days post-infection (data not shown). Due to the relatively small number of CTL in the vaginal tissue, and the need for large numbers of cells to conduct these functional analyses, we were restricted to examining only the CD8+ T cells derived from the iliac lymph nodes.
Lysosome degranulation, which can be measured by the expression of lysosomal-associated membrane-protein-1 (CD107a), is a general indicator of CTL function. Upon T cell receptor binding to a target cell presenting the appropriate peptide-MHC class I complex, CTL lysosomes migrate to the CTL-target interface and release perforin and granzymes, thereby exposing CD107a on their surface. Mice that were restrained during infection had significantly fewer CD8+ T cells expressing CD107 (Figure 5A). Impaired degranulation may result in failure to lyse infected target cells in an in vivo setting.
A second function of CTL is to produce IFN-γ, which augments the immune response by activating neighboring immune cells, such as macrophages and NK cells. Similar to what we observed in the degranulation assay, mice that were restrained had a lower number of CD8+ T cells that produced IFN-γ when stimulated with gB498-505 (Figure 5B). Although previous studies demonstrated that the synthetic glucocorticoid dexamethasone inhibits IFN-γ production by interfering with Stat4 transcription factor activities (Franchimont et al., 2000), we observed no effect of stress on levels of IFN-γ in individual CD8+ cells. This finding indicated that IFN-γ production was not impaired on an individual cell basis. This observation may be a function of the increased viral titers we observed in the vaginal washes (Figure 2), thus increasing the antigenic load supplied to the ILN. Accordingly, the immune response may have been augmented, thus compensating for any impaired transcription that may have occurred.
4.5 Restraint stress decreases the number of HSV-specific T cells within the vaginal tissue
Using a flow cytometric-based method to quantify vaginal HSV-specific T cells allowed us to focus on cells expressing not only the cell surface marker CD8, but also the gB498-505-specific T cell receptor. Through this approach, we were able to determine the number of these cells throughout the entire vagina, rather than in only discrete sections as a microscopy-based approach would allow us to do. The significant decrease in the number of gB498-505-specific CD8+ T cells (Figure 6D, 6F) could be the result of either poor trafficking from the lymph node to the vagina or simply the consequence of poor expansion of T cells in the lymph nodes (Figure 4). In models of delayed-type hypersensitivity and influenza virus infection, chronic stress has been shown to suppress leukocyte trafficking (Dhabhar and McEwen, 1999; Hermann et al., 1995). Our observed decrease in HSV-specific vaginal CTL could result in increased disease progression and severity, and in humans may also result in increased time that an individual is infectious. However, as we did not observe any mortality in our studies, we suspect that the immune response, although suppressed, is still above the threshold that is necessary for eventually clearing the infection.
The observation that total CD8+ T cells were not significantly reduced in vaginas of restrained mice, (Figure 6B, 6C) could result from several possibilities. The CD8+ T cells that are not specific for gB498-505 may be recognizing sub-dominant HSV-1 epitopes such as RR1822-829 (Salvucci et al., 1995), or other undefined epitopes. However, the decreased number of gB498-505-specific CD8+ T cells is of a greater concern, as these are the cells that are likely necessary in eliminating the viral infection (Bonneau et al., 1993a).
4.6 Clinical implications
Although the studies described herein were conducted in a mouse model, they offer the suggestion that psychological stress during human HSV-1 infection has important clinical implications. At six days post-infection, virus was still detected in vaginal washes from restrained mice only. Furthermore, at seven days post-infection, HSV-1 lesions were observed only in the vaginal epithelium of mice that were experiencing restraint stress. As these lesions are a source of progeny virus, the above findings suggest that psychological stress increases the risk of horizontal transmission of HSV by increasing the length of time during which an individual is infectious.
The increased levels of infectious virus in mice experiencing stress may result in a greater number of latently infected neurons, and thus an increased incidence of virus reactivation. This possibility is supported by studies which show that lowering the level of infectious virus during primary HSV-1 infection through acyclovir therapy results in fewer neurons harboring viral genome, fewer copies of the viral genome per neuron, and a lower reactivation frequency (Sawtell et al., 2001). A more recent study demonstrated that increasing the dose of a primary HSV-2 infection results in greater levels of latent virus (Hoshino et al., 2008). Taking these findings into consideration, psychological stress at the time of primary HSV-1 vaginal infection may ultimately result in an increased frequency and severity of recurrent HSV infections.
