Abstract
Oxidation of L[1-13C]methionine ([13C]-Met) in liver mitochondria can be quantified by measuring exhaled 13CO2. We hypothesized that 13CO2 recovery after i.v. administered [13C]-Met would provide a noninvasive measure of liver function in pediatric intestinal failure-associated liver disease (IFALD). After Institutional Review Board (IRB) approval, 27 patients underwent L[1-13C]-Met breath tests ([13C]-MBTs), five of whom underwent repeat testing after clinical changes in liver function. Sterile, pyrogen-free [13C]-Met was given i.v. Six breath samples collected during 120 min were analyzed for 13CO2 enrichment using isotope ratio mass spectrometry. Pediatric end-stage liver disease (PELD) scores were recorded, and total carbon dioxide (CO2) production was measured by indirect calorimetry. Twenty-seven patients (median age = 5.3 mo) underwent a total of 34 [13C]-MBTs without adverse events. Fourteen patients had documented liver biopsies (five with cirrhosis and nine with cholestasis or fibrosis). The [13C]-MBT differentiated patients with and without cirrhosis (medians 210 and 350, respectively, p = 0.04). Serial [13C]-MBTs in five patients reflected changing PELD scores. i.v. administering the stable isotope [13C]-Met with serial breath sampling provides a useful, safe, and potentially clinically relevant evaluation of hepatic function in pediatric IFALD. The [13C]-MBT may also help quantify progression or improvement of IFALD.
Intestinal failure (IF) is a clinical condition in which the body is incapable of supporting growth and/or fluid and electrolyte homeostasis because of inadequate functional intestinal surface area. In the neonatal population, recent reports estimate an incidence of up to 0.7% in very low birth weight infants, and up to 1.1% in extremely low birth weight infants. The associated mortality of IF in very low birth weight infants is thought to be as high as 20% (1).
The mainstay of modern medical therapy for IF is parenteral nutrition (PN), but unfortunately, this therapy can be accompanied by liver injury that is termed intestinal failure-associated liver disease (IFALD). The etiology of IFALD can be multifactorial, and beyond its association with PN, IFALD has been linked with prematurity, number of infectious episodes, ability to tolerate enteral nutrition, number of laparotomies, number of days on antibiotics, and other yet to be identified factors (2).
IFALD begins with the development of hepatic periportal inflammation and cholestasis and can, after extended periods of exposure to PN, progress to bile duct proliferation, fibrosis, and cirrhosis (3). In cases of severe, irreversible liver damage associated with IFALD, isolated liver transplantation or, more commonly, a combined intestinal/liver transplant may be required (4 – 6).
Although liver biopsy remains the gold standard for the evaluation of IFALD, the invasive nature of this test precludes its use as a frequent measure of disease progression or recovery. Conventional static biochemical liver tests (transaminase, bilirubin, alkaline phosphatase and albumin plasma levels, or prothrombin time) have been used to assess injury and function but vary widely in specificity and sensitivity (7–9). For example, patients with IFALD with normal or mildly elevated bilirubin can often have cirrhosis as determined by liver biopsy (10).
In recognition of these deficiencies, the Child-Turcotte and Pugh (CTP) scoring system was initially developed in 1964 to assess the severity of chronic liver disease (11) and more recently, another widely used scoring system, the model for end-stage liver disease (MELD), was developed using serum creatinine, international normalized ratio (INR), and bilirubin to predict survival in adult patients with chronic liver disease (12). The pediatric end-stage liver disease (PELD) score is similar to MELD but designed as a tool for predicting mortality and morbidity in children with chronic disease awaiting liver transplantation (13). PELD uses some different criteria from the MELD to recognize the specific growth and development needs of children including 1) bilirubin, which measures how effectively the liver excretes bile, 2) INR, which measures the liver’s ability to make blood clotting factors, 3) albumin, which measures the liver’s ability to synthesize a visceral protein from precursor amino acids, 4) growth failure, and 5) age (whether the child is younger than 1 year).
These scoring systems are potentially better than biochemical tests alone because they address a range of factors of reflecting hepatic reserve and function. However, the MELD and the CTP were not designed for children (13), and although the PELD score is specific to the pediatric population, it has limitations when used to assess IFALD. It has not been validated as a surrogate marker for the clinical assessment of liver disease in patients who do not have end-stage liver disease. Additionally, four of the five components of PELD (weight, height, INR, and albumin) can be altered by conditions other than IFALD. For example, prematurity, gestational age, malabsorption, intestinal losses, intestinal length, and under nutrition may contribute to abnormalities of growth, vitamin K absorption, and low albumin independent of liver function.
A noninvasive dynamic test for IFALD may better address these above limitations. Previously in adult populations, non-invasive dynamic tests have been designed to measure the liver’s metabolic capacity at a particular time and when assessed longitudinally can be used to quantify the liver’s functional reserve (9). Substrates used in these dynamic tests for assessment of hepatic function must fulfill criteria including safe, rapid and consistent absorption, specific hepatic metabolism, a well-known metabolic pathway, and rapid appearance of the metabolite in exhaled breath or other sampling site (14).
