Abstract
BACKGROUND
Angiotension receptor blockers (ARB), telmisartan and valsartan were compared for renal protection in spontaneously hypertensive rats (SHR) fed high fat diet. We hypothesized that in cardiometabolic syndrome, telmisartan an ARB with PPAR-γ activity will offer better renal protection.
METHODS
SHR were fed either normal (SHR-NF, 7% fat) or high fat (SHR-HF, 36% fat) diet and treated with an ARB for 10 weeks.
RESULTS
Blood pressure was similar between SHR-NF (190±3 mmHg) and SHR-HF (192±4 mmHg) at the end of the 10 week period. Telmisartan and valsartan decreased blood pressure to similar extents in SHR-NF and SHR-HF groups. Body weight was significantly higher in SHR-HF (368±5g) compared to SHR-NF (328±7g). Telmisartan but not valsartan significantly reduced the body weight gain in SHR-HF. Telmisartan was also more effective than valsartan in improving glycemic and lipid status in SHR-HF. Monocyte chemoattractant protein-1 (MCP-1), an inflammatory marker, was higher in SHR-HF (24±2 ng/d) compared to SHR-NF (14±5 ng/d). Telmisartan reduced MCP-1 excretion in both SHR-HF and SHR-NF to a greater extent than valsartan. An indicator of renal injury, urinary albumin excretion increased to 85±8 mg/d in SHR-HF compared to 54±9 mg/d in SHR-NF. Telmisartan (23±5 mg/d) was more effective than valsartan (45±3 mg/d) in lowering urinary albumin excretion in SHR-HF. Moreover, telmisartan reduced glomerular damage to a greater extent than valsartan in the SHR-HF.
CONCLUSIONS
Collectively, our data demonstrate that telmisartan was more effective than valsartan in reducing body weight gain, renal inflammation, and renal injury in a rat model of cardiometabolic syndrome.
Keywords: cardiometabolic syndrome, renin-angiotensin system, high fat diet, hypertension, inflammation, renal injury
INTRODUCTION
Cardiometabolic syndrome generally occurs with obesity and consists of a constellation of patho-physiological factors including insulin resistance, hypertension and hyperlipidemia that significantly increase the risk for cardiovascular diseases.1 The Adult treatment Panel II of the National Cholesterol Education Program defines metabolic syndrome as any three of the following five traits: visceral obesity, hypertension, hypertriglyceridemia, low HDL cholesterol, and impaired fasting glucose.2 Moreover, cardiometabolic syndrome increases the risk of chronic kidney diseases,3,4 hence, obese individuals with hypertension and insulin resistance usually develop renal dysfunction.5 Animal models of metabolic syndrome such as diet induced obesity models exhibit significant glomerular injury, including mesangial cell expansion, increased mesangial matrix and basement membrane thickening.6-8
In cardiometabolic syndrome, the compromised glycemic and lipid status leading to inflammation and oxidative stress contributes to the glomerular damage.6,9-11 It has also been demonstrated that hypertension per se can contribute to the renal injury as elevated blood pressure can cause mesangial cell hypertrophy, extracellular matrix production and glomerular basement thickening.12 Consequently, there has been a growing interest in antihypertensive drugs with properties that are helpful in ameliorating pathophysiological consequence of cardiometabolic syndrome and, any such drug would, indeed, be effective in slowing the progression of kidney disease in cardiometabolic syndrome.
The renin-angiotensin system (RAS) plays a pivotal role in the patho-physiological consequence of cardiometabolic syndrome and large clinical trials have demonstrated significant benefits of the blockade of RAS for end-organ protection in cardiometabolic syndrome.13-15 A number of angiotensin receptor blockers (ARBs) are available for treating hypertension in patients either with or without a co-morbid condition like diabetes. Telmisartan, an ARB with partial peroxisome proliferators activated receptor-γ (PPAR-γ) agonist activity has the ability to improve glycemic and lipid status in rats fed a high fat, high carbohydrate diet.16 There is a growing body of evidence that activators of PPAR-γ exert anti-inflammatory, anti-oxidative and anti-proliferative effects on vasculature.17-18 Hence, being a potent ARB with partial PPAR-γ agonist property telmisartan holds promise as a drug of choice for organ protection in cardiometabolic syndrome.
With this background, in the present study we aim to compare the possible renal protective effect of telmisartan to that of an ARB without PPAR-γ activity, valsartan, in a rat model of obesity and hypertension. We hypothesized that in cardiometabolic syndrome, telmisartan with an additional PPAR-γ modulating activity will offer better renal protection than an ARB without PPAR-γ activity.
METHODS
Animals
All of the animal studies were approved by the Medical College of Wisconsin Institutional Review Committee according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Eight week-old male spontaneously hypertensive rats (SHR) were purchased from Charles River Laboratories (Wilmington, MA, USA) and were housed with a 12 hour light-dark cycle and free access to water. Rats were acclimatized for 7 days before use. Animals were divided into six groups (n=6 in each group) and fed either normal rat chow containing 7% fat or a high fat diet containing 36% fat (No. F2685, BioServ, Frenchtown, NJ) ad libitum for 10 weeks. Apart from this difference in total fat content, the other components and their quantities were similar between normal rat chow and the high-fat diet. Groups 1-3 received normal rat chow (SHR-NF) and were treated with either vehicle (Group 1), valsartan (10/mg/kg/day, Group 2) or telmisartan (10 mg/kg/day, Group 3) orally for 10 weeks. Groups 4-6 received high fat diet (SHR-HF) and also were treated with vehicle (vehicle, Group 4), valsartan (10/mg/kg/day, Group 5) or telmisartan (Group 6, 10/mg/kg/day) orally for 10 weeks. Every 7 days, rats of all groups were weighed and tail vain blood samples were taken for blood glucose measurements using a glucometer. After an initial training period, systolic blood pressure was also measured in all groups on every 7 days using tail-cuff plethysmography throughout the experimental period.
