Summary
Psychological stress and its associated increases in corticosterone are generally immunosuppressive and contribute to increased herpes simplex virus (HSV)-associated pathogenicity. However, the impact of stress on local control of the initial mucosal-based HSV infection has not been elucidated, nor have the ramifications of such failures of the immune response in terms of viral spread. To address these gaps in knowledge, the studies described herein sought to determine how psychological stress and associated increases in corticosterone may increase susceptibility to HSV encephalitis by allowing for increased viral titers at the site of initial infection. We have shown that in mice intranasally infected with HSV-1, a cell-mediated immune response occurs in the nasopharyngeal-associated lymphoid tissue (NALT), mediastinal lymph nodes (MLN), and superficial cervical lymph nodes (CLN). However, psychological stress induced by restraint decreased the number of lymphocytes in these tissues in HSV-infected mice. Surprisingly, the effects of this restraint stress on HSV-specific CTL function varied by immune tissue. Increased viral titers were found in the nasal cavity of stressed mice, an observation which correlated with an increased CD8+ cell response in the CLN. These findings led us to extend our studies to also determine the ramifications of decreased numbers of locally-derived lymphocytes on viral titers following infection. Using an approach in which the NALT was surgically removed prior to infection, we confirmed that decreased numbers of NALT-derived lymphocytes at the time of infection allows for increased viral replication. We conclude that the increased viral titers observed in mice experiencing psychological stress are the consequence of a glucocorticoid-mediated reduction in the numbers of lymphocytes responsible for resolving the initial infection.
Keywords: Stress, Glucocorticoids, HSV-1, CD8+ T Lymphocytes, Mucosal immunity
Introduction
The mucosal lining of the respiratory tract is one of the most common routes of infection. Understanding the immune response to inhaled pathogens is of growing interest with the recent advent of intranasal vaccines. We and others have long been interested in determining the effects of psychological stress on the immune response to viral pathogens (reviewed in Bailey et al., 2003; Bonneau and Hunzeker, 2007; Bonneau et al., 2007). Neuroendocrine-derived peptides and hormones, including those associated with psychological stress, are clearly recognized as immune modulators. In particular, glucocorticoids have been shown to have multiple detrimental effects on the immune response to viral challenge. Studies by others have shown that psychological stress also impairs vaccine-elicited immune responses (Glaser et al., 1998; Jabaaij et al., 1993; Kiecolt-Glaser et al., 1996; Vedhara et al., 1999), and cytokine production (Connor et al., 2005; Goujon et al., 1995; Ortiz et al., 2003). Given the importance of the immune response to inhaled pathogens, in terms of both accidental environmental exposure and intentional vaccination, we sought to determine the impact of psychological stress on the number and function of CD8+ cells responding to intranasally-acquired pathogens.
Previous studies have focused on the impact of psychological stress on the immune response to HSV-infection when administered via the footpad (Bonneau et al., 1991; Brenner and Moynihan, 1997; Leo et al., 1998), eye (Freeman et al., 2007; Kip et al., 2001) and vagina (Wonnacott and Bonneau, 2002). Although previous studies have used an intranasal model of HSV-infection, these studies have focused on the outcome of viral spread to the brain, and susceptibility to herpes simplex encephalitis (Anglen et al., 2003; Nair et al., 2007), and did not examine the impact of stress on the immediate, local control of HSV at the site of infection.
In rodents, the immune response to intranasal pathogens occurs within the nasopharyngeal-associated lymphoid tissue (NALT) and the lymph nodes that drain the upper and lower respiratory tract (Heritage et al., 1998; Wu et al., 1997). Specifically, the superficial cervical lymph nodes (CLN) drain the nasal cavity and upper respiratory tract, and the mediastinal lymph nodes (MLN) drain the lower respiratory tract and lungs. The NALT is located on the posterior surface of the hard palate within the nasal cavity of rodents (Spit et al., 1989). Although humans lack a NALT in the same anatomic location as in rodents, it is generally accepted that the NALT is the rodent homologue to tonsils and adenoids, and provides the first line of immunological defense against inhaled pathogens (Hameleers et al., 1991; Wu et al., 1996; Zuercher et al., 2002). However, despite its presumed importance in the initial defense against inhaled pathogens, the anti-viral immune response within the NALT remains relatively undefined.
Though generally associated with either an oral or genital infection, HSV can be acquired intranasally, particularly during childbirth. While such infections are generally controlled by locally residing CD8+ T cells (Liu et al., 2000; Reading et al., 2006), the virus can spread to the brain in neonates as well as immunosuppressed adults, and result in herpes simplex encephalitis (HSE). As glucocorticoids are known to induce apoptosis of T lymphocytes (Blewitt et al., 1983; Caron-Leslie et al., 1991; Cohen and Duke, 1984; Wyllie, 1980), heightened levels of these hormones during HSV infection are associated with immunosuppression, viral spread to the CNS, reactivation of latent virus, and HSE (Freeman et al., 2007; Kusnecov et al., 1992; Nair et al., 2007). Therefore, HSE-associated pathogenesis could be better controlled, if not averted, by a clearer understanding of the immune response following this route of infection, as well as steps at which glucocorticoid-mediated failures in such a response occur.
To determine the effects of psychological stress on an immune response following intranasal infection, we examined the impact of physical restraint during intranasal HSV-1 infection on HSV-antigen-specific CD8+ T lymphocyte number and function. Additionally, we determined the effects of this stress on viral replication within the nasal cavity. Our initial findings support and extend the findings of previous studies, in that the observed alterations in the immune response, at least in terms of cell numbers, result from glucocorticoid-mediated mechanisms. Additionally, we present data suggesting that it is the reduced numbers of lymphocytes that account for both the increased viral load and the anatomical differences in CD8+ T cell function in stressed mice.
Methods
Animals
Male C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were obtained at 5–6 weeks of age. All mice were group-housed at 4 mice per cage, maintained on a 12/12 hour light/dark cycle (lights on at 0000 and off at 1200), and were given access to food and water ad libitum. No experiments were conducted until mice were acclimated to these conditions for at least 1 week. All experimental procedures were carried out according to guidelines set by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International) and the National Institutes of Health.
Virus
HSV-1 strain McIntyre was prepared by infection of Vero cells at a multiplicity of infection of 0.01. Virus titers were assessed by plaque assay on Vero cells and viral stocks were stored at −70°C.
Intranasal infection of mice
Mice were infected as previously described (Anglen et al., 2003). Briefly, 6–8 week old male mice were anesthetized with a 0.2 mL intraperitoneal (i.p.) injection of ketamine/xylazine (1.5 mg/kg ketamine; 0.1 mg/kg xylazine) prepared in sterile, distilled water. Mice were then infected with 1×107 plaque forming units (PFU) of HSV-1 McIntyre in 20 µL of phosphate-buffered saline (PBS) containing 1% (v/v) fetal bovine serum (FBS).