Traditionally, substrates have been categorized by the particular liver compartment where metabolism takes place (mitochondrial, microsomal, or cytosolic) (14) and can, therefore, be selected based on the compartment that is most affected in a given type of liver disease. Hepatic mitochondrial dysfunction is the result of a wide range of liver pathologies and is a useful marker of overall liver function. In cases of liver cirrhosis, fatty liver, or injury from xenobiotics, oxidative metabolism of various substrates is impaired due to electron transport chain dysfunction in hepatic mitochondria (15,16), with evidence suggesting that the assessment of mitochondrial function may be an appropriate marker for liver function during PN (17).
One substrate that is specifically metabolized in hepatic mitochondria and fulfills the other criteria noted above is the amino acid methionine (9,18). The principal site of methionine metabolism is in the liver (19) with several pathways leading to carbon dioxide (CO2) production. In the first steps of methionine metabolism, methionine-adenosyltransferase catalyzes the conversion from methionine to S-adenosylme- thionine (SAMe) (20). CO2 is produced when SAMe is decarboxylated in the pathway to polyamine synthesis (21,22). SAMe is also involved in transmethylation reactions that lead to S-adenosylhomocysteine and subsequent formation of homocysteine (19). The most abundant methyltransferase in these transmethylation reactions is glycine N-methyltransferase, which converts glycine into N-methylglycine (sarcosine) (21). Sarcosine is then oxidized by sarcosine dehydrogenase, which is found exclusively in the liver mitochondria; a subsequent set of reactions including formation of intermediates formaldehyde and formate result in the formation of CO2 (18,23). Methionine transamination is another potential pathway of methionine catabolism that results in the conversion of the methyl or carboxyl carbon to CO2 and occurs primarily in the liver. However, this pathway does not occur under normal metabolic conditions in mammals (18,24).
Each of the pathways, as noted earlier, produces CO2 in the intermediary metabolism of methionine. By relying on the pathways of methionine degradation to CO2, previous studies suggest that a 13C-labeled methionine ([13C]-Met) breath test ([13C]-MBT) could be used to evaluate the methionine oxidative capacity of the liver (9).
In this breath test, the patient is administered [1-13C]-Met, in which one of the 12C atoms in methionine has been replaced by a stable 13C isotope. The 13C stable isotope is enzymatically cleaved during one of the metabolic pathways noted earlier and eventually is incorporated into CO2 and exhaled through the breath (9). The 13C enrichment of expired CO2 is analyzed by means of isotope ratio mass spectrometry. Hence, by using a stable isotope tracer, one can follow methionine oxidative capacity in the mitochondria of hepatocytes (9).
The development of the i.v. [13C]-MBT offers the potential for a safe, noninvasive, and reliable means of evaluating liver disease specifically in children with IFALD (25). An oral version has been used safely in adults for nearly a decade (9), and the i.v. [13C]-MBT method avoids any error introduced by variable gastrointestinal absorption that may be inherent with patients with IF.
We sought to use the i.v. [13C]-MBT as an index of liver function in a pediatric cohort of patients with IF. Specifically, the 13CO2 recovery in the breath was correlated with biochem- ical tests of liver disease, PELD score, and histopathology in pediatric patients with IFALD. Furthermore, we investigated the feasibility of this test as an easily repeated serial measurement of liver function in our study population.
METHODS
After Institutional Review Board (IRB) approval and written informed consent was obtained, 27 patients followed up by the Center for Advanced Intestinal Rehabilitation on the inpatient ward of Children’s Hospital, Boston, were enrolled between July 2006 and February 2009. Inclusion criteria were 1) a diagnosis of IF (defined as dependence on PN for ≥ 30 d); 2) corrected gestational age ≥ 36 wk; and 3) the provision of PN to fulfill part or all energy and protein requirements. The diagnosis of IFALD was made by 1) serum direct bilirubin (DB) ≥ 2 mg/dL or 2) liver histopathology showing moderate to marked fibrosis in the setting of exposure to PN for >30 d. Exclusion criteria included 1) receiving parenteral antimicrobials and/or vasopressors; 2) inadequate nutrition support (<80 kcal/kg/d); 3) history of general anesthesia within the previous 48 h; 4) mechanical ventilation; or 5) history with inborn errors of metabolism affecting the methionine metabolic pathway. Inadequate nutrition was considered as an exclusion criterion to obviate spuriously low rates of methionine oxidation caused by deficiency states. Patients were eligible to receive parenteral fish oil based on our institutional protocol and criteria as defined previously (26). Direct bilirubin, INR, alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, gamma glutamyl transpeptidase (GGTP), and pertinent clinical data and liver biopsy results were obtained from the medical chart. Liver biopsies were not stipulated within the study protocol; all liver biopsies were obtained using a core technique and were performed at the clinical discretion of the operating surgeon. Liver function tests and clinical information were used to calculate the PELD score of each patient (12). This score has been used to evaluate a patient’s candidacy and urgency for liver transplantation. In our study, in addition to the conventional biochemical tests, we used the PELD score as a clinical marker of liver disease. We monitored the following types of adverse events: pyrexia, hypotension, hypoglycemia, line infection, bacteremia, and evidence of anaphylaxis.