Acute pressure response to angiotensin II (Ang II) in rats treated with telmisartan or valsartan
Three groups (n=3) of rats received either telmisartan or valsartan orally at a dose of 10 mg/kg/day for three weeks. At the end of the treatment, rats were used for measuring acute pressure response to a single bolus intravenous injection of Ang II. Animals were anesthetized (Thiobutabarbital, 100 mg/kg), jugular vein and carotid artery were catheterized for drug administration and continuous measurement of the arterial pressure, respectively. The mean arterial pressure (MAP) was measured using a pressure transducer attached to the carotid artery catheter. After a 20 minute stabilization period, a single bolus injection of Ang II (30ng/kg) was given and after baseline recording of MAP, recording was continued on each minute for 10 minutes followed by every 5 minutes recording until 30 minutes.
Enzyme-linked immunoassays for renal injury markers
Rats were housed in metabolic cages for 24h at the end of the 10 week treatment period to collect urine for biochemical analysis. After urine volume was measured gravimetrically, urine samples were aliquoted and stored at -80°C until analyzed. Urinary albumin concentration was determined by a conventional direct competitive enzyme-linked immunosorbant assay (ELISA) using Nephrat II albumin kit according to the manufacturer’s protocol (Exocell, Philadelphia, PA, USA). Urinary monocyte chemoattractant protein -1 (MCP-1) concentration was measured using a commercially available kit (BD Biosciences, San Jose, CA, USA).
Measurement of blood glucose and lipid profile
After 10 weeks of treatment, rats were humanely euthanized by an intraperitoneal injection of sodium pentobarbital. Plasma was separated for the measurement of cholesterol and triglycerides using commercially available kits (Wako Chemicals, Richmond, VA, USA). Kidneys were excised, de-capsulated and sectioned longitudinally and then preserved in neutral buffered formalin for histopathological examination.
Histopathological examination of the kidney
Formalin fixed kidney sections were embedded in paraffin, cut into 5μm thick sections and then stained with Periodic acid-Schiff (PAS) staining for histopathological analysis. Slides were examined for glomerular injury by an observer blinded to the treatment protocol of the animals.
Statistical analysis
All values were presented as mean±SE. Data were analyzed using one-way ANOVA, followed by Tukey’s post-hoc test for multiple group comparisons. Differences were considered statistically significant with p<0.05. Analyses were performed using GraphPad Prism® version 4.0 software (GraphPad Software, Inc., USA).
RESULTS
Acute blood pressure response to Ang II in rats treated with telmisartan or valsartan
Acute bolus intravenous injection of Ang II (30ng/kg) caused a maximum increase of 80.0±11.0 mmHg in MAP. In rats chronically treated with telmisartan or valsartan for three weeks, the peak increase in MAP was greatly attenuated in response to acute bolus intravenous injection of Ang II (30ng/kg) and was not different between telmisartan and valsartan. Ang II increases MAP by 21.2±5.2 mmHg in telmisartan and 24.0±4.6 mmHg in valsartan treated rats. These data suggest that telmisartan and valsartan chronically administered at the doses used in this study have a similar AT1 receptor blocking activity.
Body weight gain and blood pressure in SHR-NF and SHR-HF treated with telmisartan or valsartan
Figure 1 shows the mean body weight in SHR-NF and SHR-HF treated with vehicle, valsartan or telmisartan. The mean body weights were measured at baseline and after 10 weeks of treatment. After 10 weeks, SHR-HF treated with vehicle or valsartan demonstrate a ~20g increase in their body weight compared to SHR-NF treated similarly. When compared to vehicle treated groups, valsartan treatment did not affect the body weight gain in either SHR-NF or SHR-HF (p>0.05). Interestingly, in the telmisartan treated groups there were significant reductions in body weight gain compared to the groups treated with either vehicle or valsartan, and this phenomenon was observed in both SHR-NF and SHR-HF (p<0.05). It is also observed that telmisartan was more effective in blunting the weight gain in SHR-HF (~19%) than in SHR-NF (~8%) compared to that observed in the SHR vehicle groups on the same fat diet.
Figure 1.