Acknowledgments
The authors thank Dr. Kang Li for preparation of the histological samples, and Dr. Timothy Cooper for assistance in interpreting the histological data. We also acknowledge the excellent technical assistance of Jennifer Mellinger.
This work was supported by PHS Grant R01 HD39262 (RHB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anglen CS, Truckenmiller ME, Schell TD, Bonneau RH. The dual role of CD8+ T lymphocytes in the development of stress-induced herpes simplex encephalitis. J Neuroimmunol. 2003;140:13–27. doi: 10.1016/s0165-5728(03)00159-0. [DOI] [PubMed] [Google Scholar]
- Ashcraft KA, Hunzeker J, Bonneau RH. Psychological stress impairs the local CD8+ T cell response to mucosal HSV-1 infection and allows for increased pathogenicity via a glucocorticoid receptor-mediated mechanism. Psychoneuroendocrinology. 2008 doi: 10.1016/j.psyneuen.2008.04.010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avitsur R, Padgett DA, Sheridan JF. Social interactions, stress, and immunity. Neurol Clin. 2006;24:483–491. doi: 10.1016/j.ncl.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Betts MR, Koup RA. Detection of T-cell degranulation: CD107a and b. Methods Cell Biol. 2004;75:497–512. doi: 10.1016/s0091-679x(04)75020-7. [DOI] [PubMed] [Google Scholar]
- Blewitt RW, Abbott AC, Bird CC. Mode of cell death induced in human lymphoid cells by high and low doses of glucocorticoid. Br J Cancer. 1983;47:477–486. doi: 10.1038/bjc.1983.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau RH, Salvucci LA, Johnson DC, Tevethia SS. Epitope specificity of H-2Kb-restricted, HSV-1-, and HSV-2-cross-reactive cytotoxic T lymphocyte clones. Virology. 1993a;195:62–70. doi: 10.1006/viro.1993.1346. [DOI] [PubMed] [Google Scholar]
- Bonneau RH, Sheridan JF, Feng N, Glaser R. Stress-induced modulation of the primary cellular immune response to herpes simplex virus infection is mediated by both adrenal-dependent and independent mechanisms. J Neuroimmunol. 1993b;42:167–176. doi: 10.1016/0165-5728(93)90007-l. [DOI] [PubMed] [Google Scholar]
- Bonneau RH, Sheridan JF, Feng NG, Glaser R. Stress-induced suppression of herpes simplex virus (HSV)-specific cytotoxic T lymphocyte and natural killer cell activity and enhancement of acute pathogenesis following local HSV infection. Brain Behav Immun. 1991;5:170–192. doi: 10.1016/0889-1591(91)90015-3. [DOI] [PubMed] [Google Scholar]
- Brenner GJ, Moynihan JA. Stressor-induced alterations in immune response and viral clearance following infection with herpes simplex virus-type 1 in BALB/c and C57B1/6 mice. Brain Behav Immun. 1997;11:9–23. doi: 10.1006/brbi.1997.0480. [DOI] [PubMed] [Google Scholar]
- Campbell T, Meagher MW, Sieve A, Scott B, Storts R, Welsh TH, Welsh CJ. The effects of restraint stress on the neuropathogenesis of Theiler’s virus infection: I. Acute disease. Brain Behav Immun. 2001;15:235–254. doi: 10.1006/brbi.2000.0598. [DOI] [PubMed] [Google Scholar]
- Caron-Leslie LM, Schwartzman RA, Gaido ML, Compton MM, Cidlowski JA. Identification and characterization of glucocorticoid-regulated nuclease(s) in lymphoid cells undergoing apoptosis. J Steroid Biochem Mol Biol. 1991;40:661–671. doi: 10.1016/0960-0760(91)90288-g. [DOI] [PubMed] [Google Scholar]
- Christian LM, Graham JE, Padgett DA, Glaser R, Kiecolt-Glaser JK. Stress and wound healing. Neuroimmunomodulation. 2006;13:337–346. doi: 10.1159/000104862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JJ, Duke RC. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984;132:38–42. [PubMed] [Google Scholar]