Isotopic methods
Subjects were fasted for 2 h before i.v. L[1-13C]-Met administration. Weight-appropriate infusions of dextrose with ½ or normal saline were provided throughout the fasting and breath samples collection periods to prevent hypoglycemia and/or hypovolemia. After 2 hours of fasting, a baseline duplicate breath sample was obtained, followed by the bolus administration of a 2 mg/kg i.v. dose of sterile and pyrogenfree L[1-13C]-Met solution through a preexisting central venous catheter. Additional duplicate breath samples were then obtained at 20-min intervals (20, 40, 60, 80, 100, and 120 min after the dose was given). This technique has been published previously (25). The breath samples were batched and then analyzed by a gas isotope ratio mass spectrometer (Thermo Breath Mat, Model 9706, Thermo Finnigan Inc., Ringoes, NJ) for 13CO2 enrichment. Total CO2 production was measured by indirect calorimetry (Vmax Legacy, Viasys Healthcare Inc., Conshohocken, PA). The accumulated total 13CO2 production [V13CO2 (time)] was calculated based on the rate of 13CO2 production at each time point of air sample collection, was calculated as: V13CO2 (time) =V13CO2 × VE13CO2 (time), where VCO2 is the total CO2 production rate (in μ mol/kg/min) measured by the indirect calorimetry, and VECO2 is the isotopic enrichment of 13CO2 measured on the air sample collected at each time point = {0, 20, 40, 60, 80, 100, 120} min. The calculation of 13 CO2 production was then accomplished using established methods (25).
Statistical methods
The 13CO2 enrichment over baseline was plotted as a function of time for each patient. The area under the curve of each patient was then obtained using the Lagrange method (27).
All continuous patient characteristics were summarized using medians and interquartile ranges (IQRs), and means and standard deviations as appropriate. Categorical patient characteristics were summarized as proportions. Spearman correlation coefficients were calculated to assess the association between the [13C]-MBT results and the following variables: INR, ALT, AST, albumin, GGTP, PELD score, and duration of PN therapy at the time of testing. Comparisons of the [13C]-MBT results in subjects with and without cirrhosis were assessed by the Wilcoxon test.
RESULTS
Twenty seven patients underwent a total of 34 [13C]-MBTs without any adverse events. Patient age ranged from 5.0 to 34.4 mo (median = 5.3 mo; IQR = 3.6 – 8.0) with 16 (59%) males. The median duration of PN exposure at the time of [13C]-MBT was 5 mo (IQR: 3–7). All patients received i.v. omega-3 fatty acids at 1 g/kg/d as hepatoprotective strategy during the course of therapy. The most common diagnoses leading to IF were necrotizing enterocolitis (33%), gastroschisis (26%), and intestinal atresia (22%) (Table 1). At the time of the study, none of the patients required supplemental oxygen.
Table 1.
Patient characteristics at the time of the [13C]-MBT in the 27 patients
| Descriptive variables | All patients (N = 27) | Patients with biopsy results (N = 14) |
|---|---|---|
| Demographic variables | ||
| Age at [13C]-MBT, months: median (IQR) | 5.1 (3.3–7.3) | 6.7 (4.8 –7.7) |
| Gestational age, weeks: median (IQR) | 33 (29 –36) | 29 (25–35) |
| Gender, male: n (%) | 16 (59%) | 9 (64%) |
| Primary diagnosis, n (%) | ||
| NEC | 9 (33) | 9 (64) |
| Gastroschisis | 7 (26) | 1 (7) |
| Intestinal atresia | 6 (22) | 3 (21) |
| Other | 5 (19) | 1 (7) |
| Residual bowel length (cm) 3SD3 | 76 (89) | 49 (55) |
| Citrulline (μmol/L) 3SD3 | 13.2 (8.4) | 11.4 (7.3) |
| Nutritional variables | ||
| Weight, kg: mean (SD) | 5.3 (2.7) | 5.4 (2.1) |
| Height, cm: mean (SD) | 58 (10) | 60 (8.7) |
| PN intake (%) | 77 (23) | 73 (28) |
| REE (kcal/kg/day) | 50.1 (14.2) | 53.9 (15.4) |
| Duration of PN, months: median (IQR) | 5 (3–7) | 7 (4 –7) |
| Laboratory values | ||
| Direct bilirubin, mg/dL: median (IQR) | 2.9 (0.5–7.8) | 2.6 (0.3–5.7) |
| ALT, unit/L: median (IQR) | 76 (44 –175) | 67 (43–99) |
| GGTP, unit/L: median (IQR) | 125 (66 –178) | 113 (73–130) |
| Albumin, g/dL: median (IQR) | 3.2 (2.9 –3.3) | 3.2 (2.8 –3.4) |
| INR: median (IQR) | 1.13 (1.07–1.26) | 1.21 (1.13–1.35) |
| IFALD: histology | ||
| Liver biopsy, n (%) | ||
| Performed | 14 | 14 |
| Cholestasis or fibrosis | 9 | 9 |
| Cirrhosis | 5 | 5 |
| PELD: median (IQR) | 12 (6 –16) | 10 (6 –18) |
IQR, interquartile range; SD, standard deviation; [13C]-MBT, [13C]-methionine breath test; NEC, necrotizing enterocolitis; PN, parenteral nutrition; REE, resting energy expenditure; ALT, alanine aminotransferase; GGTP, gamma glutamyl transpeptidase; INR, international normalization ratio; IFALD, intestinal failure associated liver disease; PELD, Pediatric End stage Liver Disease score.
