Abstract
Background
To assess the influence of therapy crossovers on treatment comparisons and mortality at 5 years in patients with ischemic heart disease and heart failure randomly assigned to medical therapy alone (MED) or to MED and coronary artery bypass surgery (CABG) in the Surgical Treatment for Ischemic Heart Failure (STICH) trial.
Methods and Results
The influence of early crossover (within the first year following randomization) on 5-year mortality was assessed using time-dependent multivariable Cox models. CABG was performed in 65/602 (10.8%) patients assigned to MED and 55/610 (9.0%) patients assigned to CABG received MED only. Common reasons for crossover from MED to CABG were progressive symptoms or acute decompensation. MED-assigned patients who underwent CABG had lower 5-year mortality than those who received MED only (25% vs. 42%; hazard ratio (HR) 0.50 confidence interval (CI) 0.30-0.85, p=0.008). The main reason for crossover from CABG to MED was patient/family decision. Five patients did not undergo their assigned CABG within a year but died before receiving surgery without status change. They were deemed crossover to MED. The CABG to MED crossover population had higher 5-year mortality compared to those treated with CABG per protocol (59% vs. 33%; HR: 2.01; CI 1.36-2.96, p<0.001). CABG was associated with lower mortality compared to MED in per protocol and several time-dependent analyses (all p<0.05).
Conclusions
CABG reduced mortality in both the per protocol and crossover STICH patient populations. Crossover from assigned therapy therefore diminished the impact of CABG on survival in STICH when analyzed by intention to treat.
Keywords: coronary bypass surgery, medical therapy, heart failure
Patient enrollment into trials evaluating the effect of a major surgical procedure is challenging. Doctors, patients, and patient families often have strong views on the merits of specific treatments that may change treatment choice in response to changing circumstances. The characteristics of patients selected for trials influence their enrollment. Even with careful study design and conduct, a substantial proportion of patients may deviate from their assigned treatment after randomization.1, 2
The surgical revascularization hypothesis of the Surgical Treatment for Ischemic Heart Failure trial (STICH) compared a strategy of guideline-indicated medical therapy alone (MED) to a similar strategy combined with coronary artery bypass graft (CABG) surgery in 1,212 patients with left ventricular systolic dysfunction (LVSD) and coronary artery disease (CAD).3 STICH is an NIH-funded, international multi-center trial conducted at 96 hospitals with documented expertise in the treatment of patients with heart failure. Based on a median follow-up of 56 months, intention-to-treat analysis demonstrated a trend toward reduced all-cause mortality in those assigned to CABG (HR 0.86, 95 % CI 0.72-1.04, p=0.123) but an a priori as-treated comparison suggested a survival benefit for CABG (HR 0.70, 95% CI 0.58-0.84, P < 0.001).3 The STICH Extension Study (STICHES) will follow patients for five additional years and will provide definitive information in due course. In the interim, physicians and surgeons must use the best available evidence in order to advise patients about the need for coronary angiography and revascularization. Since the difference between the intention-to-treat and the as-treated analyses is most likely caused by the patients not following their assigned treatment (crossovers), we analyzed all crossover events specifically for their reasons of crossover. We here report these reasons and the subsequent outcome after crossover and the effect of those crossovers on the primary intention-to-treat analysis in the STICH trial.
Methods
Trial Design Provision for Crossover
In the STICH trial, the informed consent process used standardized videos, written information and discussions with investigators to inform patients that consenting to the study meant they were willing to accept either medical treatment alone or medical treatment with CABG. Patients who declined to participate were free to choose their preferred treatment strategy. Patients who did consent were also informed that they were able to withdraw consent at any time.
The STICH protocol specified that pharmacological treatments should be optimized early after randomization for all patients.4 For patients assigned to CABG, the operation should be done within 14 days of randomization. Randomization was accomplished using a telephone-based interactive voice response system. As set by the trial’s protocol, the reasons for crossover were recorded only in the first year and the clinical information requested from the sites within the first year was free text and not prestructured responses guided by pre-specified definitions. The rationale for this protocol set up was the expected imbalance in early mortality between the MED and CABG cohorts. The current report therefore addresses all crossover events that occurred in the first year (78,4% of all crossovers).
Detection and Documentation of Treatment Crossover
An early report was obtained in all patients at hospital discharge or 30 days after randomization. Subsequent follow-up clinical data were collected at 4, 8, and 12 months and at six-month intervals thereafter for the study duration. Patients assigned to CABG who did not receive surgery within one year after randomization were defined as crossover from CABG-to-MED. Patients who were assigned to MED who had CABG within one year after randomization were defined as an early crossover from MED-to-CABG. For each early crossover event, free text narrative documents were collected by the investigative teams. Categorization of crossover reasons was performed based on these documents. No attempt was made to identify the reasons for late cross-over after one year from MED-to-CABG and such events were not considered in this analysis.