- DeLano RM, Mallery SR. Stress-related modulation of central nervous system immunity in a murine model of herpes simplex encephalitis. J Neuroimmunol. 1998;89:51–58. doi: 10.1016/s0165-5728(98)00087-3. [DOI] [PubMed] [Google Scholar]
- Della Chiesa M, Romagnani C, Thiel A, Moretta L, Moretta A. Multidirectional interactions are bridging human NK cells with plasmacytoid and monocyte-derived dendritic cells during innate immune responses. Blood. 2006;108:3851–3858. doi: 10.1182/blood-2006-02-004028. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci U S A. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs CM, Feng N, Beck FM, Sheridan JF. Neuroendocrine regulation of cytokine production during experimental influenza viral infection: effects of restraint stress-induced elevation in endogenous corticosterone. J Immunol. 1996;157:1870–1877. [PubMed] [Google Scholar]
- Engler A, Roy S, Sen CK, Padgett DA, Sheridan JF. Restraint stress alters lung gene expression in an experimental influenza A viral infection. J Neuroimmunol. 2005;162:103–111. doi: 10.1016/j.jneuroim.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Feng N, Pagniano R, Tovar CA, Bonneau RH, Glaser R, Sheridan JF. The effect of restraint stress on the kinetics, magnitude, and isotype of the humoral immune response to influenza virus infection. Brain Behav Immun. 1991;5:370–382. doi: 10.1016/0889-1591(91)90032-6. [DOI] [PubMed] [Google Scholar]
- Ferlazzo G, Pack M, Thomas D, Paludan C, Schmid D, Strowig T, Bougras G, Muller WA, Moretta L, Munz C. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci U S A. 2004;101:16606–16611. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchimont D, Galon J, Gadina M, Visconti R, Zhou Y, Aringer M, Frucht DM, Chrousos GP, O’Shea JJ. Inhibition of Th1 immune response by glucocorticoids: dexamethasone selectively inhibits IL-12-induced Stat4 phosphorylation in T lymphocytes. J Immunol. 2000;164:1768–1774. doi: 10.4049/jimmunol.164.4.1768. [DOI] [PubMed] [Google Scholar]
- Freeman ML, Sheridan BS, Bonneau RH, Hendricks RL. Psychological Stress Compromises CD8+ T Cell Control of Latent Herpes Simplex Virus Type 1 Infections. J Immunol. 2007;179:322–328. doi: 10.4049/jimmunol.179.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallichan WS, Rosenthal KL. Effects of the estrous cycle on local humoral immune responses and protection of intranasally immunized female mice against herpes simplex virus type 2 infection in the genital tract. Virology. 1996;224:487–497. doi: 10.1006/viro.1996.0555. [DOI] [PubMed] [Google Scholar]
- Gerosa F, Gobbi A, Zorzi P, Burg S, Briere F, Carra G, Trinchieri G. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol. 2005;174:727–734. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- Gillgrass AE, Ashkar AA, Rosenthal KL, Kaushic C. Prolonged exposure to progesterone prevents induction of protective mucosal responses following intravaginal immunization with attenuated herpes simplex virus type 2. J Virol. 2003;77:9845–9851. doi: 10.1128/JVI.77.18.9845-9851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillgrass AE, Fernandez SA, Rosenthal KL, Kaushic C. Estradiol regulates susceptibility following primary exposure to genital herpes simplex virus type 2, while progesterone induces inflammation. J Virol. 2005;79:3107–3116. doi: 10.1128/JVI.79.5.3107-3116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Glaser R. Stress-induced immune dysregulation: implications for wound healing, infectious disease and cancer. J Neuroimmune Pharmacol. 2006;1:421–427. doi: 10.1007/s11481-006-9036-0. [DOI] [PubMed] [Google Scholar]