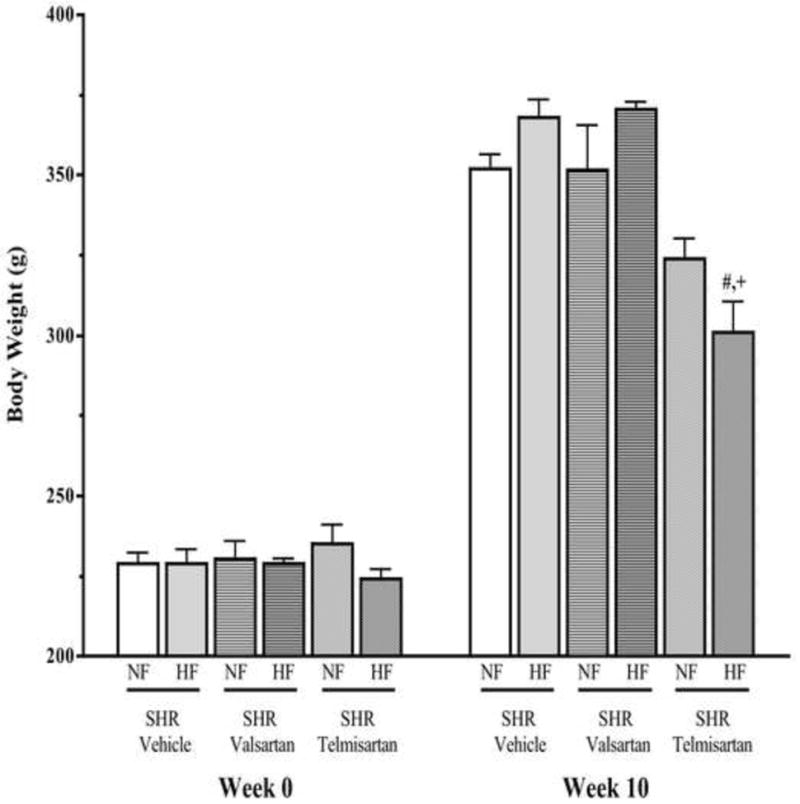
Body weight gain in 10 weeks high and normal fat diet fed SHR treated with telmisartan or valsartan. Values are presented as mean±SE. *p<0.05 vs. normal fat diet, #p<0.05 vs. vehicle and +p<0.05 vs. valsartan.
High fat feeding for 10 weeks did not affect the systolic blood pressure in SHR as compared to that in the SHR received normal diet (Table 1). It was observed that compared to vehicle treatment, SHR treated with valsartan and telmisartan had a significantly lower systolic blood pressure and this phenomenon was observed in both SHR-NF and SHR-HF. It was also observed that the systolic blood pressure of valsartan treated SHR-NF and SHR-HF was approximately 25% lower than those treated with vehicle (p<0.05). In both diet groups, an identical blood pressure lowering trend was observed in the telmisartan treated rats (p<0.05). It is further observed that the blood pressure lowering effect of valsartan and telmisartan were similar between SHR-NF and SHR-HF (Table 1).
Table 1.
Systolic blood pressure, glycemic and lipid status in SHR fed with normal or high fat diet as well as received valsartan or telmisartan for 10 weeks.
| Parameters | SHR-normal fat diet | SHR-high fat diet | SHR-high fat diet | |
|---|---|---|---|---|
|
| ||||
| Vehicle | Vehicle | Valsartan | Telmisartan | |
| Systolic blood pressure (mmHg) (n=6) | 190±3 | 192±4 | 141±5† | 126±14† |
| Blood glucose (mg/dL) (n=6) | 105±3 | 132±4* | 119±11† | 117±5†,‡ |
| Triglycerides (mg/dL) (n=6) | 65±6 | 99±8* | 96±5 | 69±7†,‡ |
| Cholesterol (mg/dL) (n=6) | 61±5 | 73±2* | 72±4 | 74±5 |
Values are presented as mean±SE; n= number of rats;
p<0.05 vs. normal fat diet;
p<0.05 vs. high fat diet;
p<0.05 vs. valsartan.
Blood glucose and lipid profile in SHR-NF and SHR-HF treated with telmisartan or valsartan
Glycemic and lipid status of SHR-NF and SHR-HF treated with valsartan and telmisartan are provided in Table 1. As shown in Table 1, SHR-HF has significantly elevated blood glucose levels compared to SHR-NF. In SHR-HF, the elevated blood glucose level was significantly reduced by both valsartan and telmisartan compared to vehicle treated rats. SHR-HF had significantly elevated triglyceride and cholesterol levels compared to SHR-NF. Valsartan did not affect the elevated triglyceride level in SHR-HF, while telmisartan significantly reduced triglyceride levels in these rats. Neither telmisartan nor valsartan affected the cholesterol level in SHR-HF.
Renal inflammation and injury in SHR-NF and SHR-HF treated with telmisartan or valsartan
Urinary excretion of MCP-1
It is well acknowledged that inflammation plays a pivotal role in the development of renal injury and MCP-1 excretion is often used as an indicator of renal inflammatory status.5,6 In the present study, it was observed that urinary MCP-1 excretion in SHR-HF was almost 50% greater than SHR-NF (p<0.05) (Figure 2). This elevated excretion of MCP-1 in the urine indicated renal inflammation in SHR-HF. It was observed that in SHR-HF but not in SHR-NF valsartan treatment caused almost 50% reduction in urinary MCP-1 excretion compared to those treated with vehicle (p<0.05). Telmisartan treatment showed a potent effect on the markedly elevated MCP-1 excretion observed in SHR-HF. This strong inhibitory effect of telmisartan on MCP-1 excretion was observed in both SHR-HF and SHR-NF (Figure 2).
Figure 2.