Categorization of Crossover Reasons
Three authors (TD, JR, RJ) used a two-step Delphi process to develop four categories of MED-to-CABG crossover reasons and four categories of reasons for CABG-to-MED crossovers based on the perceived susceptibility of the early crossover event to reflect possible bias of the enrolling investigators. The four categories created for MED-to-CABG crossover were: 1) progressive symptoms (i.e., worsening of angina or of heart failure or of the combination), 2) acute decompensation (i.e., heart failure, myocardial infarction or angina; cardiac arrest or ventricular arrhythmias, or endocarditis), 3) clinician decision despite stable symptoms, 4) patient/family decision. The least to most susceptible to bias categories of reasons created for CABG-to-MED crossover from least to most susceptible to investigator bias were: 1) patient/family decision, 2) died before operation, 3) clinical decision, 4) research staff/provider miscommunication.
Documentation of Risk at Randomization
A risk at randomization (RAR) index was calculated for each patient enrolled in the study to assess the treatment effect of CABG beyond that of MED.5 This predicted risk of 5-year mortality, assuming MED only treatment, was based on prognostic factors identified from a multivariable Cox model analysis developed in a completely independent database of patients, namely STICH-eligible patients in the Duke Databank for Cardiovascular Diseases.5 In the present report, the 1,212 patients of the surgical revascularization hypothesis of the STICH trial were clustered into one of three tertile RAR groupings (RAR 1-6; 7-16; 17-32) to assess the influence of baseline risk on crossover occurrence. In addition, the 5-year mortality rates of the 1,092 patients who received their assigned randomized treatment of MED (N = 537) or CABG (N = 555) per protocol and the 5-year mortality of the MED-to-CABG crossovers and the CABG-to-MED crossovers were tabulated by RAR grouping to help define the relationship of baseline risk to treatment effect on mortality.
Statistical Methods
Hazard ratios and associated 95% confidence intervals for comparing CABG vs. MED with respect to all-cause mortality were calculated with CABG as a time-dependent variable expanding on our previously presented analysis in the primary report.4 A total of five different methods to account for death before CABG and for early crossover to CABG of patients assigned to MED are presented (Figure 1). A 0/1 time-dependent covariate was created to reflect the CABG-free interval after randomization and used with the Cox model to indicate whether and when a patient received CABG, thereby allowing an assessment of the CABG treatment effect from multiple different clinical scenarios. One of the modeling strategies initially set the time-dependent covariate to 0 for all 1,212 H1 patients (no CABG) and changed the covariate value to 1 (indicating CABG was performed) on the day the operation occurred. This approach can be viewed as an “as treated” analysis, and survival time prior to CABG (in the patients who undergo CABG) is thus credited to medical therapy. A second and closely related strategy assigned a value of 1 at the time of randomization to all patients randomized to CABG who actually underwent the operation. Two additional analyses were performed in which patients randomized to CABG but who died early before receiving CABG (within 30 days or within 60 days of randomization respectively) were credited to CABG (i.e., early deaths among patients randomized to CABG that occurred before CABG was performed were attributed to the CABG arm). A fifth analysis was done by assigning 1 to all patients randomized to CABG at the time of randomization regardless of whether or not CABG was ever performed. In this latter analysis, all patients randomized to CABG were thus counted with the CABG patients regardless of whether they received CABG, whereas the CABG variable was set to 0 for MED-assigned patients at the time of randomization and only changed to 1 on the day of CABG as a crossover operation. This family of models provides a range of assessments of the treatment effects of CABG depending on various different ways of accounting for patients randomized to MED who crossed over to CABG and of delay or failure to undergo a timely CABG operation in patients randomized to the CABG arm.
Figure 1.
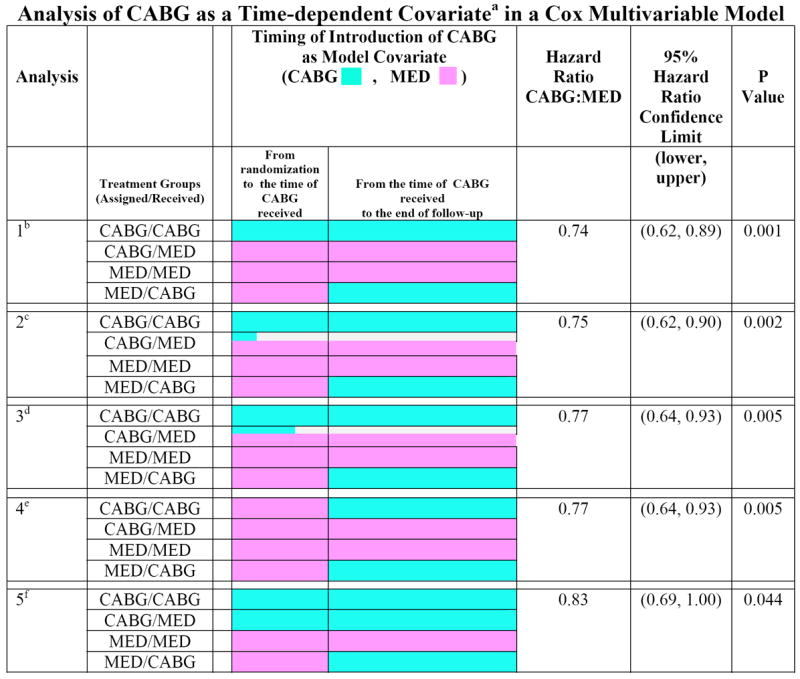
Time-dependent covariate Cox multivariable analysis of CABG vs. MED.