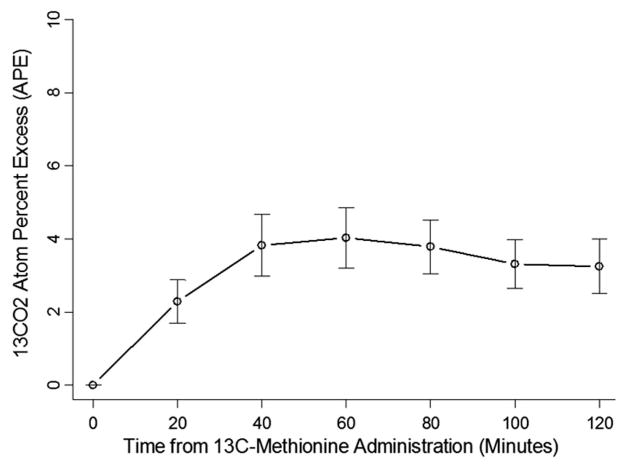
Figure 1 demonstrates the 13CO2 atom percent excess of the 27 patients. Correlations between parameters of liver function tests in the 27 patients and the [13C]-MBT were generally quite weak. The strongest correlations with the area under the curve of the enrichment over baseline of the [13C]-Met (13CO2 AUC) were gestational age (Spearman correlation = 0.39, p = 0.04), weight (Spearman correlation = 0.36, p = 0.06), height (Spearman correlation = 0.36, p = 0.07), the INR (Spearman correlation = −0.36, p = 0.07), and the PELD scores (Spearman correlation = −0.31, p = 0.12). Age at the time of test and residual bowel length were not associated with the [13C]-MBT.
Figure 1.
13 CO2 atoms percent excess (APE), of the 27 patients. The APE is an expression of stable isotope enrichment within the total CO2 pool and reflects 13C-methionine tracer oxidation in the liver. It can be defined as: APE = tracer/(tracer + tracee) × 100, where the tracer (in this case, 13CO2), is a compound that is chemically and functionally identical to the naturally occurring compound of interest, the tracee (12CO2) (40). As time from 13C-methionine administration increases, the appearance of 13CO2 increases, which is in accordance with expectations because as discussed in the introduction, metabolism of 13C-methionine is anticipated to result in production of 13CO2.
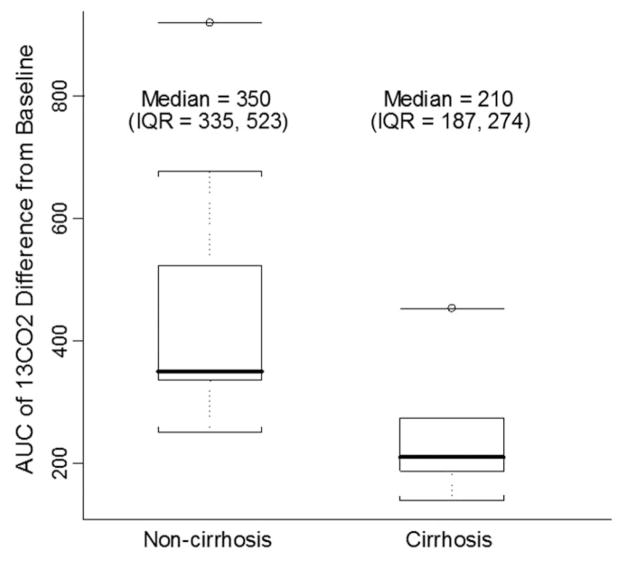
Of the 27 patients, 14 had liver biopsies. These patients varied from individuals without liver biopsy in regards to duration of PN exposure at time of testing, age at time of testing, primary diagnosis, and gestational age (Table 1). Of the 14 patients with liver biopsies, five had cirrhosis and nine had cholestasis or fibrosis. The median 13CO2 AUC was 210 for patients with cirrhosis and 350 for patients without cirrhosis (p = 0.04) (Fig. 2).