Restraint stress protocol
Beginning one day prior to HSV-1 infection, mice were physically restrained (without squeezing or compression) in well-ventilated, 50 mL conical tubes containing approximately one-hundred 0.4 cm diameter holes (Anglen et al., 2003; Nair and Bonneau, 2006; Nair et al., 2007). This procedure induces psychological stress because of the animal’s confinement. Mice were able to move forward and backward, but were unable to turn around. Mice were restrained for 16 hours (1700 to 0900) per session beginning 5 hours into the dark cycle. Since restrained mice were not able to obtain food and water during this 16-hour stress session, non-stressed control mice were deprived of food and water during the same period of time. All mice received equal access to food and water following the termination of each stress session.
Serum collection and corticosterone radioimmunoassay
Mice were anesthetized in a saturated atmosphere of halothane (Halocarbon Laboratories, River Edge, NJ) and blood was collected via retroorbital puncture. Blood samples were allowed to clot, after which time they were centrifuged at 16,000 × g. The resulting serum was removed and stored at −70°C. Serum corticosterone was determined using a corticosterone 125I radioimmunoassay kit (MP Biomedicals, Costa Mesa, CA). Corticosterone values were calculated from a standard curve which was generated using 0 to 1000 ng/mL corticosterone standards.
Exogenous corticosterone administration
Corticosterone (ICN Pharmaceuticals) was dissolved in HBC (2-hydroxypropyl-β-cyclodextrin; Sigma, St. Louis, MO) at a concentration of 30% (w/v) and then diluted to 100 µg/mL with tap water. The final concentration of HBC in the drinking water was 0.3%. Water was changed every 2 days.
Pharmacological blockade of the type II glucocorticoid receptor
Mice were administered RU486 (Sigma) subcutaneously (s.c.) in sesame oil (MP Biomedicals) at a dosage of 25 mg/kg. RU486 injections were administered 24 hours prior to the beginning of the first stress session and then two hours before beginning the restraint sessions on every day thereafter. This dose of RU486 has been used previously in our laboratory and has been shown to be effective in blocking the type II glucocorticoid receptor (Nair and Bonneau, 2006).
Isolation of lymphoid cells from NALT
NALT was isolated as described previously (Asanuma et al., 1997), with slight modifications. Mice were administered sodium pentobarbital (100 mg/kg; Nembutal®, Abbott Laboratories, North Chicago, IL) and exsanguinated via cardiac puncture. The jaw muscles were severed and the mouths were opened to reveal the palate. An incision was made along the toothline with a scalpel and the palate was peeled back from the incisors toward the molars and placed in Iscove’s-modified Dulbecco’s media (IMDM) (Gibco; Carlsbad, CA) supplemented with 10% (v/v) fetal bovine serum (FBS), 50 µM 2-mercaptoethanol, 2 mM glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin sulfate. To dissociate lymphoid cells from the palates, the palates were incubated in 2 mg/mL collagenase D (Roche, Indianapolis, IN) for 40 minutes at 37°C and then rubbed against a 70 µm nylon cell strainer (BD Biosciences, Bedford, MA). This method allowed for the disruption of NALT-derived lymphocytes from the palate without homogenizing the palate itself. The resulting cell suspension was washed with supplemented IMDM. The number of viable cells was determined by trypan blue dye exclusion.
Isolation of cells from lymph nodes
Mice were anesthetized and exsanguinated as described above. Superficial cervical (CLN) and mediastinal (MLN) lymph nodes were removed and placed in Iscove’s-modified Dulbecco’s media (IMDM) (Gibco) supplemented with 10% (v/v) fetal bovine serum (FBS), 50 µM 2-mercaptoethanol, 2 mM glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin sulfate. Lymph nodes were then mechanically dissociated by passage through a 70 µm nylon cell strainer (BD Biosciences) and the resulting cell suspension was washed with supplemented IMDM. The number of viable cells was determined by trypan blue dye exclusion.
Analysis of cell surface markers and tetramer specificity
Flow cytometric analysis of cell surface markers was determined as described previously (Anglen et al., 2003; Nair and Bonneau, 2006; Zhang et al., 2004) with slight modifications. Briefly, CD16/CD32 Fcγ receptors on isolated mononuclear cells were blocked with antibody in a 2.4G2 hybridoma cell culture supernatant supplemented with 20% mouse serum (Sigma) (Anglen et al., 2003). Cell surface expression of CD8 was detected using anti-CD8 APC antibody (clone 53-6.7; eBioscience). Cell surface expression of CD4 was detected using anti-CD4 FITC antibody (clone GK1.5; eBioscience). For the detection and quantification of gB498–505 epitope-specific T lymphocytes, cells were incubated with a PE-labeled tetramer that was prepared as described previously (Altman et al., 1996) and was a kind gift from Dr. Satvir Tevethia, (The Pennsylvania State University College of Medicine). This tetramer detects the H-2Kb-restricted, gB498–505-specific T cell receptor complex (Blaney et al., 1998), which has previously been described as the immunodominant epitope in C57BL/6 mice (Bonneau et al., 1993a; Hanke et al., 1991). Following washes with FACS buffer (PBS supplemented with 1% [v/v] FBS, 0.02% [w/v] sodium azide), cells were resuspended in 2% (w/v) paraformaldehyde (prepared in PBS) prior to analysis by flow cytometry.
Degranulation assay for T cell lytic function
CD107a (LAMP-1) expression was determined as described previously (Betts and Koup, 2004), with slight modifications. Lymphocytes were resuspended in supplemented IMDM and incubated with 1 µM gB498–505 peptide and a 1:100 dilution of anti-CD107a FITC antibody (clone 1D4B; BD Pharmingen) for 1 hour at 37°C. All cells were then treated with 10 mM of ammonium chloride for three hours at 37°C to prevent acidification of endosomes and the subsequent loss of the FITC signal (Hoppe et al., 2004; Jin et al., 2005). Following this incubation, cells were washed twice with FACS buffer and the CD16/CD32 Fcγ receptors were blocked with 2.4G2 cell culture supernatant supplemented with 20% mouse serum. The cells were then incubated with anti-CD8 APC antibody, resuspended in 2% paraformaldehyde, and analyzed by flow cytometry.