a. A numeric (0, 1) time-dependent covariate was created in the Cox model to indicate whether and when a patient received CABG, with the format of 1 = CABG and 0= MED. This variable allows an assessment of the CABG treatment effect to begin at the time that a patient actually received CABG. A patient is counted in the MED group until the CABG variable is switched to 1.
b. Analysis 1 has the CABG variable initially set to 1 for all patients who were assigned to CABG and actually received CABG. For patients assigned to MED who crossed over to CABG, the CABG variable is started as 0 and set to 1 at the time of the crossover. For all other patients (i.e., those assigned to MED who received MED, and assigned to CABG but did not receive CABG), the time-dependent CABG variable remains as 0 in the Cox model.
c. Analysis 2 is the same as Analysis 1 except that early deaths in patients randomized to CABG are handled differently. In this analysis, patients who were assigned to CABG but died within 30 days after randomization without receiving CABG are counted as CABG=1. These patients are not counted as MED patients (as in Analysis 1) even though they never received CABG. Thus, these early deaths are credited to the CABG arm.
d. Analysis 3 is the same as Analysis 2 except that patients who died within 60 days after randomization before receiving CABG are all counted as CABG=1.
e. Analysis 4 has the CABG variable started as 0 (MED) for all 1212 Hypothesis 1 patients. For patients who received CABG treatment, the CABG variable is set to 1 on the day of surgery.
f. Analysis 5 has the CABG variable started as 1 for all patients who were randomized to CABG regardless of whether they ever received the CABG. For all the other patients (i.e., MED patients), the CABG variable is started as 0 and switched to 1 at the time of CABG for any patients who crossed over from MED to CABG.
Results
Of 602 patients assigned to MED, 537 (89%) remained in their assigned group and 65 crossed over to CABG within the first year following randomization. There were 35 additional MED patients who received CABG later during follow up. Of 610 patients assigned to CABG, 555 (91%) received CABG within the first year after randomization at a median of 10 days (interquartile range 5-16 days). The 55 CABG-assigned patients who did not receive CABG within 1 year of randomization were considered to have crossed over to MED.
Figure 2 shows the Kaplan-Meier (K-M) curves for the per protocol and the crossover cohorts. Patients who were assigned to and received CABG in the first year had a lower five-year mortality than MED patients who remained in their assigned group (HR 0.76, 95% CI 0.62-0.92, p=0.005). The 65 patients who were assigned to MED but received CABG had a lower five-year mortality (14 deaths; K-M 5-year rate 25%) than the 537 patients (208 deaths; K-M 5-year rate 42%) randomized to MED who remained in their assigned group (HR 0.50, 95% CI 0.30-0.85, p=0.008). In contrast, the 55 patients assigned to CABG who did not receive surgery within one year of randomization had a higher five-year mortality (29 deaths; K-M 5-year rate 59%) than the 555 patients (167 deaths; K-M 5-year rate 33%) who were randomized to and received CABG within one year (HR 2.01, 95% CI 1.36-2.96, P<0.001). In order to address the question why the CABG to MED crossovers had the worst and the MED to CABG crossovers had the best outcomes, we analyzed in details the reasons for crossover (Figure 3) and the influence of individual risks as assessed by the RAR score (Table 1).
Figure 2.