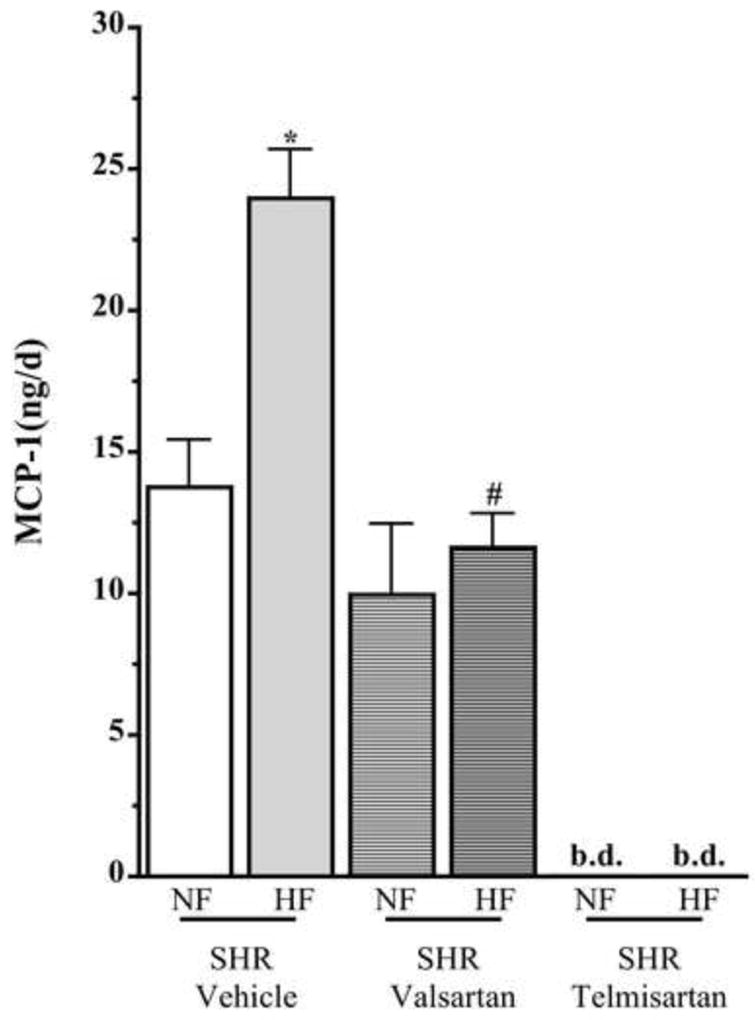
Urinary excretion of MCP-1 in 10 weeks high and normal fat diet fed SHR treated with telmisartan or valsartan. Values are presented as mean±SE. *p<0.05 vs. normal fat diet, #p<0.05 vs. vehicle and +p<0.05 vs. valsartan.
Urinary excretion of albumin
Urinary albumin excretion was measured as a marker of renal damage in SHR-HF and SHR-NF and treated with vehicle, valsartan or telmisartan for 10 weeks (Figure 3A). It is observed that the urinary albumin excretion was almost 40% greater in SHR-HF compared to SHR-NF. In SHR-NF, telmisartan and valsartan treatments demonstrate a similar inhibitory effect on urinary excretion of albumin compared to that of vehicle treatment. As in SHR-NF, valsartan and telmisartan markedly reduced the elevated level of urinary albumin excretion in SHR-HF (p<0.05). However, in SHR-HF telmisartan showed stronger inhibitory effect on the elevated albuminuria compared to that caused by valsartan (p<0.05) (Figure 3A).
Figure 3.
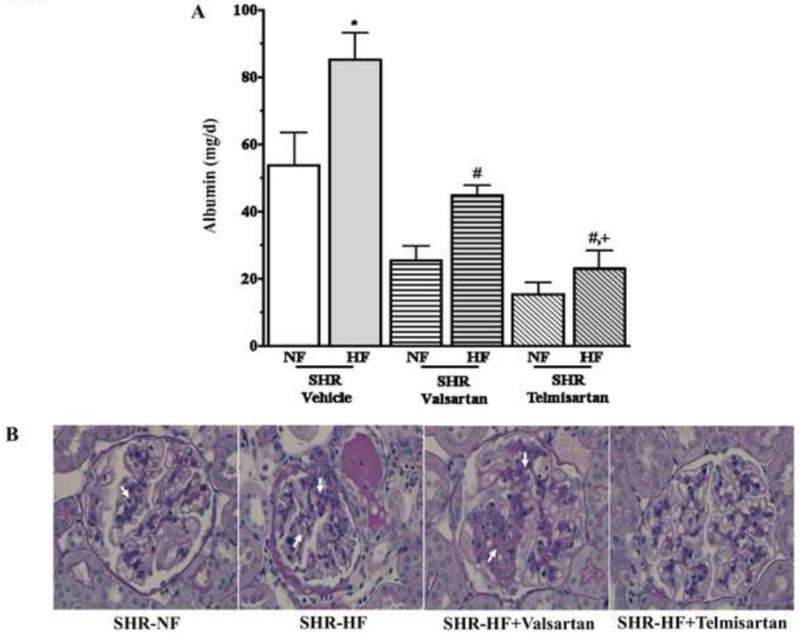
(A) Urinary excretion of albumin in 10 weeks high (HF) and normal fat (NF) diet fed SHR treated with telmisartan or valsartan. Values are presented as mean±SE. *p<0.05 vs. normal fat diet, #p<0.05 vs. vehicle and +p<0.05 vs. valsartan. (B) Representative photomicrographs illustrating glomerular injury (arrows) in SHR-NF, SHR-HF, SHR-HF + valsartan and SHR-HF + telmisartan groups.