Intracellular cytokine staining for IFN-γ
The NALT- and lymph node-derived lymphocytes were isolated as described above. These cells were resuspended in supplemented IMDM and incubated with 1 µM gB498–505 (SSIEFARL) peptide (synthesized at The Pennsylvania State University College of Medicine Macromolecular Core Facility) for 2 hours at 37°C. Cells were treated with brefeldin A (Sigma) (final concentration of 5 µg/mL) to prevent the secretion of cytokines and incubated for an additional 4 hours at 37°C. Cells were then washed twice with FACS buffer and the CD16/CD32 Fcγ receptors blocked with 2.4G2 cell culture supernatant supplemented with mouse serum as described above. To identify CD8+ T lymphocytes, cells were incubated with anti-CD8 APC antibody. Following staining for CD8, cells were fixed in 2% paraformaldehyde and incubated at room temperature for 20 minutes in the dark. Cells were then washed twice with FACS buffer and incubated with anti-IFN-γ FITC antibody (clone XMG1.2; eBioscience) diluted in 0.5% (w/v) saponin (Sigma) prepared in FACS buffer. Subsequently, cells were washed twice in 0.5% saponin, resuspended in 2% paraformaldehyde, and analyzed by flow cytometry.
Viral plaque assays from nasal washes
Mice were anesthetized with Nembutal® (100 mg/kg) and exsanguinated via cardiac puncture. An incision along the toothline was made with a scalpel, and the palate was peeled back to expose the nasal cavity. The nasal cavity was washed 5 times with 200 µL of PBS containing 1% (v/v) FBS for a total volume of 1.0 mL. The recovered PBS was stored at −70°C until plaque assays were performed. The PBS washes underwent three freeze/thaw cycles, and were sonicated in a water bath sonicator (Sinosonic Industrial Co., Taiwan) three times for one minute each. These washes were centrifuged at 130 × g for 10 minutes to remove cell debris, and virus levels were determined by standard plaque assay on Vero cells. Cells were fixed and stained with 5% (v/v) formaldehyde/0.5% (w/v) crystal violet. Plaques were counted and normalized for the total volume of PBS retrieved from each mouse following the nasal wash procedure.
Surgical removal of NALT
The NALT was surgically removed as described in detail elsewhere (Wiley et al., 2005), with slight modifications. Briefly, mice were anesthetized with Nembutal® (70 mg/kg) and their mouths held open with a plastic ring (outer diameter = 1.0 cm; inner diameter = 0.5 cm). An incision was made along the hard palate. A 0.5 mm microcurette (Fine Science Tools, Foster City, CA) was inserted into the incision and used to scrape the nasal cavity on both sides of the nasal septum. Pressure was applied to the incision site until bleeding stopped. Analgesia was provided by Buprenex® (Reckitt Benckiser; Richmond, VA) (0.2 mg/kg), administered intraperitoneally every 12 hours, for 48 hours following surgery. Mice were allowed to recover for 20 days prior to being used for further experimentation. Control mice were only anesthetized and received analgesia.
Flow cytometry analysis
Flow cytometric analysis was conducted using a FACSCalibur flow cytometer (Becton Dickinson, San Diego, CA). Using forward-angle light scatter and 90° light scatter profiles, electronic gates were set around the live cells and at least 50,000 events were collected per sample. Dot plots and histograms were analyzed using FlowJo Software (TreeStar, Inc.; Ashland, OR). The total number of cells per sample was determined as follows: [percentage of specific cell type in sample] × [number of viable cells in sample].
Statistical analysis
Statistical significance was determined by analysis of variance (ANOVA) using StatView 5.0.1 software (SAS Institute Inc, Cary, NC). Comparisons between groups were performed using unpaired t-test and p values < 0.05 were considered significant.
Results
Psychological stress and corticosterone decrease the number of lymphoid cells in the secondary lymphoid tissues
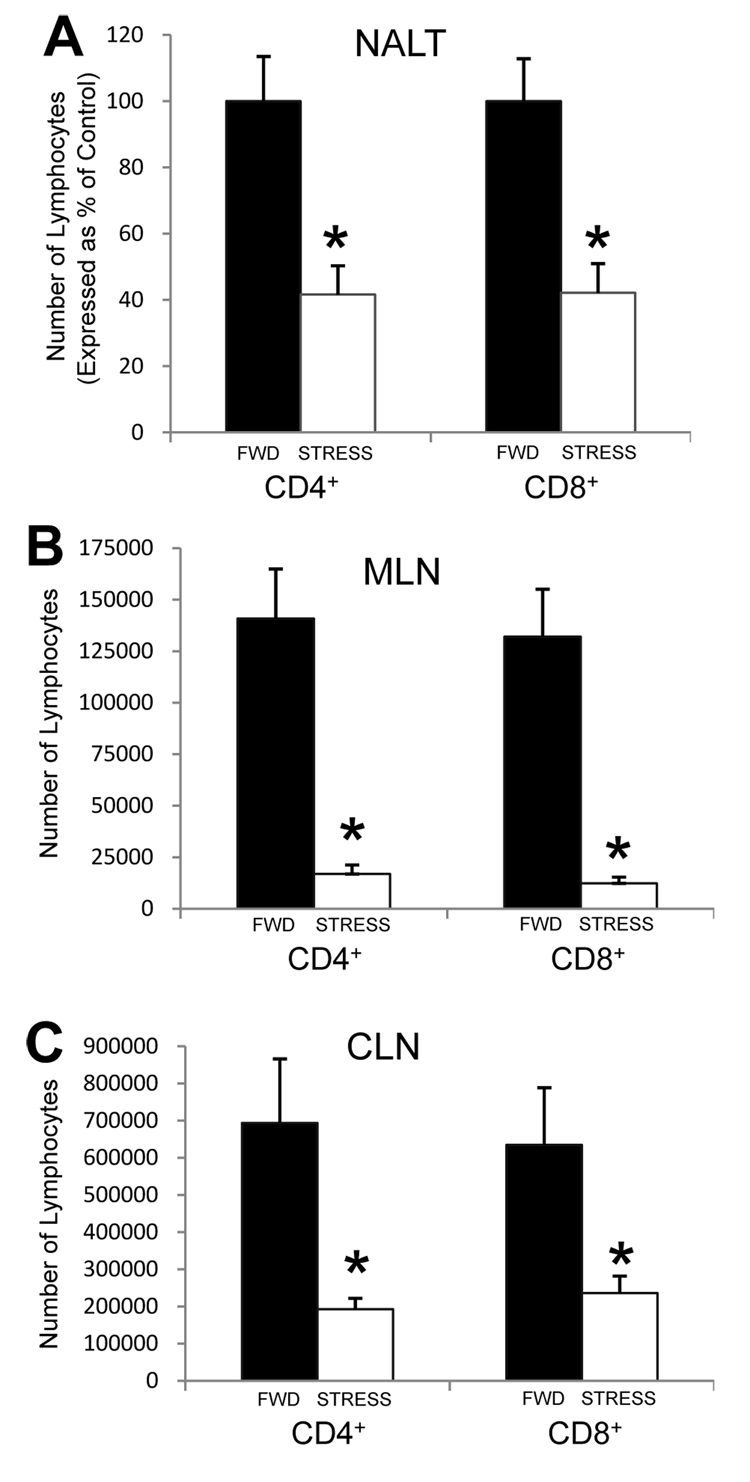
To determine the effects of stress on the T lymphocyte composition of respiratory tract-associated secondary lymphoid tissues in non-infected mice, mice underwent daily restraint stress for six consecutive days. After six sessions of restraint, peripheral blood from a subset of mice was collected and serum corticosterone levels were measured. As was expected, mice experiencing restraint stress had an average serum corticosterone level of approximately 300 ng/mL, whereas serum from food-and-water-deprived mice contained less than 100 ng/mL. Immediately following the last session of restraint, mice were euthanized and the NALT, CLN, and MLN were removed and assessed for the numbers of both CD4+ and CD8+ lymphocytes. Stressed mice exhibited a significant decrease in the number of lymphocytes expressing these markers in the NALT (Figure 1A), MLN (Figure 1B), and CLN (Figure 1C) as compared to control mice that were deprived of food and water (FWD) during the same time periods. This decrease was due to a combination of a decreased total number of lymphocytes within the tissues, as well as a decrease in the percentages of CD4+ and CD8+ cells (data not shown).