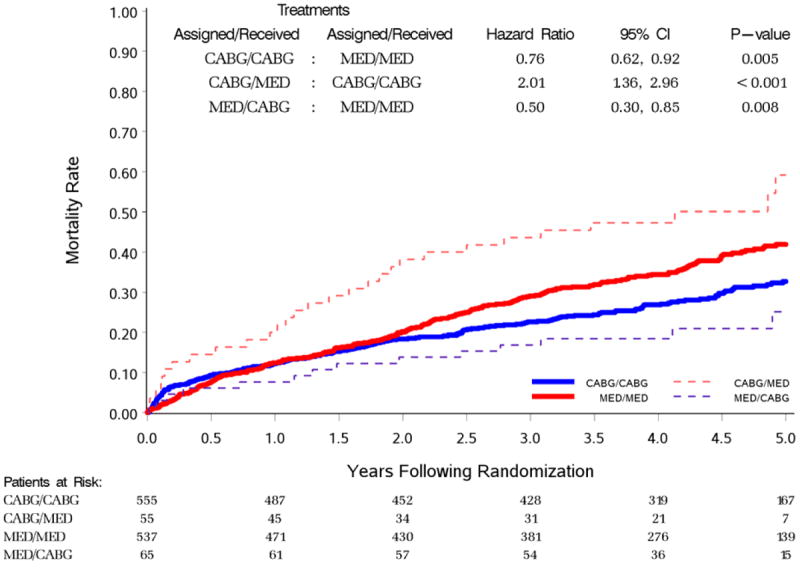
Kaplan-Meier analysis of patients assigned to CABG (blue lines) or MED (red lines) either adhering (per protocol) or not adhering (crossover) to their randomly assigned treatment.
Figure 3.
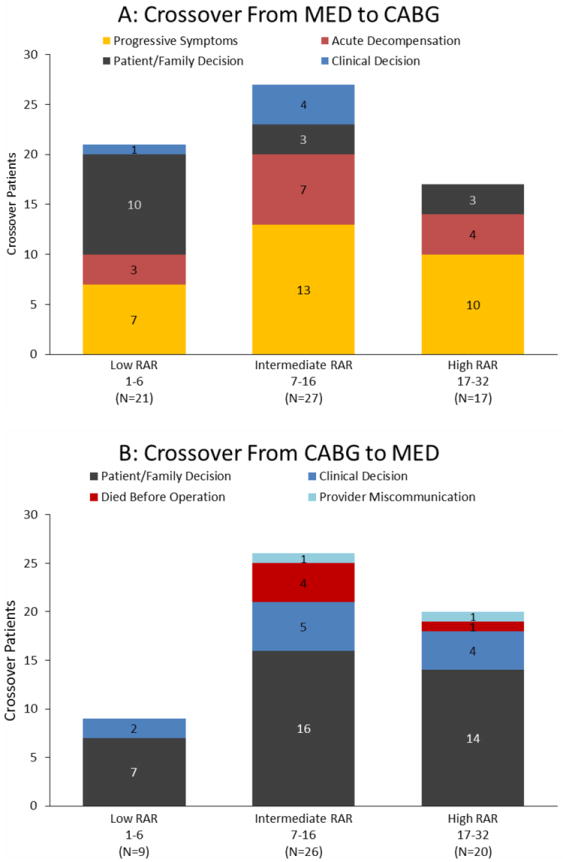
Crossover by reason (as adjudicated by a committee) with increasing risk at randomization (RAR) indices. The reasons for crossover are shown for MED to CABG crossovers (panel A) and CABG to MED crossovers (panel B).
Table 1.
Influence of Risk at Randomization on Difference in 5-Year Mortality Rate of 1212 Patients Randomized MED (n=602) or CABG (n=610) Treatment
| Patient Population | Parameters | Treatment Group | Low 1-6 | RAR Tertile Groups Intermediate 7-16 | High 17-32 |
|---|---|---|---|---|---|
| Patients Received Randomized Treatment Per Protocol | Number of Patients | ALL (N=1092) | N=349 | N=376 | N=367 |
| Kaplan-Meier Estimates of 5-Year Death Rate1 | MED | 34% (±4%) | 41% (±4%) | 50% (±4%) | |
| CABG | 21% (±3%) | 30% (±4%) | 46% (±4%) | ||
|
| |||||
| Crossover Patients | Number of Patients | ALL (N=120) | N=30 | N=53 | N=37 |
| Kaplan-Meier Estimates of 5-Year Death Rate1 | Crossover to MED | 22% (±14%) | 69% (±11%) | 56% (±11%) | |
| Crossover to CABG | 10% (±6%) | 29% (±12%) | 37% (±12%) | ||
Kaplan-Meier rates are reported in the format of “Death rate (± Standard Error)”.
The adjudicated reasons for early crossover from MED to CABG are shown in Figure 3A and from CABG to MED in Figure 3B. Patients were grouped according to their RAR score as low (1-6), intermediate (7-16) and high risk (17-32). The main reason for crossover from MED to CABG was acute decompensation or progressive worsening of status/symptoms (44 of 65 patients). The most common reason for crossover from CABG to MED was a decision change by the patients or their families (37 of 55 patients). The clinical investigator responsible for the care of each patient made the decision to crossover from CABG to MED in only 11 cases. In 5 patients, the reason for crossover was deemed “died before operation,” because no unavoidable reason for the interval between randomization and death was described in the free-text documents. Only 2 of these deaths occurred before the 14-day interval after randomization specified by protocol as the acceptable interval between randomization and CABG. Appendix 1 provides a listing of all 120 early crossover patients with extraction of phrases from free text documents provided by STICH investigators that best reflect the rationale for crossover of each patient.