Glomerular injury
In the present study, SHR-HF had significantly greater glomerular injury (1.6±0.1, p<0.05) than SHR-NF (1.2±0.1). It was apparent that valsartan treatment did not provide any protection from the deleterious effects of a high fat diet in SHR since the glomerular injury score was 1.5±0.2 in the valsartan treated SHR-HF group. On the other hand, SHR-HF treated with telmisartan had a significantly lower glomerular injury score (0.8±0.2, p<0.05) compared to vehicle treated SHR-HF. This finding demonstrates that telmisartan exhibited a stronger renal protective effect than valsartan in SHR-HF. Representative photomicrographs illustrating glomerular injury in SHR-NF, SHR-HF either treated or untreated with telmisartan or valsartan are shown in Figure 3B.
DISCUSSION
Telmisartan, an ARB with potent AT1 receptor binding affinity has been shown to act as a partial activator of PPAR-γ 19 and this PPAR-γ agonist activity of telmisartan is independent of its AT1 receptor blocking action.18 By virtue of its unique PPAR-γ activator activity, telmisartan decreases advanced glycation end products and C-reactive protein formation, as well as, offering metabolic benefits in metabolic syndrome.20 Valsartan, the other ARB used in this study does not possess PPAR-γ modulating activity but is known to have anti-inflammatory15 and anti-albuminuric properties.21 Considering the dual ARB/PPAR-γ modulator activity of telmisartan, we hypothesized that in high fat diet fed SHR (SHR-HF), a rat model of cardiometabolic syndrome, telmisartan may offer better renal protection than an ARB such as valsartan by reducing inflammation, oxidative stress and, also by correcting the metabolic abnormalities in cardiometabolic syndrome. In the present study we have treated the animals with similar doses (10mg/kg/day) of telmisartan and valsartan. We determined that telmisartan and valsartan have similar effect to block the acute blood pressure response to Ang II at this dose. This finding would support the concept that the differences between telmisartan and valsartan on body weight, inflammation, and renal injury observed in the current study are not due to differences in their ability to inhibit AT1 receptors.
We recognize that there are limitations associated with animal models of human disease including cardiometabolic syndrome. It is now well acknowledged that hypertension and obesity are the major hallmarks of metabolic syndrome in humans and these can be caused by consumption of fat rich diet. Many recognized animal models of metabolic syndrome like ob/ob mice, obese Zucker rats or high fructose diet have many of the characteristics required but are not hypertensive. Others and we have used the SHR-high fat model of metabolic syndrome that includes hypertension, metabolic abnormalities, inflammation and renal injury.6,22-25
In the current study, 10 weeks of high fat feeding in SHR resulted in body weight gain, increased excretion of renal inflammatory marker MCP-1, marked albuminuria, and glomerular injury without causing any appreciable change in the systolic blood pressure. These results are in agreement with our previous findings in normotensive Wistar-Kyoto (WKY) rats and in SHR. In this earlier study, we have observed that 10 weeks feeding of high fat diet did not alter blood pressure; however, SHR and WKY rats fed a high fat diet had increased MCP-1 excretion.6 In SHR-HF, the observed increase in renal inflammation and injury without any change in blood pressure suggested that a diet high in fat can cause renal damage via pressure-independent mechanisms. Previous rat studies have shown that a high fat diet increases oxidative stress and superoxide production.26 Such increase in oxidative stress in turn activates the intracellular transcription factor NF-κB, which then translocates from the cytoplasm to the nucleus to activate downstream inflammatory cytokines perturbing the inflammatory cycle.26-28
Telmisartan, but not valsartan, markedly decreased body weight gain in the high fat fed SHR. It was also observed that telmisartan improved the glycemic and lipid status of these animals. Indeed, it is earlier reported that telmisartan significantly attenuated body weight gain and reduced glucose, insulin, and triglyceride levels in rats fed a high fat, high carbohydrate diet compared to losartan.17 Further, it was shown that telmisartan, but not valsartan, increased caloric expenditure, reduced accumulation of visceral fat and decreased adipocyte size to a much greater extent than valsartan in animal models of metabolic syndrome.20 Apart from our observations in high fat fed SHR, we further observed that telmisartan demonstrated decreased body weight gain and inflammation in SHR fed a normal chow diet. It is conceivable that with a strong anti-inflammatory, anti-proliferative and organ protective effect, telmisartan could provide similar protective effect in SHR where oxidative stress and inflammation are known to contribute to the pathophysiology. 35,36,37