Figure 1.
Effect of stress on CD4+ and CD8+ T lymphocyte populations within the NALT (A), MLN (B), and CLN (C). Mice were subjected to food-and-water deprivation (FWD; black bars) or restraint stress (STRESS; white bars) for six consecutive sessions. Immediately following the sixth session, mice were euthanized and lymphocytes from the NALT, MLN, and CLN were isolated and quantified as described in the Methods. The numbers of NALT-derived lymphocytes for restraint mice are expressed as a percentage of the FWD control mice, since the numbers of NALT-derived lymphocytes varied among experiments. n = 5–9 for all groups. * Significant difference (p < 0.05) as compared to FWD control mice.
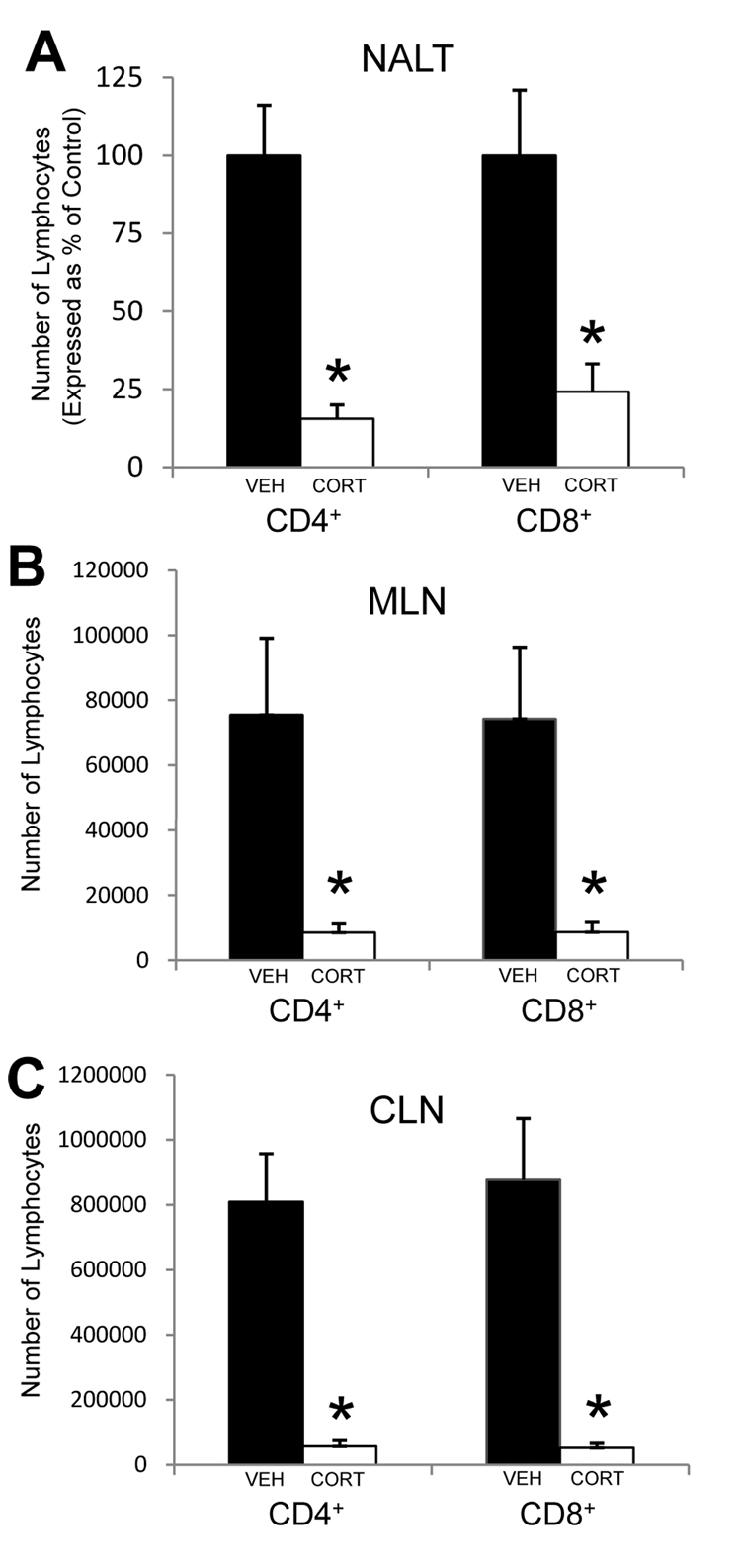
We next mimicked the stress-induced increase in corticosterone by providing mice with exogenous corticosterone in their drinking water. The concentration used (100 µg/mL) has been shown to increase serum corticosterone to levels similar to those seen in mice undergoing restraint stress (approximately 300 ng/mL). Mice were provided access to corticosterone-containing drinking water beginning six days prior to analysis of the lymphoid tissues. NALT, MLN, and CLN were removed and assessed for the numbers of both CD4+ and CD8+ T lymphocytes. Mice that were provided exogenous corticosterone in their drinking water had a significant decrease in the number of CD4+ and CD8+ T cells in the NALT (Figure 2A), MLN (Figure 2B), and CLN (Figure 2C) as compared to mice provided vehicle alone.