Table 1 shows the relationship between risk at baseline (RAR score groups as in Figure 2) and five-year mortality rates for patients who received their assigned therapy (per-protocol) as well as for those having crossed over. Five-year mortality rate rose as baseline risk increased but in each case, mortality rates were lower in patients receiving CABG. Based on the information in Table 1 and Figure 3, no pattern indicative of outcome in the two crossover populations could be identified.
Table 2 shows the baseline risk spectrum of the per-protocol and the crossover patients. Patients assigned to MED who crossed over to CABG had more severe symptoms of angina (p=0.004), heart failure (p=0.003), and a lower 6-minute walk distance (p=0.024) compared to the patients assigned to MED who did not cross over. In contrast, patients assigned to CABG that crossed over to MED included more patients with previous bypass surgery (p=0.01), more prior PCI (p=0.056), and larger LVESVI (p=0.036) compared to the patients assigned to CABG who did not cross over. However, none of these differences explain the outcome, because the majority of patients crossing from CABG to MED did so based on a family or patient decision and none of the patients differed in the individual risk (as assessed by the RAR score) from the per protocol patients at the time of randomization.
Table 2.
Patient Characteristics According to Management
| Randomized to MED | Randomized to CABG | |||||
|---|---|---|---|---|---|---|
| Rec. MED (per protocol) (n=537) | Rec. CABG (crossover) (n=65) | P | Rec. MED (crossover) (n=55) | Rec. CABG (per protocol) (n=555) | p | |
| Age (years) | 59(54, 67) | 57 (51, 65) | 0.170 | 61 (57, 69) | 60 (54, 68) | 0.237 |
| Women (%) | 11.9% | 16.9% | 0.249 | 10.9% | 12.1% | 0.800 |
| White (%) | 70.2% | 66.2% | 0.502 | 61.8% | 67.2% | 0.419 |
| Black or other(%) | 29.8% | 33.8% | 38.2% | 32.8% | ||
| Body mass index, median (kg/m2) | 27 (24, 30) | 26 (24, 29) | 0.842 | 26 (24, 29) | 27(24, 30) | 0.226 |
| Medical history(%) | ||||||
| Myocardial infarction | 78.4% | 78.5% | 0.991 | 74.5% | 75.9% | 0.829 |
| Hyperlipidemia | 61.5% | 62.5% | 0.871 | 47.3% | 60.3% | 0.061 |
| Hypertension | 62.6% | 52.3% | 0.108 | 50.9% | 59.5% | 0.219 |
| Diabetes | 40.8% | 29.2% | 0.072 | 40.0% | 39.3% | 0.917 |
| Current smoker | 19.2% | 29.2% | 0.058 | 27.3% | 20.7% | 0.258 |
| Previous percutaneous coronary intervention | 12.3% | 12.3% | 0.997 | 21.8% | 12.6% | 0.056 |
| Chronic renal Insufficiency | 7.8% | 4.6% | 0.460 | 14.5% | 7.4% | 0.071 |
| Stroke | 6.7% | 7.7% | 0.793 | 5.5% | 8.6% | 0.609 |
| PVD | 14.2% | 29.2% | 0.002 | 10.9% | 15.0% | 0.418 |
| Previous CABG | 2.6% | 0.0% | 0.383 | 10.9% | 2.9% | 0.010 |
| Current CCS angina class | ≤0.0014 | 0.255 | ||||
| No angina | 39.5% | 20.0% | 29.1% | 36.2% | ||
| I | 15.5% | 12.3% | 16.4% | 15.7% | ||
|
| ||||||
| II | 41.2% | 60.0% | 47.3% | 43.1% | ||
| III / IV | 4.0% | 7.7% | 7.2% | 5.0% | ||
|
| ||||||
| Current NYHA heart failure class | 0.003 | 0.069 | ||||
| I | 13.0% | 6.2% | 9.1% | 10.8% | ||
| II | 52.3% | 40.0% | 41.8% | 53.3% | ||
| III / IV | 34.6% | 53.9% | 49.1% | 35.9% | ||
| Systolic blood pressure, median mm Hg | 120 (110, 130) | 120 (110, 130) | 0.224 | 120 (110, 140) | 120 (110, 130) | 0.836 |
| Pulse, median beats/min | 72 (65, 80) | 74 (68, 80) | 0.858 | 72 (65, 84) | 74 (66, 82) | 0.502 |
| Able to perform 6-minute walk | 89.2% | 92.2% | 0.455 | 72.2% | 84.7% | 0.019 |
| 6-minute walk distance, median ft | 1139 (878, 1348) | 984 (738, 1247) | 0.024 | 984 (853, 1290) | 1148 (875, 1325) | 0.356 |
| No. diseased vessels (≥ 75%) | 0.648 | 0.859 | ||||
| ≤1 | 26.1% | 29.3% | 26.0% | 24.2% | ||
| 2 | 38.4% | 35.4% | 37.0% | 38.4% | ||
| 3 | 35.6% | 35.4% | 37.0% | 37.5% | ||
| Proximal LAD≥ 75% | 68.7% | 70.8% | 0.735 | 61.1% | 68.1% | 0.295 |
| LVEF (site-reported) | 27 (22, 30) | 30 (25, 33) | 0.003 | 27 (24, 31) | 27 (22, 31) | 0.993 |
| LVEF (core lab + site) | 28 (21, 34) | 27 (23, 32) | 0.579 | 25 (19, 33) | 27 (22, 33) | 0.306 |
