Abstract
Pathogens develop creative ways to undermine host defenses. In this issue of Cell Host & Microbe, Bakowski et al. (2010) have unveiled a mechanism by which Salmonella evades lysosomal fusion by using a bacterial protein, SopB, that depletes the phagosomal membrane of negative charge.
Children commonly begin to test the boundaries of their knowledge of electricity by performing one simple experiment: rubbing a balloon vigorously over their head and then sticking it to their mother’s back. Remarkably, in this issue of Cell Host & Microbe, Bakowski et al. (2010) report that the pathogen Salmonella Typhimurium coopts the intracellular environment of its host using a mechanism analogous to that underlying the balloon experiment.
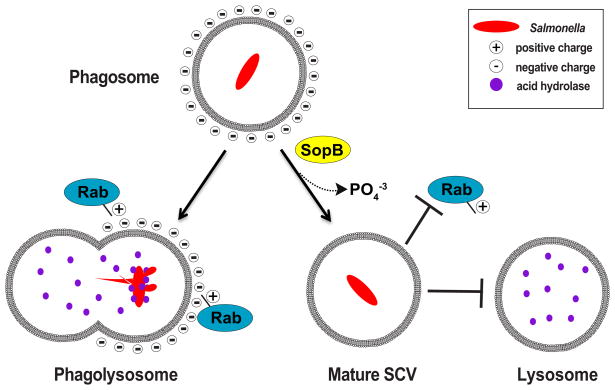
Bacteria in the genus Salmonella are Gram-negative facultative intracellular pathogens that cause food-borne illnesses such as typhoid fever and severe gastroenteritis (Steele-Mortimer, 2008). Upon contact with the intestinal epithelium, these pathogens induce their own uptake into nonphagocytic epithelial cells. Once inside the cell, Salmonella orchestrates a cell signaling program aimed at hijacking the endolysosome-trafficking environment of its host. Such a stealthy pathogenic strategy allows the bacterium to develop and maintain a unique phagocytic compartment called the Salmonella-containing vacuole (SCV) (Figure 1). From the pathogen’s point of view, coopting the membrane-trafficking network is an opportunity to find a replicative niche within a cell, thus avoiding the induction of an immunological response. Under normal circumstances, however, lysosomes routinely fuse with incoming phagosomes, presenting Salmonella with a second antimicrobial challenge (Figure 1). Bakowski et al. (2010) now propose a new model for how Salmonella defends itself from lysosomal fusion. Much like the balloon analogy above, the pathogen modulates the electrical charge of the SCV membrane, neutralizing the onslaught of antimicrobial attack within the host cell.
Figure 1.
SopB Depletes the SCV Membrane of Negative Charge, Thereby Blocking the Recruitment of Rab GTPases and Lysosomal Fusion with the Phagosome
It is well known that Salmonella gains control of intracellular trafficking events by injecting “effector” proteins into the cytosol of the host cell via two distinct type III secretion systems, designated SPI-1 and SPI-2. In their study, Bakowski et al. (2010) focused on a SPI-1 Salmonella effector, SopB, to evaluate its role in inhibiting lysosomal fusion with the SCV. SopB is a phosphoinositide (PI) phosphatase that dephosphorylates PI(4,5)P2 to produce PI(5)P. Through its phosphatase activity, SopB has previously been shown to localize to the host membrane early in infection and assists in bacterial internalization in an actin-dependent manner (Zhou et al., 2001). Once Salmonella is inside the cell, SopB dissociates from the plasma membrane through a polymonoubiquitinated signal and is retargeted to the phagosome (Marcus et al., 2002; Patel et al., 2009). SopB then aids in SCV maturation by changing the phosphorylation state of the PIs on the membrane of the SCV, which is thought to impact vesicle fusion, although the mechanism was previously unknown (Mallo et al., 2008; Yeung et al., 2008).
Here, Bakowski et al. (2010) specifically investigated the molecular consequences of dephosphorylating phospholipids at the SCV membrane. In particular, they focused on the ability of SopB to regulate the surface charge created by the polyanionic lipid PI(4,5)P2. Through an elegant series of cell biological and bacterial genetic studies, the authors convincingly showed that Salmonella neutralizes the negative charge on SCV membranes, and in doing so prevents the formation of a phagolysosome (Figure 1).
At the onset of their study, the authors found that the SCV membrane in cells infected with a wild-type Salmonella strain was electrically neutral, as it did not co-localize with the polycationic membrane probe, RpreRed. In contrast, the SCV was negatively charged in cells infected with a mutant Salmonella strain carrying a deletion of the sopB gene (ΔsopB). To further confirm that the loss of negative charge was due to SopB phosphatase activity, the authors probed the lipid environment using fluorescently labeled plekstrin homology domains, PLC-PH and LactC2, that recognize and bind PI(4,5)P2 or phosphotidylserine (PS), respectively. They found lower levels of both lipids present on the SCV of wild-type Salmonella compared to the SCV of a ΔsopB strain. The consequence of a loss of negative charge on the membrane was further shown when a series of Ras family small GTPases that share a positively charged polycationic targeting domain were absent on the SCV membrane of wild-type but present on the SCV membrane of ΔsopB mutants (Heo et al., 2006). Importantly, the GTPases excluded from the SCV belonged to the Rab subclass of membrane traffic control molecules such as Rab8, Rab13, Rab23, and Rab35 (Smith et al., 2007). It appears that the absence of negative charge on the SCV prevents lysosomal fusion events by restricting the accumulation of Rab GTPases at these sites.