- Hanke T, Graham FL, Rosenthal KL, Johnson DC. Identification of an immunodominant cytotoxic T-lymphocyte recognition site in glycoprotein B of herpes simplex virus by using recombinant adenovirus vectors and synthetic peptides. J Virol. 1991;65:1177–1186. doi: 10.1128/jvi.65.3.1177-1186.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson G, Peng W, Jin L, Perng GC, Nesburn AB, Wechsler SL, Jones C. Regulation of caspase 8- and caspase 9-induced apoptosis by the herpes simplex virus type 1 latency-associated transcript. J Neurovirol. 2002;8 Suppl 2:103–111. doi: 10.1080/13550280290101085. [DOI] [PubMed] [Google Scholar]
- Hermann G, Beck FM, Sheridan JF. Stress-induced glucocorticoid response modulates mononuclear cell trafficking during an experimental influenza viral infection. J Neuroimmunol. 1995;56:179–186. doi: 10.1016/0165-5728(94)00145-e. [DOI] [PubMed] [Google Scholar]
- Hoppe HC, van Schalkwyk DA, Wiehart UI, Meredith SA, Egan J, Weber BW. Antimalarial quinolines and artemisinin inhibit endocytosis in Plasmodium falciparum. Antimicrob Agents Chemother. 2004;48:2370–2378. doi: 10.1128/AAC.48.7.2370-2378.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y, Qin J, Follmann D, Cohen JI, Straus SE. The number of herpes simplex virus-infected neurons and the number of viral genome copies per neuron correlate with the latent viral load in ganglia. Virology. 2008;372:56–63. doi: 10.1016/j.virol.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunzeker J, Padgett DA, Sheridan PA, Dhabhar FS, Sheridan JF. Modulation of natural killer cell activity by restraint stress during an influenza A/PR8 infection in mice. Brain Behav Immun. 2004;18:526–535. doi: 10.1016/j.bbi.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Inagaki-Ohara K, Daikoku T, Goshima F, Nishiyama Y. Impaired induction of protective immunity by highly virulent herpes simplex virus type 2 in a murine model of genital herpes. Arch Virol. 2000;145:1989–2002. doi: 10.1007/s007050070035. [DOI] [PubMed] [Google Scholar]
- Jarillo-Luna A, Rivera-Aguilar V, Garfias HR, Lara-Padilla E, Kormanovsky A, Campos-Rodriguez R. Effect of repeated restraint stress on the levels of intestinal IgA in mice. Psychoneuroendocrinology. 2007;32:681–692. doi: 10.1016/j.psyneuen.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Jarillo-Luna A, Rivera-Aguilar V, Martinez-Carrillo BE, Barbosa-Cabrera E, Garfias HR, Campos-Rodriguez R. Effect of restraint stress on the population of intestinal intraepithelial lymphocytes in mice. Brain Behav Immun. 2008;22:265–275. doi: 10.1016/j.bbi.2007.08.004. [DOI] [PubMed] [Google Scholar]
- John M, Keller MJ, Fam EH, Cheshenko N, Hogarty K, Kasowitz A, Wallenstein S, Carlucci MJ, Tuyama AC, Lu W, Klotman ME, Lehrer RI, Herold BC. Cervicovaginal secretions contribute to innate resistance to herpes simplex virus infection. J Infect Dis. 2005;192:1731–1740. doi: 10.1086/497168. [DOI] [PubMed] [Google Scholar]
- Kaushic C, Ashkar AA, Reid LA, Rosenthal KL. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J Virol. 2003;77:4558–4565. doi: 10.1128/JVI.77.8.4558-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King NJ, Parr EL, Parr MB. Migration of lymphoid cells from vaginal epithelium to iliac lymph nodes in relation to vaginal infection by herpes simplex virus type 2. J Immunol. 1998;160:1173–1180. [PubMed] [Google Scholar]
- Kip KE, Cohen F, Cole SR, Wilhelmus KR, Patrick DL, Blair RC, Beck RW. Recall bias in a prospective cohort study of acute time-varying exposures: example from the herpetic eye disease study. J Clin Epidemiol. 2001;54:482–487. doi: 10.1016/s0895-4356(00)00310-3. [DOI] [PubMed] [Google Scholar]