Figure 2.
Effect of exogenous corticosterone on CD4+ and CD8+ T lymphocyte populations within the NALT (A), MLN (B), and CLN (C). Mice were provided drinking water containing either vehicle (VEH; black bars) or 100 µg/mL corticosterone (CORT; white bars) for six consecutive days. On the sixth day, mice were euthanized and lymphocytes from the NALT, MLN, and CLN were isolated and quantified as described in the Methods. The numbers of NALT-derived lymphocytes for CORT mice are expressed as a percentage of control (VEH) mice, since the numbers of NALT-derived lymphocytes varied among experiments. n = 4–7 for all groups. * Significant difference (p < 0.01) as compared to VEH control mice.
The glucocorticoid receptor antagonist, RU486, blocks stress-associated decreases in lymphoid cells
We further tested our hypothesis that stress mediates the decrease in lymphocyte numbers via a glucocorticoid-receptor mediated mechanism by treating mice with the glucocorticoid receptor antagonist, RU486. RU486 or vehicle was administered daily, beginning 24 hours before the start of the first stress session. Mice underwent daily restraint stress for six consecutive days. Immediately following the last session of restraint, mice were euthanized and the NALT, MLN, and CLN were removed and assessed for the numbers of both CD4+ and CD8+ lymphocytes. As was expected, mice that experienced restraint stress while receiving only vehicle demonstrated a significant decrease in the number of CD4+ and CD8+ T cells within the NALT, MLN, and CLN (Table 1). However, mice that received RU486 while experiencing restraint stress demonstrated a less extensive decrease in lymphoid cell numbers (Table 1).
TABLE 1.
Treatment with the type II glucocorticoid receptor antagonist RU486 partially alleviates the stress-induced decrease in T lymphocytes
| NALTa | MLNb | CLNc | ||||
|---|---|---|---|---|---|---|
| CD4+ | CD8+ | CD4+ | CD8+ | CD4+ | CD8+ | |
| FWD, VEHICLE | 25.1 ± 2.8 | 16.6 ± 2.2 | 11.3 ± 3.3 | 11.1 ± 3.1 | 6.1 ± 0.6 | 5.4 ± 0.5 |
| STRESS, VEHICLE | 9.9 ± 2.9 | 6.2 ± 2.1 | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.4 ± 0.2 | 1.3 ± 0.2 |
| STRESS, RU486 | 22.0 ± 5.5 | 9.7 ± 2.0 | 4.4 ± 0.7 | 4.0 ± 0.6 | 4.0 ± 0.6 | 3.8 ± 0.6 |
Number of cells × 104 ± SEM. n = 6 for all groups
Number of cells × 104 ± SEM. n = 8 for all groups
Number of cells × 105 ± SEM. n = 8 for all groups
Psychological stress reduces the number of immune cells associated with the response to HSV infection
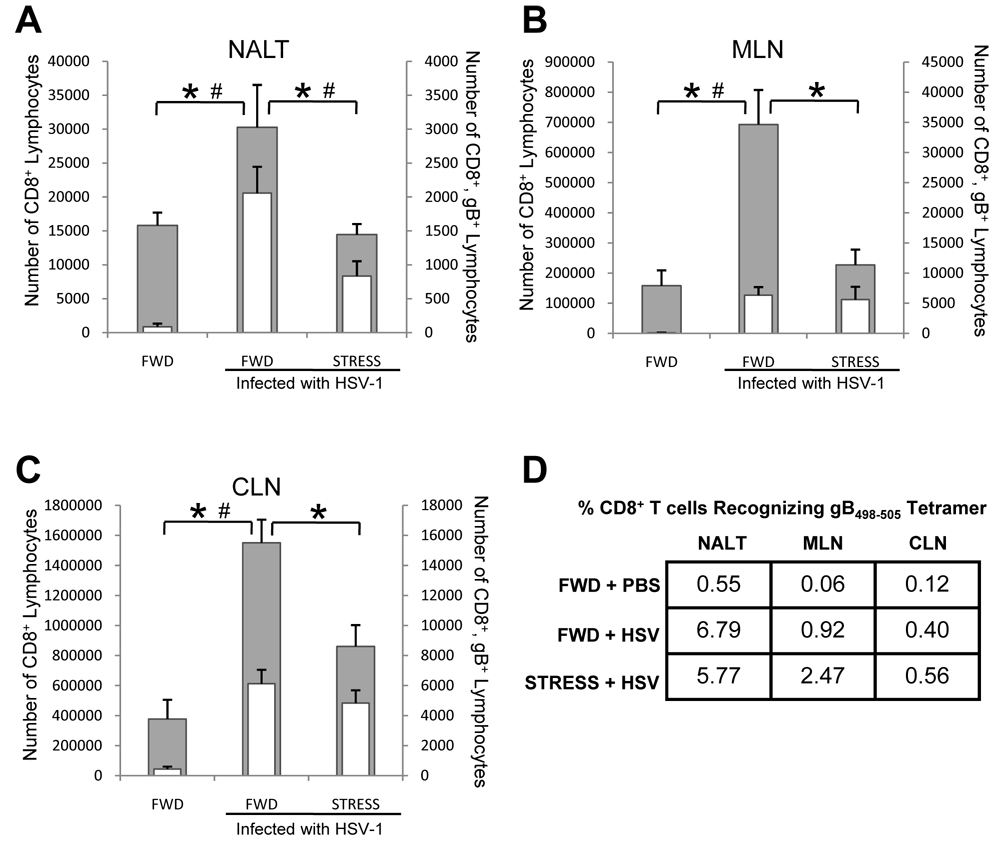
Having shown that psychological stress decreases the number of T cells within the NALT, MLN, and CLN, we hypothesized that the immune response to intranasal HSV infection would be compromised in stressed mice. To test this hypothesis, mice were restrained beginning one day prior to intranasal infection with 1 × 107 PFU HSV-1. Restraint stress sessions continued each day thereafter, and mice were euthanized seven days post infection. As was expected, mice that were HSV-infected but not stressed at the time of infection had a significant increase in the number of both total CD8+ and HSV-1 gB498–505-specific CD8+ T cells in the NALT (Figure 3A), MLN (Figure 3B), and CLN (Figure 3C) as compared to mice that were not infected with HSV. However, we observed significantly fewer total CD8+ lymphocytes within the NALT (Figure 3A), MLN (Figure 3B) and CLN (Figure 3C) of HSV-1 infected mice undergoing restraint stress. Furthermore, in the NALT, the number of gB498–505-specific CD8+ T cells was significantly fewer than in non-stressed mice (Figure 3A). Despite the fewer number of total CD8+ T cells within the MLN (Figure 3B) and CLN (Figure 3C), the percentage of those cells specific for gB498–505 was greater than in those mice that were not stressed (Figure 3D). As a result, the number of CD8+, gB498–505-specific T cells in these lymph nodes was the same in both stressed and non-stressed mice (Figure 3B, 3C).