|
| ||||||
| LVESVI (core lab + site) | 79 (58, 108) | 78 (64, 95) | 0.888 | 92 (73, 111) | 78 (61, 101) | 0.036 |
|
| ||||||
| Mitral regurgitation (site-reported) | 0.333 | 0.446 | ||||
| None or trace | 38.0% | 29.2% | 27.3% | 35.7% | ||
| Mild | 42.5% | 52.3% | 49.1% | 47.9% | ||
| Moderate (3+) | 16.7% | 13.8% | 18.2% | 13.2% | ||
| Severe (4+) | 2.8% | 4.6% | 5.5% | 3.2% | ||
| RAR score | 11 (5, 20) | 10 (5, 17) | 0.588 | 12 (7, 20) | 11 (5, 19) | 0.136 |
Data shown are median and inter-quartile range or proportions as a percentage.
In order to assess the influence of time of crossover on outcome, we performed various Cox multivariable statistical models using CABG as a time-dependent variable. The results are graphically illustrated in Figure 1. Relative to the time of randomization, the figure depicts when the time-dependent covariate in the multivariable model was set or changed for a given patient to reflect the period of follow-up in which the patient was counted as a CABG patient. In the first analysis depicted in the figure, patients randomized to receive CABG were included in the CABG group from the time they were randomized as long as they had CABG at some time within the following year. Patients randomized to MED were all initially included in the MED group, but those who crossed over to CABG within the first year were shifted to the CABG cohort on the date of surgery. This strategy produced the lowest CABG: MED hazard ratio (0.74) and the smallest p-value (P = 0.001), which suggests that the MED to CABG crossover event did not detract from the survival advantage contributed by CABG to the total 1,212-patient cohort. Analysis strategy 5 in Figure 1 represents a scenario in which all patients randomized to CABG, including patients who “died before surgery” and all other CABG to MED crossovers, were counted in the CABG group. Among patients randomized to MED, the MED to CABG crossovers were counted with the CABG group once they received surgery. Even in this analysis, which may be considered the least biased towards surgery and which differed from the primary outcome report only by treating the MED/CABG crossovers differently, the HR was 0.83 (CI 0.69-1.00) and still significant (p=0.044). The other analysis scenarios depicted in Figure 1 considered different time frames of counting patients in one or the other group. They produced results that were intermediate between those of analyses 1 and 5, all showing a significant favorable effect of CABG. In other words, the figure shows that significance level of the investigated difference is the greater the earlier the crossover event was considered in the analysis.
Discussion
Coronary artery disease (CAD) is the most common cause of heart failure associated with left ventricular systolic dysfunction (LVSD).3 Effective treatment of CAD should retard or reverse the progression of LVSD and heart failure. Medical treatments, such as beta-blockers6 and ACE inhibitors,7 appear to be effective for both heart failure and CAD. However, interventions directed solely at CAD, including aspirin8, 9 and statins,10, 11 have met with little success when applied to patients enrolled in trials on the basis of heart failure. Historically, trials of coronary revascularization have excluded patients who had either heart failure or substantial LVSD.
Recently, the STICH trial failed to show a statistically significant reduction in the primary endpoint of all-cause mortality by intention to treat analysis.3 However, there were a number of treatment crossovers during patient follow-up in the trial, and the as treated analysis demonstrated that all patients who received CABG had a lower mortality. Furthermore CABG showed a significant improvement compared to MED for secondary endpoints such as survival free of cardiac hospitalization.3 These observations suggest that CABG may reduce mortality but that crossovers between assigned groups during the trial diluted the effect of the intervention.