- Konstantinos AP, Sheridan JF. Stress and influenza viral infection: modulation of proinflammatory cytokine responses in the lung. Respir Physiol. 2001;128:71–77. doi: 10.1016/s0034-5687(01)00266-3. [DOI] [PubMed] [Google Scholar]
- Linehan MM, Richman S, Krummenacher C, Eisenberg RJ, Cohen GH, Iwasaki A. In vivo role of nectin-1 in entry of herpes simplex virus type 1 (HSV-1) and HSV-2 through the vaginal mucosa. J Virol. 2004;78:2530–2536. doi: 10.1128/JVI.78.5.2530-2536.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund JM, Linehan MM, Iijima N, Iwasaki A. Cutting Edge: Plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J Immunol. 2006;177:7510–7514. doi: 10.4049/jimmunol.177.11.7510. [DOI] [PubMed] [Google Scholar]
- Malkin JE. Epidemiology of genital herpes simplex virus infection in developed countries. Herpes. 2004;11 Suppl 1:2A–23A. [PubMed] [Google Scholar]
- Mbopi-Keou FX, Belec L, Dalessio J, Legoff J, Gresenguet G, Mayaud P, Brown DW, Morrow RA. Cervicovaginal neutralizing antibodies to herpes simplex virus (HSV) in women seropositive for HSV Types 1 and 2. Clin Diagn Lab Immunol. 2003;10:388–393. doi: 10.1128/CDLI.10.3.388-393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi W, Prentice TW, Young CR, Johnson RR, Sieve AN, Meagher MW, Welsh CJ. Restraint stress decreases virus-induced pro-inflammatory cytokine mRNA expression during acute Theiler’s virus infection. J Neuroimmunol. 2006;178:49–61. doi: 10.1016/j.jneuroim.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Morahan PS, Breinig MC, McGeorge MB. Immune responses to vaginal or systemic infection of mice with herpes simplex virus type 2. IARC Sci Publ. 1978:759–763. [PubMed] [Google Scholar]
- Moretta L, Ferlazzo G, Bottino C, Vitale M, Pende D, Mingari MC, Moretta A. Effector and regulatory events during natural killer-dendritic cell interactions. Immunol Rev. 2006;214:219–228. doi: 10.1111/j.1600-065X.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- Nair A, Bonneau RH. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol. 2006;171:72–85. doi: 10.1016/j.jneuroim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Nair A, Hunzeker J, Bonneau RH. Modulation of microglia and CD8(+) T cell activation during the development of stress-induced herpes simplex virus type-1 encephalitis. Brain Behav Immun. 2007;21:791–806. doi: 10.1016/j.bbi.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Ortiz GC, Sheridan JF, Marucha PT. Stress-induced changes in pathophysiology and interferon gene expression during primary HSV-1 infection. Brain Behav Immun. 2003;17:329–338. doi: 10.1016/s0889-1591(03)00027-8. [DOI] [PubMed] [Google Scholar]
- Parr MB, Kepple L, McDermott MR, Drew MD, Bozzola JJ, Parr EL. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest. 1994;70:369–380. [PubMed] [Google Scholar]
- Puzanov IJ, Bennett M, Kumar V. IL-15 can substitute for the marrow microenvironment in the differentiation of natural killer cells. J Immunol. 1996;157:4282–4285. [PubMed] [Google Scholar]
- Reading PC, Whitney PG, Barr DP, Smyth MJ, Brooks AG. NK cells contribute to the early clearance of HSV-1 from the lung but cannot control replication in the central nervous system following intranasal infection. Eur J Immunol. 2006;36:897–905. doi: 10.1002/eji.200535710. [DOI] [PubMed] [Google Scholar]
- Roberts CM, Pfister JR, Spear SJ. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex Transm Dis. 2003;30:797–800. doi: 10.1097/01.OLQ.0000092387.58746.C7. [DOI] [PubMed] [Google Scholar]