Figure 2.
Boxplots of [13C]-MBT results, comparing patients with (n = 5) and without (n = 9) cirrhosis on liver biopsy. The distribution is based on the 13CO2 AUC. The solid bar within the box represents the median value; upper boundary of the box: the 75th percentile; lower boundary of the box: the 25th percentile; and whiskers extend to the most extreme observation within 1.5 interquartile range units of the 25th and 75th percentiles. The median 13CO2 AUC was 210 for patients with cirrhosis and 350 for patients without cirrhosis (p = 0.04). p value was estimated by Wilcoxon tests.
Five patients had repeated [13C]-MBT measurements, three showed increased 13CO2 excretion and decreased PELD scores. One patient had a decreased PELD score with a [13C]-MBT result that remained essentially unchanged. The fifth patient underwent the [13C]-MBT four times. The 13CO2 AUC decreased between the first and third tests, reflecting the patient’s worsening clinical status (development of significant stomal bleeding and deterioration of medical status with increased INR). Meanwhile, the PELD score during this episode improved slightly. Ultimately, the patient stabilized by cessation of stomal bleeding, correction of coagulopathy, and this improved clinical condition was reflected in both an improved (decreased) PELD score and an improved (increased) 13CO2 AUC.
DISCUSSION
Although PN remains a vital therapeutic bridge for patients undergoing intestinal rehabilitation, prolonged exposure is a risk for developing the multifactor pathology of IFALD. IFALD remains a primary cause of morbidity and mortality in patients receiving long-term PN, particularly in the susceptible neonatal population (2). The gold standard for the assessment of IFALD continues to be liver biopsy. This is an invasive procedure, however, with measurable morbidity and is not well suited for frequent assessments of alterations in liver status.
We sought to develop an i.v. [13C]-MBT as a safe, noninvasive test for liver injury in pediatric patients with IF. Our initial pilot study of this i.v. technique demonstrated the feasibility of this approach in children with limited enteral absorption (4). The enteral version of the MBT has been shown to correlate with both hepatic steatosis and cirrhosis in adults (27). Similarly, adult liver transplant outcomes (28) and HIV patient drug-induced hepatic toxicity (18) have been predicted by an orally administered [13C]-MBT. Furthermore, in HIV-infected adults, oral [13C]-MBT results were shown to be an earlier marker of hepatic mitochondrial dysfunction than either standard liver function tests or patient symptomatology (29).
Although these previous reports have demonstrated the safety and clinical applicability of the [13C]-MBT, they have relied on the oral administration of [13C]-Met. In our population of children with IF, however, reliable enteral absorption cannot be assumed. Hence, we modified the original technique to an i.v. administered test using the preexisting central venous catheters present in our cohort of patients with IF. This study supports our previous report (25) that the i.v. administered [13C]-MBT is well tolerated in children. No adverse events were recorded in the 34 tests performed on 27 children. The safety of this particular stable isotope test is consistent with the existing literature on the safe clinical application of stable isotope techniques in the neonatal and pediatric populations (30,31).
We report a strong relationship between the 13CO2 AUC and the presence or absence of cirrhosis on liver biopsy (p = 0.04). The ability to accurately recognize the presence of cirrhosis is of clinical importance. All patients with a diagnosis of cirrhosis must be carefully monitored for signs of hepatic decompensation, and long-term survivors living with compensated cirrhosis must be followed for possible malignant degeneration (32,33). We believe that the ability of the [13C]-MBT to noninvasively distinguish the presence or absence of cirrhosis in children with IFALD offers a promising alternative to the current gold standard of liver biopsy.
Finally, this study demonstrates the feasibility of performing serial i.v. [13C]-MBT measurements in individuals with concomitant intestinal and liver disease. In three of the five patients subjected to serial testing, changes in the patient’s PELD scores correlated well with their [13C]-MBT results. Interestingly, in the one patient followed up with four tests, an acute worsening of the patient’s clinical status, marked by increased INR and presence of stomal bleeding, was not reflected in the patient’s PELD score. However the [13C]-MBT results did show this decompensation. This observation might be explained by the fact that the [13C]-MBT traces an essential hepatic mitochondrial metabolic pathway that is particularly sensitive to liver injury (9). It may, therefore, prove to be an earlier marker of hepatic dysfunction than the PELD score. Although the PELD score is a widely used liver disease scoring system, it is based largely on clinical parameters of plasma liver function tests, which are, at best, considered indirect measures of liver function. It is also important to take into consideration increasingly used hepatoprotective strategies such as the use of omega-3 fatty acids and the practice of lowering the dose of i.v. fats (1 g/kg) The ability of these strategies to protect hepatocytes from injury and decrease cholestasis (34 –36) might further undermine the utility of the PELD score.