While the first series of studies showed that SopB could affect the charge distribution and protein localization to the SCV, the next experiments directly tested the ability of SopB to inhibit lysosomal fusion with the phagosome. Through the use of a proteolytically activated fluorescent conjugate of BSA, the authors showed that the wild-type strain did not colocalize with degradative compartments, whereas the ΔsopB strains did. To further their case, the authors used a clever set of experiments to chemically couple the yeast 5-phosphatase, Inp54p, to the plasma membrane. Local accumulation of Inp54p rescued the ΔsopB strain by preventing the SCV from fusing with the lysosomal compartment. This experiment shows that dephosphorylation PI(4,5)P2 at either the 4- or 5-phosphate position is functionally equivalent. Thus, it appears that the overall charge density on the phospholipids, and not the specific identity of the PI second messenger, is the major contributing factor to SCV maintenance during Salmonella infection.
PI and PS are the major negatively charged lipids associated with the eukaryotic plasma membrane and endosomal compartments (Heo et al., 2006; McLaughlin and Murray, 2005; Yeung et al., 2008). While SopB specifically lowers the negative charge accumulation on PI(4,5)P2 through dephosphorylation, the authors unexpectedly found that PS is also restricted from the SCV. As the authors pointed out, it is currently unclear how PS is restricted from these sites, since SopB does not directly alter its turnover. Because PS is vastly more abundant than PI(4,5)P2 at the plasma membrane, there must be an active mechanism to restrict its accumulation at the sites of bacterial internalization. Future studies will be needed to address this important molecular mechanism. Nevertheless, Bakowski et al. (2010) uncovered a pathogenic strategy of host cell regulation that has broad implications both for eukaryotic cell signaling and for bacterial regulation of intracellular trafficking events.
The spatial and temporal dynamics of the SCV maturation process is a fascinating event that still holds many mysteries. It is currently unclear how Salmonella balances inhibition of lysosomal fusion through electrical charge remodeling with its need to promote membrane fusion with organelles that provide nutrients to the dividing bacterium. Although the goal of this new study was not to address this problem, it is likely that additional type III effectors facilitate membrane fusion events at late time points of infection when Salmonella has already established its replicative niche. In addition, all intracellular bacterial pathogens avoid lysosomal degradation. Using the techniques and molecular probes developed by these groups, further work can be done to see if alteration in the electric potential of membranes is a conserved molecular mechanism by which important human pathogens inhibit lysosomal fusion. Such studies will be instrumental to unravel the complexities by which numerous pathogens compromise the host cell traffic network.
References
- Bakowski MA, Braun V, Lam GY, Yeung T, Do Heo W, Meyer T, Finlay BB, Grinstein S, Brumell JH. Cell Host Microbe. 2010;7:453–462. doi: 10.1016/j.chom.2010.05.011. this issue. [DOI] [PubMed] [Google Scholar]
- Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, Meyer T. Science. 2006;314:1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallo GV, Espina M, Smith AC, Terebiznik MR, Aleman A, Finlay BB, Rameh LE, Grinstein S, Brumell JH. J Cell Biol. 2008;182:741–752. doi: 10.1083/jcb.200804131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus SL, Knodler LA, Finlay BB. Cell Microbiol. 2002;4:435–446. doi: 10.1046/j.1462-5822.2002.00202.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin S, Murray D. Nature. 2005;438:605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- Patel JC, Hueffer K, Lam TT, Galan JE. Cell. 2009;137:283–294. doi: 10.1016/j.cell.2009.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Heo WD, Braun V, Jiang X, Macrae C, Casanova JE, Scidmore MA, Grinstein S, Meyer T, Brumell JH. J Cell Biol. 2007;176:263–268. doi: 10.1083/jcb.200611056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele-Mortimer O. Curr Opin Microbiol. 2008;11:38–45. doi: 10.1016/j.mib.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung T, Gilbert GE, Shi J, Silvius J, Kapus A, Grinstein S. Science. 2008;319:210–213. doi: 10.1126/science.1152066. [DOI] [PubMed] [Google Scholar]
- Zhou D, Chen LM, Hernandez L, Shears SB, Galan JE. Mol Microbiol. 2001;39:248–259. doi: 10.1046/j.1365-2958.2001.02230.x. [DOI] [PubMed] [Google Scholar]