Figure 3.
Effect of stress on total and gB498–595-specific CD8+ T lymphocytes. Mice were subjected to restraint stress (STRESS) or food-and-water deprivation (FWD) and infected with 1 × 107 PFU HSV-1 as described in the Methods. Seven days post-infection, mice were euthanized and lymphocytes from the NALT (A), MLN (B), and CLN (C) were collected and quantified for CD8+ expression (gray bars), and assessed for gB498–505-specificity (white bars) using a gB498–505/H-2Kb tetramer. The percentage of CD8+ T cells with receptors specific for the gB498–505 epitope is illustrated in panel D. n = at least 5 for all groups. * Significant difference (p < 0.05) in total CD8+ cell numbers between the indicated groups. # Significant difference (p < 0.05) in gB498–505-specific T cells between the indicated groups.
Stress-mediated changes in CD8+ T cell function varies by lymphoid tissue
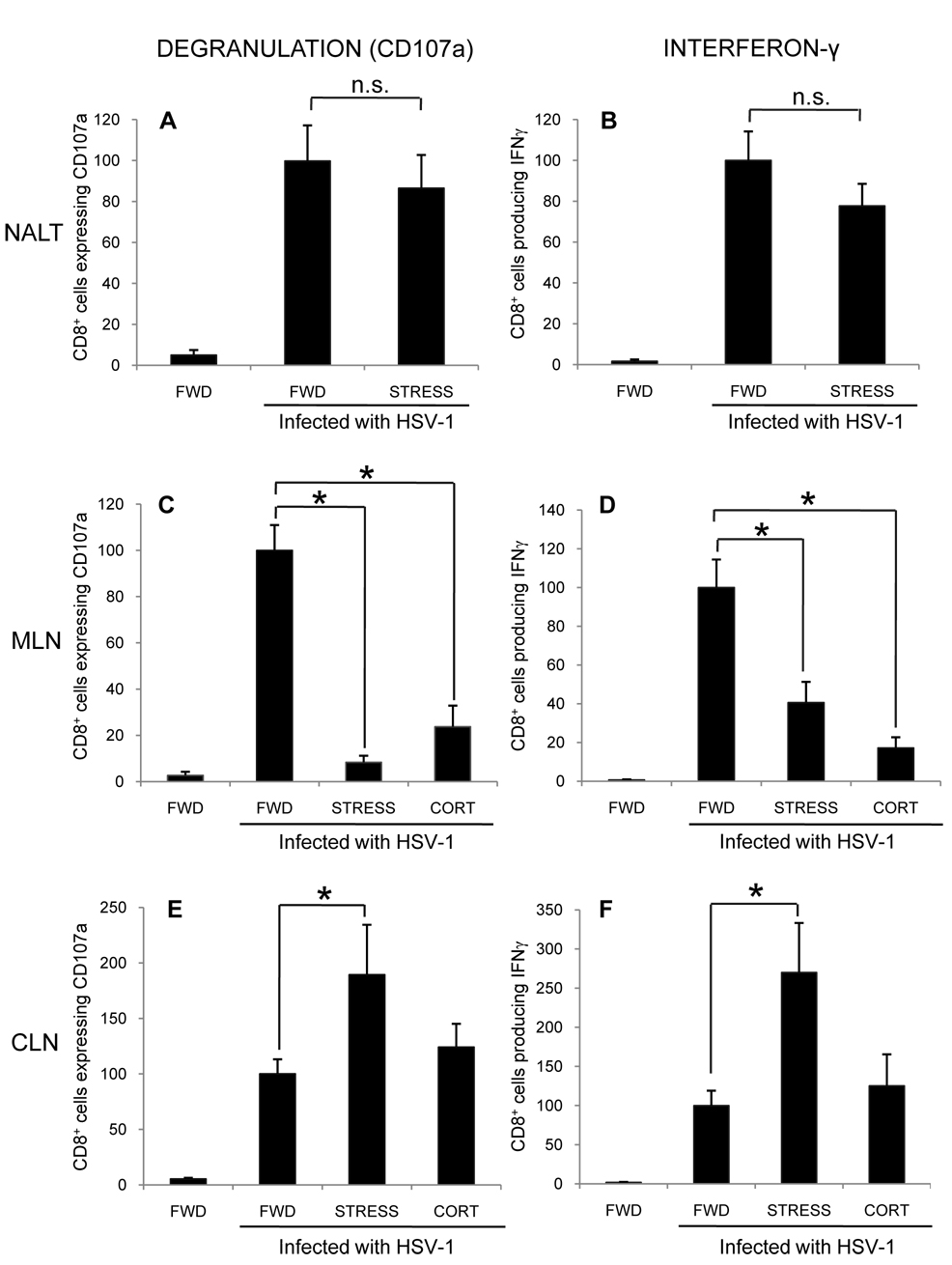
Although restraint stress did not alter the number of CD8+, gB498–505-specific T cells in the MLN and CLN, it was important to determine their function, and thus, possibly their ability to limit the extent of HSV infection. To determine the effects of stress on this function, mice were infected intranasally with 1 × 107 PFU HSV-1, and subjected to restraint stress sessions beginning one day prior to infection and continuing daily. At seven days post infection, mice were euthanized and the NALT, MLN, and CLN were collected. The CD8+ T cells within these tissues were assessed for their ability to express the degranulation marker CD107a and produce intracellular IFN-γ, both in response to stimulation with the gB498–505 peptide.
As was expected, relatively few cells in the NALT (Figures 4A, 4B), MLN (Figure 4C, 4D), and CLN (Figure 4E, 4F) of uninfected, control mice expressed CD107a and produced IFN-γ. In HSV-infected mice, there was a significant increase in the number of cells responding to peptide stimulation in terms of both CD107a expression and IFN-γ production at all three sites examined (Figure 4A–F). These findings indicate that intranasal HSV infection elicits an HSV-specific CD8+ T cell response in each of these anatomic locations. In HSV-infected mice subjected to stress, there was no difference in the number of NALT-derived CD8+ T cells that degranulated (Figure 4A) or produced IFN-γ (Figure 4B) as compared to FWD control mice. However, there was a significant decrease in the number of MLN-derived CD8+ cells that degranulated (Figure 4C) and produced interferon-γ (Figure 4D). Surprisingly, there was a significant increase in the number of CLN-derived CD8+ cells that degranulated (Figure 4E) and produced IFN-γ (Figure 4F). Therefore, despite the decrease in the number of CD8+ T cells in the CLN, there was still a significantly greater number of functional CD8+, gB498–505-specific T cells.