Finally, it is important to note that although the PELD includes the patient’s bilirubin level as an indicator of liver injury and cholestasis, an elevated bilirubin level does not seem to correlate well with histologic evidence of liver injury (37–39).
In fact, there seems to be evidence that in children with IFALD, advanced fibrosis and even cirrhosis can exist in the absence of an elevated direct bilirubin level (10). Hence the i.v. [13C]-MBT offers a novel and clinically relevant test for the evaluation of IFALD. In this study, we confirm the safety of the i.v. technique and the feasibility of repeat testing in children. Furthermore, we demonstrate a significant relationship between the [13C]-MBT results and the presence or absence of liver cirrhosis in children with IFALD.
The study has several limitations including a relatively small sample size (27 patients), the lack of consistent cotemporal liver biopsies, and the absence of a comparator cohort. Although a significant advantage of the [13C]-MBT is as a repeated test for the longitudinal assessment of liver function, only pilot data are available for review in this investigation. Finally, the use of [13C]-MBT in older patients with IF has as yet to be assessed.
Future investigation is warranted to determine the ability of the [13C]-MBT to distinguish more subtle degrees of liver fibrosis and to evaluate the applicability of this test in predicting long-term clinical outcomes in varied IF cohorts.
Acknowledgments
Supported by the Institutional Grant from NIH T32-HD43034-05A1, Pilot Feasibility Grant from the Clinical Nutrition Research Center, NIH #P30 DK40561-13, and by a Junior Faculty Career Development Award from Children’s Hospital, Boston [D.D.] and by the Howard Hughes Medical Institute Medical Student Fellowship [to C.-F.J.Y.]. The study isotopic analyses were supported by NIH #P30DK040561 [D.D., Y.M.Y., C.D., T.J.].
We thank patients and families who participated and Ms. Flourence Lin B.S. for her mass spectrometry analyses.
Abbreviations
- ALT
alanine aminotransferase
- [13C]-Met
L[1-13C]methionine
- [13C]-MBT
L[1-13C]methionine breath test
- 13 CO2 AUC
area under the curve of the enrichment over baseline of the [13C]-Met
- GGTP
gamma glutamyl transpeptidase
- IF
intestinal failure
- IFALD
intestinal failure-associated liver disease
- INR
international normalized ratio
- IQR
Interquartile range
- PELD
pediatric end-stage liver disease
- PN
parenteral nutrition
References
- 1.Cole CR, Hansen NI, Higgins RD, Ziegler TR, Stoll BJ. Very low birth weight preterm infants with surgical short bowel syndrome: incidence, morbidity and mortality, and growth outcomes at 18 to 22 months. Pediatrics. 2008;122:e573– e582. doi: 10.1542/peds.2007-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly DA. Intestinal failure-associated liver disease: what do we know today? Gastroenterology. 2006;130:S70–S77. doi: 10.1053/j.gastro.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 3.Zambrano E, El-Hennawy M, Ehrenkranz RA, Zelterman D, Reyes-Mugica M. Total parenteral nutrition induced liver pathology: an autopsy series of 24 newborn cases. Pediatr Dev Pathol. 2004;7:425– 432. doi: 10.1007/s10024-001-0154-7. [DOI] [PubMed] [Google Scholar]
- 4.Muiesan P, Dhawan A, Novelli M, Miele-Vergani G, Rela M, Heaton ND. Isolated liver transplant and sequential small bowel transplantation for intestinal failure and related liver disease in children. Transplantation. 2000;69:2323–2326. doi: 10.1097/00007890-200006150-00017. [DOI] [PubMed] [Google Scholar]
- 5.Horslen SP, Sudan DL, Iyer KR, Kaufmann SS, Iverson AK, Fox IJ, Shaw BW, Langnas AN. Isolated liver transplantation in infants with end-stage liver disease associated with short bowel syndrome. Ann Surg. 2002;235:435– 439. doi: 10.1097/00000658-200203000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan JD, Rudolph JA, Kocoshis SA, Alonso MH, Ryckman FC, Tiao GM. Isolated liver and multivisceral transplantation for total parenteral nutrition–related end-stage liver disease. J Pediatr Surg. 2007;42:143–147. doi: 10.1016/j.jpedsurg.2006.09.049. [DOI] [PubMed] [Google Scholar]