In the present study we have also assessed therapeutic effect of valsartan and telmisartan on the renal injury in high fat fed SHR. As evident from marked albuminuria, elevated urinary MCP-1 level and glomerular injury index, the high fat feeding caused substantial renal damage in the SHR. These results are in agreement with our earlier report which showed a marked renal inflammation, increased albuminuria and renal injury in SHR-HF.6-7 Telmisartan and valsartan used in the present study markedly inhibited the progression of renal injury as evident by a decreased albuminuria and renal inflammation in high fat fed SHR. It was also observed that this inhibitory effect of telmisartan on the renal damage in SHR fed a high fat diet was greater than valsartan treatment. As far as renal inflammation is concerned, we observed a pronounced anti-inflammatory effect of telmisartan that lowered urinary MCP-1 excretion to very low levels. We have previously observed that urinary MCP-1 levels are much lower than plasma MCP-1 levels and that extremely low urinary levels occur in normal rodents.6,7,38 Thus in the present study, the strong anti-inflammatory effect of telmisartan lowered urinary MCP-1 levels to levels observed in normotensive rodents. Indeed, such strong anti-inflammatory effect of telmisartan is reported in murine experimental autoimmune uveoretinitis, a moderate inflammatory condition.39
The renal protective effects of ARBs are mostly related to the improvement of hemodynamic status and direct blockade of pro-sclerotic effects stimulated by angiotensin II.29 However, the enhanced renal protective effect of telmisartan in SHR fed a high fat diet support the concept that this might be attributable to its additional PPAR-γ agonist activity and, indeed, there is growing body of evidence that activators of PPAR-γ exert anti-inflammatory, anti-oxidative and anti-proliferative effects.29-30 It was also observed that telmisartan, but not valsartan, markedly inhibited the events leading to glomerular injury in high fat fed SHR. Considering the observation that telmisartan but not valsartan attenuated glomerular injury, the present observation in the high fat fed SHR raise the possibility that the protective effects of telmisartan on renal inflammation and injury may go beyond blockade of the AT1 receptor. Our findings are consistent with PPAR-γ agonist activity of telmisartan contributing to the greater inhibition of glomerular injury in the high fat fed SHR. Indeed, the PPAR-γ agonist pioglitazone has been found to inhibit cell growth and reduce matrix production in human kidney fibroblasts through mechanisms that included a reduction in the secretion of type IV collagen and fibronectin, reduction in the activity of tissue inhibitors metalloproteinase-1 (TIMP-1), TIMP-2, and metalloproteinase-9 (MMP-9).31,32 Similar to pioglitazone, telmisartan possesses anti-proliferative effect33 and exhibited an ability to inhibit TGF-β-mediated activation of mesangial cells.34 Indeed, it is observed that activation of PPAR-γ by telmisartan decreased type IV collagen expression in the extracelluar matrix.34 Hence, our experimental findings are consistent with concept that the dual ARB/PPAR-γ agonist telmisartan has greater ability to protect the kidney from any potential injury than other ARBs like valsartan.
CONCLUSIONS
The present study showed the important therapeutic potential of telmisartan in an experimental model of cardiometabolic syndrome. Indeed, this potential of telmisartan makes it a particularly stronger therapeutic option in treating hypertensive renal disease and the patho-physiological events frequently occurring in cardiometabolic syndrome. This study also confirmed that the renal protective effects of telmisartan were, at least, partly independent of antagonism of the AT1 receptor. Thus, the findings of the current study support the concept that telmisartan due to its PPAR-γ activation properties as well as its potent AT1 receptor blocking activity may be more efficient than other ARBs in offsetting the sequels of cardiometabolic syndrome.
Acknowledgments
We are grateful to Dr. Christine Teutsch of Boehringer Ingelheim GmbH for providing telmisartan and valsartan. We gratefully acknowledge the technical assistance provided by Dr. Sean M. Shaw, Joshua Steinbert and Jessica Meyer of Department of Pharmacology and Toxicology, Medical College of Wisconsin, Milwaukee, WI 53226, USA.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant HL-59699 and Advancing a Healthier Wisconsin to John D. Imig.
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Scheen AJ. Management of the metabolic syndrome. Minerva Endocrinol. 2004;29(2):31–45. [PubMed] [Google Scholar]
- 2.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 3.Sowers JR, Frohlich ED. Insulin and insulin resistance: impact on blood pressure and cardiovascular disease. Med Clin North A. 2004;88(1):63–82. doi: 10.1016/s0025-7125(03)00128-7. [DOI] [PubMed] [Google Scholar]