Figure 4.
Effect of stress and exogenous corticosterone on CD8+ T cell function within the NALT, MLN, and CLN. Mice were subjected to restraint stress (STRESS) or provided with corticosterone (CORT) beginning one day prior to infection with 1 × 107 PFU HSV-1. Seven days post-infection, the CD8+ cells within the NALT (A, B), MLN (C, D), or CLN (E, F) were assessed for degranulation (A, C, E) or interferon-γ production (B, D, F) in response to stimulation with gB498–505 peptide. Results were normalized within individual experiments to the number of functioning cells in HSV-1 infected, food-and-water-deprived mice. n = at least 4 for all groups. * Significant difference (p < 0.05) as compared to infected, FWD control mice.
To assess the contribution of glucocorticoids alone, additional mice were provided with corticosterone in their drinking water beginning one day prior to infection and continuing until mice were euthanized seven days post-infection. As restraint stress did not alter the function of CD8+ cells within the NALT (Figure 4A, 4B), we examined only the MLN and CLN. In the MLN, there was a significant decrease in both degranulation (Figure 4C) and IFN-γ production (Figure 4D) in HSV-1 infected mice that received corticosterone as compared to FWD control mice. These results were similar to those seen in mice that were restrained throughout the infection, supporting the hypothesis that stress-induced glucocorticoids are at least partly responsible for reducing the number of functional CD8+ cells in the MLN. In contrast, providing HSV-infected mice with exogenous corticosterone did not increase the number of functional CD8+ T lymphocytes as was observed in CLN of mice undergoing restraint stress (Figure 4E, 4F).
Psychological stress or exogenous corticosterone limit local control of infection
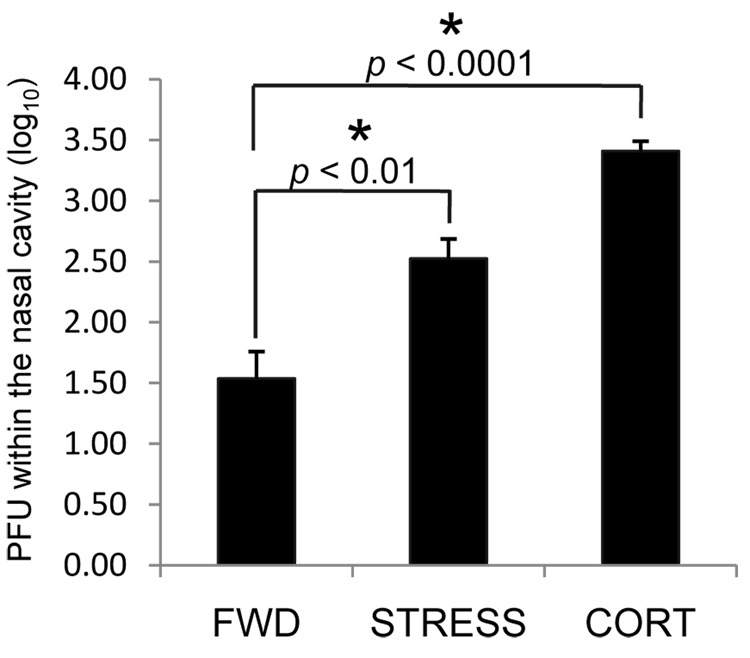
We hypothesized that the increase in functional HSV-1 specific CD8+ cells in the CLN of mice undergoing restraint stress is a consequence of increased viral antigenic load within the tissues draining these lymph nodes due to increased viral replication and/or persistence. To address this hypothesis, we determined the level of infectious HSV within the nasal cavity. Mice were infected intranasally with 1 × 107 PFU HSV-1. Restraint stress sessions began one day prior to infection, and continued daily until mice were euthanized five days post-infection. Nasal washes were collected as described in the Methods, and HSV titers determined by plaque assay. There was a significant increase in the level of infectious virus in the nasal cavity of mice experiencing restraint stress at the time of infection as compared to FWD control mice (Figure 5). To address the possible contribution of glucocorticoids in mediating these findings, we provided an additional set of mice with corticosterone via their drinking water beginning one day prior to intranasal infection. Five days post-infection, mice were euthanized and the level of HSV in the nasal cavities was determined. Mice that were provided corticosterone also had significantly higher levels than FWD control mice. These studies suggest that increased levels of infectious virus in the nasal cavity are a function of increased levels of glucocorticoids and associated decreases in NALT-derived lymphocytes.
Figure 5.
Effect of stress and exogenous corticosterone on the control of intranasal HSV-1 infection. Mice were intranasally infected with 1 × 107 PFU HSV-1. Restraint stress (STRESS) or providing corticosterone in the drinking water (CORT) began one day prior to infection; control mice were deprived of food and water (FWD) in lieu of restraint. Five days post-infection, mice were euthanized and nasal washes were collected as described in the Methods. Virus within the washes was titered and is expressed in terms of log10 PFU/nasal cavity. n = 6–14 for all groups. * Significant difference between the indicated groups.