The NIH-funded Coronary Artery Surgery Study (CASS) compared survival of 780 patients with LVEF of ≥0.35 randomized to MED or CABG.2 During 10 years of follow-up of the cohort randomized to MED, CABG was performed in 6% of patients within 6 months of randomization2 compared to the 11% crossover of STICH patients randomized to MED who had CABG within 1 year. Of the CASS patients assigned to CABG, 11% remained on MED only at 6 months after randomization2 compared to 9% of CABG-assigned patients in STICH who remained on MED 1 year after randomization. Therefore, the rate of crossover in STICH was comparable to that of CASS despite the management challenges imposed in STICH by the patients with more severe LV dysfunction. Moreover, in response to comments of others regarding outcomes of MED-to-CABG crossovers in CASS stating “the introduction of moribund patients into the surgical group would bias results against operation,” Fisher, et al12 countered by pointing out that in the CASS trial, MED-to-CABG crossovers had a lower mortality rate than the original patients who remained compliant with the CABG treatment assignment. This concordance in outcome of crossover events between MED-to-CABG crossover cohorts of CASS and STICH patients most likely reflects the entry criteria at baseline that required knowledge of the coronary anatomy at the time of randomization. Because patients in both clinical trials were known by investigators responsible for their clinical care, appropriate evaluation and treatment could be expedited in response to deterioration of clinical status of the patient.
Management of patients with long-term medical conditions, such as heart failure, requires continuing evaluation and adjustment of treatment according to changing circumstances. This is also true in clinical trials. Randomization reflects a decision at a particular time to implement a certain strategy, but if the patient’s condition changes from baseline after randomization, the management strategy must also change to reflect the usual standard of care for patient safety. In this respect, crossover remains part of the original design of STICH. STICH is a study comparing the two treatment strategies of CABG versus no CABG. Retrospective analysis of reasons for early crossover now also allows STICH to be considered a trial of early routine compared to delayed and highly selective surgical revascularization. We found that crossover events could not be pinpointed to a specific subset of patients who in retrospect might have been inappropriate for randomization upon initial evaluation. Table 2 demonstrates the baseline clinical profiles to reflect a broad spectrum of risk of the crossover patients. The few patients (1.8%) who did cross from CABG to MED because the responsible clinician felt CABG was no longer in the patient’s best interest had similar baseline RAR scores to patients crossing over from MED to CABG. Thus, as there was no way to easily identify patients who were treated medically that would eventually decompensate and require CABG without a demonstrable increased mortality, the results of STICH should not be interpreted as suggesting that a delay to proceeding with CABG is warranted in routine clinical practice. However, in common with many other clinical trials, the median age of patients in STICH was substantially lower than in epidemiological cohorts of patients with heart failure and coronary disease. The results of STICH should therefore be extrapolated with care to older patients with heart failure and multiple co-morbidities where operative risk may be increased.
The STICH study was powered to show a 25% reduction in mortality with CABG compared to pharmacological therapy using an ITT analysis allowing for crossover rates of up to 20% for the duration of the study. However, use of K-M analysis for comparison of data with early crossing of survival curves has limited applicability for guiding physician and patient decisions about accepting the higher early risk of CABG with the hope of longer survival once they are safely through the operative risk. Technically, K-M curves that cross violate the proportional hazards assumptions. Physicians and surgeons can confidently say about patients meeting the STICH inclusion criteria that the initial risk of CABG always will be higher than an additional day of MED treatment.
The role of this report is therefore to provide data on survival of all 1,212 STICH surgical revascularization hypothesis patients as individuals who also can be considered to be part of one of four observational cohorts – those who were and those who were not compliant with their randomized treatment assignment. This type of analysis introduces bias into the analysis. Here it is important to realize, that the bias was by definition not in the “per-protocol” patients. They were compliant with their assigned treatment. Any potential bias resided in the crossover patients, and that bias can never be understood without placing the early crossover events in the context of data available only in this current manuscript. The current manuscript therefore complements our primary publication and in no way contradicts the conclusion of the primary report.3 Nevertheless, without the message of this paper in the literature, the full message of the STICH surgical revascularization hypothesis cohort would never be complete.
Conclusion
CABG reduced mortality in both the per protocol and crossover STICH patient populations. The crossover events from randomly assigned therapy therefore diminished the impact of CABG on survival in STICH when analyzed by intention to treat. Until the 10-year outcomes (STICHES) are available, STICH-like patients should be informed about the 5-year outcome results of the STICH surgical revascularization hypothesis patients prior to making their own treatment decision.
Supplementary Material
The international, multi-center STICH trial (Surgical Treatment of IsChemic Heart failure) had identified a 14% relative risk reduction of bypass surgery (CABG) vs. optimal medical therapy (MED). However, this risk reduction was not statistically significant. We illustrate in this manuscript that crossover events within the first year of randomization diluted the difference between the two treatment options because all MED patients had higher 5-year mortality than all CABG patients. Importantly, we analyzed the reasons for such crossover events and were unable to identify predictable patterns or risk profiles that characterized the crossover patients. In other words, we provide strong support for the conclusion that crossover events were random and not associated with the patients perceived risk at the time of randomization or thereafter. This information should therefore be helpful for advising all patients with systolic heart failure and coronary artery disease amenable for bypass surgery with respect to treatment options until definitive information on all-cause mortality is available by the STICH extension study (STICHES).