- 4.Watson KE, Peters Harmel AL, Matson G. Atherosclerosis in type 2 diabetes mellitus: the role of insulin resistance. Cardiovasc Pharmacol Ther. 2003;8(4):253–60. doi: 10.1177/107424840300800402. [DOI] [PubMed] [Google Scholar]
- 5.Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D. Obesity and prevalent and incident CKD: the hypertension detection and follow-up program. Am J Kidny Dis. 2005;46(4):587–594. doi: 10.1053/j.ajkd.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Knight SF, Quigley JE, Yuan J, Roy SS, Elmarakby A, Imig JD. Endothelial dysfunction and the development of renal injury in spontaneously hypertensive rats fed a high-fat diet. Hypertension. 2008;51(2):352–359. doi: 10.1161/HYPERTENSIONAHA.107.099499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knight SF, Yuan J, Roy S, Imig JD. Simvastatin and tempol protect against endothelial dysfunction and renal injury in a model of obesity and hypertension. Am J Physiol Renal Physiol. 2010;298(1):F86–94. doi: 10.1152/ajprenal.00351.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagase M, Yoshida S, Shibata S, Nagase T, Gotoda T, Ando K, Fujita T. Enhanced aldosterone signaling in the early diabetic nephropathy of rats with metabolic syndrome: possible contribution of fat-derived factors. J Am Soc Nephrol. 2006;17(12):3438–3446. doi: 10.1681/ASN.2006080944. [DOI] [PubMed] [Google Scholar]
- 9.Reisin E, Ebenezer PJ, Liao J, Lee BS, Larroque M, Hu X, Aguilar EA, Morse SA, Francis J. Effect of the HMG-CoA reductase inhibitor rosuvastatin on early chronic kidney injury in obese Zucker rats fed with an atherogenic diet. Am J Med Sci. 2009;338(4):301–309. doi: 10.1097/MAJ.0b013e3181b27195. [DOI] [PubMed] [Google Scholar]
- 10.Dey A, Williams RS, Pollock DM, Stepp DW, Newman JW, Hammock BD, Imig JD. Altered kidney CYP2C and cyclooxygenase-2 levels are associated with obesity-related albuminuria. Obes Res. 2004;12(8):1278–89. doi: 10.1038/oby.2004.162. [DOI] [PubMed] [Google Scholar]
- 11.Gross ML, Ritz E, Schoof A, Adamczak M, Koch A, Tulp O, Parkman A, El-Shakmak A, Szabo A, Amann K. Comparison of renal morphology in the streptozotocin and the SHR/N-cp models of diabetes. Lab Invest. 2004;84(4):452–64. doi: 10.1038/labinvest.3700052. [DOI] [PubMed] [Google Scholar]
- 12.Russo LM, Osicka TM, Brammar GC, Candido R, Jerums G, Comper WD. Renal processing of albumin in diabetes and hypertension in rats: possible role of TGF-beta1. Am J Nephrol. 2003;23(2):61–70. doi: 10.1159/000068039. [DOI] [PubMed] [Google Scholar]
- 13.Ruilope LM, Segura J. Losartan and other angiotensin II antagonists for nephropathy in type 2 diabetes mellitus: a review of the clinical trial evidence. Clin Ther. 2003;25(12):3044–3064. doi: 10.1016/s0149-2918(03)90091-9. [DOI] [PubMed] [Google Scholar]
- 14.Silverstein RL, Fenves AZ, Ram CV. ARBs and target organ protection. Exploring benefits beyond their antihypertensive effects. Postgrad Med. 2004;116(2):31–38. doi: 10.3810/pgm.2004.08.1569. [DOI] [PubMed] [Google Scholar]
- 15.Galle J, Schwedhelm E, Pinnetti S, Böger RH, Wanner C VIVALDI investigators. Antiproteinuric effects of angiotensin receptor blockers: telmisartan versus valsartan in hypertensive patients with type 2 diabetes mellitus and overt nephropathy. Nephrol Dial Transplant. 2008;23(10):3174–3183. doi: 10.1093/ndt/gfn230. [DOI] [PubMed] [Google Scholar]
- 16.Kakuta H, Sudoh K, Sasamata M, Yamagishi S. Telmisartan has the strongest binding affinity to angiotensin II type 1 receptor: comparison with other angiotensin II type 1 receptor blockers. Int J Clin Pharmacol Res. 2005;25(1):41–46. [PubMed] [Google Scholar]
- 17.Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA, Kurtz TW. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPAR {gamma}-modulating activity. Hypertension. 2004;43(5):993–1002. doi: 10.1161/01.HYP.0000123072.34629.57. [DOI] [PubMed] [Google Scholar]
- 18.Schupp M, Janke J, Clasen R, Unger T, Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activity. Circulation. 2004;109(17):2054–2057. doi: 10.1161/01.CIR.0000127955.36250.65. [DOI] [PubMed] [Google Scholar]
- 19.Yamagishi S, Nakamura K. Telmisartan, its potential therapeutic implications in cardiometabolic disorders. Recent Pat Cardiovasc Drug Discov. 2006;1(1):79–83. doi: 10.2174/157489006775244281. [DOI] [PubMed] [Google Scholar]
- 20.Sugimoto K, Qi NR, Kazdová L, Pravenec M, Ogihara T, Kurtz TW. Telmisartan but not valsartan increases caloric expenditure and protects against weight gain and hepatic steatosis. Hypertension. 2006;47(5):1003–1009. doi: 10.1161/01.HYP.0000215181.60228.f7. [DOI] [PubMed] [Google Scholar]
- 21.Viberti G, Wheeldon NM. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure independent effect. Circulation. 2002;106(6):672–678. doi: 10.1161/01.cir.0000024416.33113.0a. [DOI] [PubMed] [Google Scholar]