- 7.McIntyre N. The limitations of conventional liver function tests. Semin Liver Dis. 1983;3:265–274. doi: 10.1055/s-2008-1040779. [DOI] [PubMed] [Google Scholar]
- 8.Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin Chem. 2000;46:2027–2049. [PubMed] [Google Scholar]
- 9.Armuzzi A, Marcoccia S, Zocco MA, De Lorenzo A, Grieco A, Tondi P, Pola P, Gasbarrini G, Gasbarrini A. Non-Invasive assessment of human hepatic mitochondrial function through the 13C-methionine breath test. Scand J Gastroenterol. 2000;35:650– 653. doi: 10.1080/003655200750023633. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgibbons SC, Jones BA, Hull MA, Zurakowski D, Duro D, Duggan C, Boctor D, Sigalet DL, Jaksic T. The relationship between biopsy-proven parenteral nutrition associated liver fibrosis and biochemical cholestasis in children with short bowel syndrome. J Pediatr Surg. 2010;45:95–99. doi: 10.1016/j.jpedsurg.2009.10.020. discussion 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Child CG, 3rd, Turcotte JG. Surgery and portal hypertension. In: Child CG 3rd, editor. The Liver and Portal Hypertension. Saunders; Philadelphia: 1964. p. 50. [Google Scholar]
- 12.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464– 470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 13.McDiarmid SV, Anand R, Lindblad AS. Development of a pediatric end-stage liver disease score to predict poor outcome in children awaiting liver transplantation. Transplantation. 2002;74:173–181. doi: 10.1097/00007890-200207270-00006. [DOI] [PubMed] [Google Scholar]
- 14.Candelli M, Cazzato IA, Zocco MA, Nista EC, Fini L, Armuzzi A, Camise V, Santoro M, Miele K, Grieco A, Gasbarrini G, Gasbarrini A. 13C-Breath tests in the study of mitochondrial liver function. Eur Rev Med Pharmacol Sci. 2004;8:23–31. [PubMed] [Google Scholar]
- 15.Chedid A, Mendenhall CL, Tosch T, Chen T, Rabin L, Garcia-Pont P, Goldberg SJ, Kiernan T, Seeff LB, Sorrell M, Tamburro C, Weesner RE, Zetterman R the Veterans Administration Cooperative Study of Alcoholic Hepatitis . Significance of megamitochondria in alcoholic liver disease. Gasteroenterology. 1986;90:1858–1864. doi: 10.1016/0016-5085(86)90253-2. [DOI] [PubMed] [Google Scholar]
- 16.Fréneaux E, Pessayre D. Drug-induced steatosis, phospholipidosis and pseudo-alcoholic liver disorders. Gastroenterol Clin Biol. 1993;17:H36–H43. [PubMed] [Google Scholar]
- 17.Katayama T, Tanaka M, Tanaka K, Asonuma K, Uemoto S, Okamura R, Utsunomiya H, Fujita S, Ueda J, Tanaka A, Ozawa K. Alterations in hepatic mitochondrial function during total parenteral nutrition in immature rats. JPEN J Parenter Enteral Nutr. 1990;14:640– 645. doi: 10.1177/0148607190014006640. [DOI] [PubMed] [Google Scholar]
- 18.Milazzo L, Piazza M, Sangaletti O, Gatti N, Cappelletti A, Adorni F, Antinori S, Galli M, Moroni M, Riva A. [13C]Methionine breath test: a novel method to detect antiretroviral drug-related mitochondrial toxicity. J Antimicrob Chemother. 2005;55:84– 89. doi: 10.1093/jac/dkh497. [DOI] [PubMed] [Google Scholar]
- 19.Finkelstein JD. Methionine metabolism in mammals. J Nutr Biochem. 1990;1:228–236. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- 20.Finkelstein JD. Methionine metabolism in liver diseases. Am J Clin Nutr. 2003;77:1094–1095. doi: 10.1093/ajcn/77.5.1094. [DOI] [PubMed] [Google Scholar]
- 21.Mato JM, Martinez-Chantar ML, Lu SC. Methionine metabolism and liver disease. Annu Rev Nutr. 2008;28:273–293. doi: 10.1146/annurev.nutr.28.061807.155438. [DOI] [PubMed] [Google Scholar]
- 22.Ishii Y, Asai S, Kohno T, Suzuki S, Ishii M, Hosoi I, Fujii M, Iwai S, Ishikawa K. CO2 peak value of L-[1–13C] phenylalanine breath test reflects hepatopathy. J Surg Res. 1999;86:130–135. doi: 10.1006/jsre.1999.5705. [DOI] [PubMed] [Google Scholar]
- 23.Case GL, Benvenga NJ. Evidence for S-adenosylmethionine catabolism in the rat. J Nutr. 1976;106:1721–1736. doi: 10.1093/jn/106.12.1721. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell AD, Benevenga NJ. The role of transamination in methionine oxidation in the rat. J Nutr. 1978;108:67–78. doi: 10.1093/jn/108.1.67. [DOI] [PubMed] [Google Scholar]