Acknowledgments
Sources of Funding
The STICH trial was supported by grants U01HL69015 and U01HL69013.
Footnotes
Disclosures
Dr. Doenst received fees for consulting from B. Braun, St. Jude Medical and Edwards and research grants from B.Braun and the German Research Foundation (DFG)
Dr. Ohman received consulting fees from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Gilead Sciences, Janssen Pharmaceuticals, Liposcience, Merck, Pozen Inc, Roche, Sanofi Aventis, The Medicines Company and research grants from Daiichi Sankyo, Eli Lilly & Company, Gilead Sciences
Dr. Velazquez received consulting fees from Gilead and Novartis and research grants from Abbott. Vascular and Ikaria
References
- 1.Jones RH, Velazquez EJ, Michler RE, Sopko G, Oh JK, O’Connor CM, Hill JA, Menicanti L, Sadowski Z, Desvigne-Nickens P, Rouleau JL, Lee KL. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009;360:1705–1717. doi: 10.1056/NEJMoa0900559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alderman EL, Bourassa MG, Cohen LS, Davis KB, Kaiser GG, Killip T, Mock MB, Pettinger M, Robertson TL. Ten-year follow-up of survival and myocardial infarction in the randomized Coronary Artery Surgery Study. Circulation. 1990;82:1629–1646. doi: 10.1161/01.cir.82.5.1629. [DOI] [PubMed] [Google Scholar]
- 3.Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT, Yii M, Prabhakaran D, Szwed H, Ferrazzi P, Petrie MC, O’Connor CM, Panchavinnin P, She L, Bonow RO, Rankin GR, Jones RH, Rouleau JL. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–1616. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Velazquez EJ, Lee KL, O’Connor CM, Oh JK, Bonow RO, Pohost GM, Feldman AM, Mark DB, Panza JA, Sopko G, Rouleau JL, Jones RH. The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg. 2007;134:1540–1547. doi: 10.1016/j.jtcvs.2007.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones RH, White H, Velazquez EJ, Shaw LK, Pietrobon R, Panza JA, Bonow RO, Sopko G, O’Connor CM, Rouleau JL. STICH (Surgical Treatment for Ischemic Heart Failure) trial enrollment. J Am Coll Cardiol. 2010;56:490–498. doi: 10.1016/j.jacc.2009.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleland JG. Beta-blockers for heart failure: why, which, when, and where. Med Clin North Am. 2003;87:339–371. doi: 10.1016/s0025-7125(02)00173-6. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf S, Pogue J. ACE inhibition in stable coronary artery disease. N Engl J Med. 2005;352:937–939. doi: 10.1056/NEJM200503033520919. [DOI] [PubMed] [Google Scholar]
- 8.Cleland JG, Findlay I, Jafri S, Sutton G, Falk R, Bulpitt C, Prentice C, Ford I, Trainer A, Poole-Wilson PA. The Warfarin/Aspirin Study in Heart failure (WASH): a randomized trial comparing antithrombotic strategies for patients with heart failure. Am Heart J. 2004;148:157–164. doi: 10.1016/j.ahj.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Massie BM, Collins JF, Ammon SE, Armstrong PW, Cleland JG, Ezekowitz M, Jafri SM, Krol WF, O’Connor CM, Schulman KA, Teo K, Warren SR. Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure: the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial. Circulation. 2009;119:1616–1624. doi: 10.1161/CIRCULATIONAHA.108.801753. [DOI] [PubMed] [Google Scholar]
- 10.Gullestad L, Ueland T, Kjekshus J, Nymo SH, Hulthe J, Muntendam P, Adourian A, Bohm M, van Veldhuisen DJ, Komajda M, Cleland JG, Wikstrand J, McMurray JJ, Aukrust P. Galectin-3 predicts response to statin therapy in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) European heart journal. 2012;33:2290–2296. doi: 10.1093/eurheartj/ehs077. [DOI] [PubMed] [Google Scholar]
- 11.Latini R, Gullestad L, Masson S, Nymo SH, Ueland T, Cuccovillo I, Vardal M, Bottazzi B, Mantovani A, Lucci D, Masuda N, Sudo Y, Wikstrand J, Tognoni G, Aukrust P, Tavazzi L. Pentraxin-3 in chronic heart failure: the CORONA and GISSI-HF trials. European journal of heart failure. 2012;14:992–999. doi: 10.1093/eurjhf/hfs092. [DOI] [PubMed] [Google Scholar]
- 12.Fisher LD, Kaiser GC, Davis KB, Mock MB. Crossovers in coronary artery bypass grafting trials: desirable, undesirable, or both? The Annals of thoracic surgery. 1989;48:465–466. doi: 10.1016/s0003-4975(10)66840-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