- 22.Elmarakby AA, Imig JD. Obesity is the major contributor to vascular dysfunction and inflammation in high-fat diet hypertensive rats. Clinical Science. 2010;118(4):291–301. doi: 10.1042/CS20090395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung S, Park CW, Shin SJ, Lim JH, Chung HW, Youn DY, Kim HW, Kim BS, Lee JH, Kim GH, Chang YS. Tempol or candesartan prevents high-fat diet-induced hypertension and renal damage in spontaneously hypertensive rats. Nephrol Dial Transplant. 2010;25(2):389–99. doi: 10.1093/ndt/gfp472. [DOI] [PubMed] [Google Scholar]
- 24.Shin SJ, Lim JH, Chung S, Youn DY, Chung HW, Kim HW, Lee JH, Chang YS, Park CW. Peroxisome proliferator-activated receptor-alpha activator fenofibrate prevents high-fat diet-induced renal lipotoxicity in spontaneously hypertensive rats. Hypertens Res. 2009;32(10):835–45. doi: 10.1038/hr.2009.107. [DOI] [PubMed] [Google Scholar]
- 25.Saiki R, Okazaki M, Iwai S, Kumai T, Kobayashi S, Oguchi K. Effects of pioglitazone on increases in visceral fat accumulation and oxidative stress in spontaneously hypertensive hyperlipidemic rats fed a high-fat diet and sucrose solution. J Pharmacol Sci. 2007;105(2):157–67. doi: 10.1254/jphs.fp0070619. [DOI] [PubMed] [Google Scholar]
- 26.Tian N, Moore RS, Braddy S, Rose RA, Gu JW, Hughson MD, Manning RD., Jr Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol. 2007;293(6):H3388–H3395. doi: 10.1152/ajpheart.00981.2007. [DOI] [PubMed] [Google Scholar]
- 27.Poudyal H, Panchal S, Brown L. Comparison of purple carrot juice and beta-carotene in a high-carbohydrate, high-fat diet-fed rat model of the metabolic syndrome. Br J Nutr. 2010;104(9):1322–1332. doi: 10.1017/S0007114510002308. [DOI] [PubMed] [Google Scholar]
- 28.Yuan L, Li X, Li J, Li HL, Cheng SS. Effects of renin-angiotensin system blockade on the islet morphology and function in rats with long-term high-fat diet. Acta Diabetol. 2010 Jul 14; doi: 10.1007/s00592-010-0210-8. Epub. [DOI] [PubMed] [Google Scholar]
- 29.Takano H, Hasegawa H, Zou Y, Komuro I. Pleiotropic actions of PPAR-γ activators thiazolidinediones in cardiovascular diseases. Curr Pharm Des. 2004;10(22):2779–2780. doi: 10.2174/1381612043383719. [DOI] [PubMed] [Google Scholar]
- 30.Marx N, Duez H, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors and atherogenesis. Regulators of gene expression in vascular cells. Cir Res. 2004;94(9):1168–1178. doi: 10.1161/01.RES.0000127122.22685.0A. [DOI] [PubMed] [Google Scholar]
- 31.Dong FQ, Li H, Cai WM, Tao J, Li Q, Ruan Y, Zheng FP, Zhang Z. Effects of pioglitazone on expressions of matrix metalloproteinases 2 and 9 in kidneys of diabetic rats. Chin Med J (Engl) 2004;117(7):1040–1044. [PubMed] [Google Scholar]
- 32.Zafiriou S, Stanners SR, Saad S, Polhill TS, Poronnik P, Pollock CA. Pioglitazone inhibits cell growth and reduces matrix production in human kidney fibroblasts. J Am Soc Nephrol. 2005;16(3):638–645. doi: 10.1681/ASN.2004040278. [DOI] [PubMed] [Google Scholar]
- 33.Yammamoto K, Ohishi M, Ho C, Kurtz TW, Rakugi H. Telmisartan-induced inhibition of vascular cell proliferation beyond angiotensin receptor blockade and peroxisome proliferator-activated receptor-γ activation. Hypertension. 2009;54(6):1353–1359. doi: 10.1161/HYPERTENSIONAHA.109.138750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yaho Y, Zou R, Liu X, Jiang J, Huang Q, He Y, Li M, Wang S, Zhou J, Ma D, Xu G. Telmisartan but not valsartan inhibits TGF-β-mediated accumulation of extracellular matrix via activation of PPARγ. J Huazhong Univ Sci Technol (Med Sci) 2008;28(5):543–548. doi: 10.1007/s11596-008-0512-z. [DOI] [PubMed] [Google Scholar]
- 35.Elmarakby AA, Faulkner J, Posey SP, Sullivan JC. Induction of hemeoxygenase-1 attenuates the hypertension and renal inflammation in spontaneously hypertensive rats. Pharmacol Res. 2010;62(5):400–7. doi: 10.1016/j.phrs.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Cardinale JP, Sriramula S, Pariaut R, Guggilam A, Mariappan N, Elks CM, Francis J. HDAC inhibition attenuates inflammatory, hypertrophic, and hypertensive responses in spontaneously hypertensive rats. Hypertension. 2010;56(3):437–44. doi: 10.1161/HYPERTENSIONAHA.110.154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi YX, Chen Y, Zhu YZ, Huang GY, Moore PK, Huang SH, Yao T, Zhu YC. Chronic sodium hydrosulfide treatment decreases medial thickening of intra myocardial coronary arterioles, interstitial fibrosis, and ROS production in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;293(4):H2093–100. doi: 10.1152/ajpheart.00088.2007. [DOI] [PubMed] [Google Scholar]
- 38.Manhiani M, Quigley JE, Knight SF, Tasoobshirazi S, Moore T, Brands MW, Hammock BD, Imig JD. Soluble epoxide hydrolase gene deletion attenuates renal injury and inflammation with DOCA-salt hypertension. Am J Physiol Renal Physiol. 2009;297:F74–F748. doi: 10.1152/ajprenal.00098.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okunuki Y, Usui Y, Nagai N, Kezuka T, Ishida S, Takeuchi M, Goto H. Suppression of experimental autoimmune uveitis by angiotensin II type 1 receptor blocker telmisartan. Invest Ophthalmol Vis Sci. 2009;50:2255–2261. doi: 10.1167/iovs.08-2649. [DOI] [PubMed] [Google Scholar]