- 25.Duro D, Duggan C, Valim C, Bechard L, Fitzgibbons S, Jaksic T, Yu YM. Novel intravenous (13) C-methionine breath test as a measure of liver function in children with short bowel syndrome. J Pediatr Surg. 2009;44:236–240. doi: 10.1016/j.jpedsurg.2008.10.046. discussion 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gura KM, Lee S, Valim C, Zhou J, Kim S, Modi BP, Arsenault DA, Strijbosch R, Lopes S, Duggan C, Puder M. Safety and efficacy of a fish-oil based fat emulsion in the treatment of parenteral nutrition-associated liver disease safety. Pediatrics. 2008;121:e678– e686. doi: 10.1542/peds.2007-2248. [DOI] [PubMed] [Google Scholar]
- 27.Yeh KC, Kwan KC. A comparison of numerical integrating algorithms by trapezoidal, Lagrange, and spline approximation. J Pharmacokinet Biopharm. 1978;6:79–98. doi: 10.1007/BF01066064. [DOI] [PubMed] [Google Scholar]
- 28.Di Campli C, Angelini G, Armuzzi A, Nardo B, Zocco MA, Candelli M, Santoliquido A, Cavallari A, Bernardi M, Gasbarrini A. Quantitative evaluation of liver function by the methionine and aminopyrine breath tests in the early stages of liver transplantation. Eur J Gastroenterol Hepatol. 2003;15:727–732. doi: 10.1097/01.meg.0000059158.46867.a6. [DOI] [PubMed] [Google Scholar]
- 29.Milazzo L, Riva A, Sangaletti O, Piazza M, Antinori S, Moroni M. 13C-Methionine breath test detects liver mitochondrial impairment in HIV-infected patients with antiretroviral drug-related hyperlactatemia. J Acquir Immune Defic Syndr. 2004;35:429– 432. doi: 10.1097/00126334-200404010-00015. [DOI] [PubMed] [Google Scholar]
- 30.Shew SB, Beckett PR, Keshen TH, Jahoor F, Jaksic T. Validation of a [13C] bicarbonate tracer technique to measure neonatal energy expenditure. Pediatr Res. 2000;47:787–791. doi: 10.1203/00006450-200006000-00018. [DOI] [PubMed] [Google Scholar]
- 31.Shew SB, Keshen TH, Jahoor F, Jaksic T. Assessment of cysteine synthesis in very low-birth weight neonates using a [13C6] glucose tracer. J Pediatr Surg. 2005;40:52–56. doi: 10.1016/j.jpedsurg.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Chandra RS, Kapur SP, Kelleher J, Jr, Luban N, Patterson K. Benign hepato-cellular tumors in the young. A clinicopathologic spectrum. Arch Pathol Lab Med. 1984;108:168–171. [PubMed] [Google Scholar]
- 33.Vileisis RA, Sorensen K, Gonzalez-Crussi F, Hunt CE. Liver malignancy after parenteral nutrition. J Pediatr. 1982;100:88–90. doi: 10.1016/s0022-3476(82)80242-4. [DOI] [PubMed] [Google Scholar]
- 34.Lee S, Kim S, Le HD, Meisel J, Strijbosch RA, Nose V, Puder M. Reduction of hepatocellular injury after common bile duct ligation using omega-3 fatty acids. J Pediatr Surg. 2008;43:2010–2015. doi: 10.1016/j.jpedsurg.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 35.Lee SI, Valim C, Johnston P, Le HD, Meisel J, Arsenault DA, Gura KM, Puder M. The impact of fish oil-based lipid emulsion on serum triglyceride, bilirubin, and albumin levels in children with parenteral nutrition-associated liver disease. Pediatr Res. 2009;66:698–703. doi: 10.1203/PDR.0b013e3181bbdf2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puder M, Valim C, Meisel JA, Le HD, de Meijer VE, Robinson EM, Zhou J, Duggan C, Gura KM. Parenteral fish oil improves outcomes in patients with parenteral nutrition-associated liver injury. Ann Surg. 2009;250:395– 402. doi: 10.1097/SLA.0b013e3181b36657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dahms BB, Halpin TC., Jr Serial liver biopsies in parenteral nutrition-associated cholestasis of early infancy. Gastroenterology. 1981;81:136–144. [PubMed] [Google Scholar]
- 38.Moss RL, Das JB, Raffensperger JG. Total parenteral nutrition-associated cholestasis: clinical and histopathologic correlation. J Pediatr Surg. 1993;28:1270–1274. doi: 10.1016/s0022-3468(05)80311-2. discussion 1274–1275. [DOI] [PubMed] [Google Scholar]
- 39.Hasegawa T, Sasaki T, Kimura T, Nakai H, Sando K, Wasa M, Takagi Y, Okada A, Mushiake S, Harada T. Effects of isolated small bowel transplantation on liver dysfunction caused by intestinal failure and long-term total parenteral nutrition. Pediatr Transplant. 2002;6:235–239. doi: 10.1034/j.1399-3046.2002.01074.x. [DOI] [PubMed] [Google Scholar]
- 40.Wolfe RB, Chinkes DL. Isotope Tracers in Metabolic Research. Wiley-Liss; Hoboken: 2005. pp. 27–28. [Google Scholar]