- Salvucci LA, Bonneau RH, Tevethia SS. Polymorphism within the herpes simplex virus (HSV) ribonucleotide reductase large subunit (ICP6) confers type specificity for recognition by HSV type 1-specific cytotoxic T lymphocytes. J Virol. 1995;69:1122–1131. doi: 10.1128/jvi.69.2.1122-1131.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samra Z, Scherf E, Dan M. Herpes simplex virus type 1 is the prevailing cause of genital herpes in the Tel Aviv area, Israel. Sex Transm Dis. 2003;30:794–796. doi: 10.1097/01.OLQ.0000079517.04451.79. [DOI] [PubMed] [Google Scholar]
- Sawtell NM, Thompson RL, Stanberry LR, Bernstein DI. Early intervention with high-dose acyclovir treatment during primary herpes simplex virus infection reduces latency and subsequent reactivation in the nervous system in vivo. J Infect Dis. 2001;184:964–971. doi: 10.1086/323551. [DOI] [PubMed] [Google Scholar]
- Sheridan JF, Dobbs C, Jung J, Chu X, Konstantinos A, Padgett D, Glaser R. Stress-induced neuroendocrine modulation of viral pathogenesis and immunity. Ann N Y Acad Sci. 1998;840:803–808. doi: 10.1111/j.1749-6632.1998.tb09618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan JF, Feng NG, Bonneau RH, Allen CM, Huneycutt BS, Glaser R. Restraint stress differentially affects anti-viral cellular and humoral immune responses in mice. J Neuroimmunol. 1991;31:245–255. doi: 10.1016/0165-5728(91)90046-a. [DOI] [PubMed] [Google Scholar]
- Stone AA, Bovbjerg DH. Stress and humoral immunity: a review of the human studies. Adv Neuroimmunol. 1994;4:49–56. doi: 10.1016/s0960-5428(06)80189-0. [DOI] [PubMed] [Google Scholar]
- Tseng RJ, Padgett DA, Dhabhar FS, Engler H, Sheridan JF. Stress-induced modulation of NK activity during influenza viral infection: role of glucocorticoids and opioids. Brain Behav Immun. 2005;19:153–164. doi: 10.1016/j.bbi.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Vitale M, Della Chiesa M, Carlomagno S, Pende D, Arico M, Moretta L, Moretta A. NK-dependent DC maturation is mediated by TNFalpha and IFNgamma released upon engagement of the NKp30 triggering receptor. Blood. 2005;106:566–571. doi: 10.1182/blood-2004-10-4035. [DOI] [PubMed] [Google Scholar]
- Vitale M, Della Chiesa M, Carlomagno S, Romagnani C, Thiel A, Moretta L, Moretta A. The small subset of CD56brightCD16- natural killer cells is selectively responsible for both cell proliferation and interferon-gamma production upon interaction with dendritic cells. Eur J Immunol. 2004;34:1715–1722. doi: 10.1002/eji.200425100. [DOI] [PubMed] [Google Scholar]
- Welsh CJ, Bustamante L, Nayak M, Welsh TH, Dean DD, Meagher MW. The effects of restraint stress on the neuropathogenesis of Theiler’s virus infection II: NK cell function and cytokine levels in acute disease. Brain Behav Immun. 2004;18:166–174. doi: 10.1016/S0889-1591(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Wonnacott KM, Bonneau RH. The effects of stress on memory cytotoxic T lymphocyte-mediated protection against herpes simplex virus infection at mucosal sites. Brain Behav Immun. 2002;16:104–117. doi: 10.1006/brbi.2001.0624. [DOI] [PubMed] [Google Scholar]
- Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. Jama. 2006;296:964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- Yang PC, Jury J, Soderholm JD, Sherman PM, McKay DM, Perdue MH. Chronic psychological stress in rats induces intestinal sensitization to luminal antigens. Am J Pathol. 2006;168:104–114. doi: 10.2353/ajpath.2006.050575. quiz 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Nair A, Krady K, Corpe C, Bonneau RH, Simpson IA, Vannucci SJ. Estrogen stimulates microglia and brain recovery from hypoxia-ischemia in normoglycemic but not diabetic female mice. J Clin Invest. 2004;113:85–95. doi: 10.1172/JCI200418336. [DOI] [PMC free article] [PubMed] [Google Scholar]