Surgical removal of NALT results in increased viral titers in nasal cavities of infected, unstressed mice
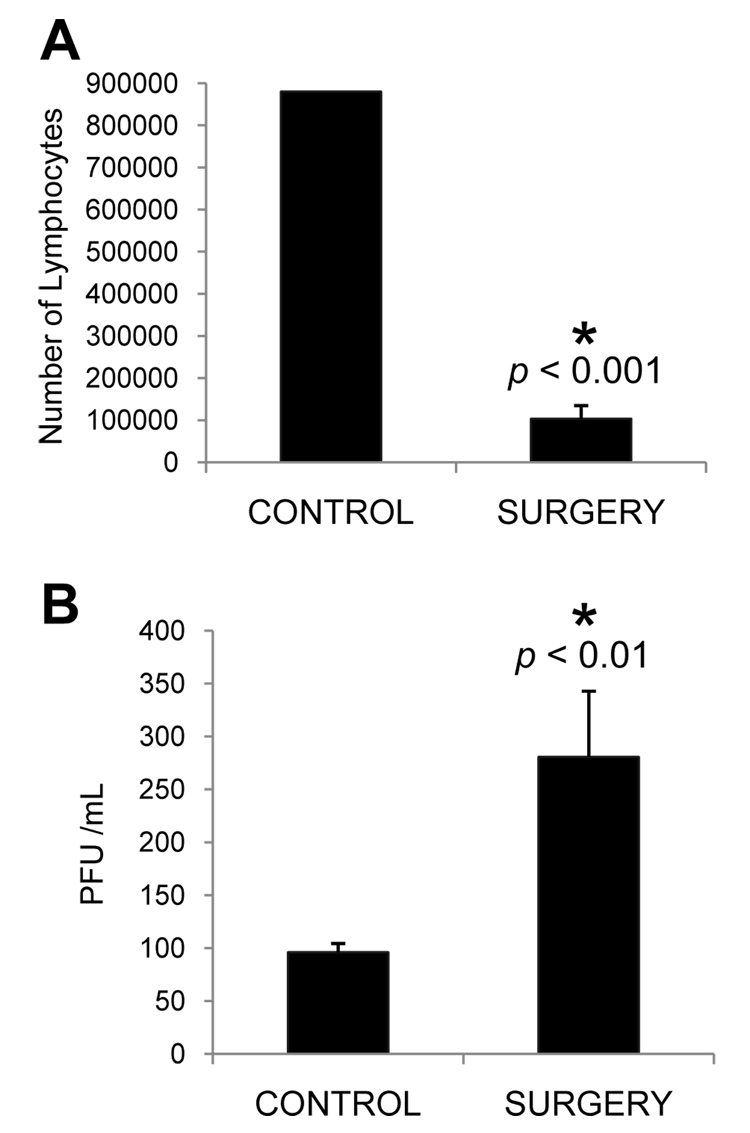
To further test the hypothesis that NALT-derived lymphocytes play an important role in controlling the levels of infectious HSV in the nasal cavity, we employed a recently described method to surgically remove the NALT (Wiley et al., 2005). Following the surgical recovery period, both NALT-depleted and control mice were infected with 1 × 107 PFU HSV-1 intranasally. At five days post-infection, mice were euthanized and nasal washes were collected as described in the Methods. In order to confirm that surgery decreased the number of NALT-derived lymphocytes, the palates from a subset of these mice were collected at the time of euthanasia, and lymphocytes were isolated and counted by trypan blue dye exclusion. As was expected, mice undergoing NALT removal surgery had significantly fewer lymphocytes as compared to control mice (Figure 6A). Nasal washes from mice whose NALT had been surgically removed contained a significantly greater amount of infectious virus as compared to control mice (Figure 6B). These findings indicate that a decrease in the number of NALT-derived lymphocytes impairs the ability to resolve an intranasal HSV-1 infection. This failure of the local immune response results in increased levels of infectious virus, and supports our earlier hypothesis that the increased number of functional CLN-derived CD8+ T cells is a consequence of an increased viral antigenic load.
Figure 6.
Effect of surgical NALT removal on HSV titers following intranasal infection. Mice underwent surgery to remove the NALT (SURGERY) or were simply administered anesthesia and analgesia (CONTROL). Three weeks after surgery, all mice were infected with 1 × 107 PFU HSV-1. Five days post-infection, the NALT was examined to confirm the surgical reduction in NALT-derived lymphocytes (A), and nasal washes were collected. The levels of infectious HSV within the washes were determined and are expressed in terms of PFU/mL (B). n = 2, 3 (A) and 7, 6 (B) for control and surgery groups, respectively. * Significant difference as compared to control mice.
Discussion
The respiratory tract is essentially an open door for pathogens that are aerosolized or introduced through exposure to bodily fluids. In rodents, the primary site of immunological responses to these pathogens is the nasopharyngeal-associated lymphoid tissue (NALT). The NALT is widely accepted as the rodent homologue to human tonsils and adenoids. The NALT contains all of the cells that are necessary to elicit an anti-viral immune response, including both CD4+ and CD8+ T lymphocytes (van der Ven and Sminia, 1993). Unfortunately, despite its presumed importance in defense against inhaled pathogens, the immune response within the NALT has only recently begun to be characterized. As the natural exposure to pathogens most often occurs at mucosal sites, a better understanding of both the components of a mucosal immune response as well as external factors that modulate this response is essential. Therefore, we sought to characterize the effects of psychological stress on the cellular immune response to an intranasal pathogen, HSV-1.
Our initial studies simply determined that restraint stress (Figure 1) and corticosterone (Figure 2) decrease the number of CD4+ and CD8+ T lymphocytes in the NALT, MLN, and CLN. These observations support the existing literature, which portrays corticosteroids as being generally immunosuppressive during intranasal infection (Hermann et al., 1995). This decrease was not surprising, as nearly three decades of research has established the fact that glucocorticoids reduce T cell numbers by inducing their apoptosis (Blewitt et al., 1983; Caron-Leslie et al., 1991; Cohen and Duke, 1984; Wyllie, 1980). Using annexin V and propidium iodide staining, we confirmed that there is indeed an increase in the number of cells undergoing apoptosis (data not shown). Mice receiving corticosterone displayed a greater degree of immunosuppression than did mice that were subjected to restraint stress. This observation is likely due to their continuous exposure to increased levels of corticosterone, in contrast to the cyclic rises and falls in corticosterone that occur in restrained mice during any 24-hour period. Although CD8+ T cells are directly involved in clearing viral infections via their destruction of virus-infected cells, our observed decrease in CD4+ T cells is also noteworthy since the CD4+ subset of T cells is involved in the generation of both primary and memory CD8+ cells (Janssen et al., 2003; Williams and Bevan, 2007).
RU486 blocks the type II glucocorticoid receptor (Emilie et al., 1984). In our studies, treatment of mice with RU486 partially blocked the decrease in lymphocyte numbers in the NALT, MLN, and CLN of mice experiencing restraint stress (Table 1). Although stressed mice receiving RU486 still had fewer lymphocytes than did non-stressed mice, there were significantly more cells in the MLN and CLN of stressed mice than in mice receiving vehicle only. We were not surprised to find that the mice receiving RU486 still demonstrated a reduction in lymphocyte numbers as RU486 is known to have agonistic capabilities in some cell types, including lymphocytes (Gruol and Altschmied, 1993). Therefore, a low level of RU486-mediated apoptosis likely contributes to the decrease in lymphocytes in stressed mice receiving RU486. The failure of RU486 to completely block the reduction of lymphocytes suggests a role for a stress-induced, sympathetic nervous system-mediated mechanism, as we have described previously (Bonneau et al., 1993b; Leo and Bonneau, 2000). This hypothesis is supported by previous findings which reported that cervical lymph nodes of rats (Giron et al., 1980) and mediastinal lymph nodes of mice (Bulloch and Pomerantz, 1984) are innervated by catecholaminergic fibers. Although the sympathetic innervation of NALT has not been studied, we suspect that this tissue is also innervated by the sympathetic nervous system due to the partial protection provided by RU486.
Intranasal viral infection leads to CTL influx and expansion in respiratory-associated lymphoid tissues (Lawrence et al., 2005; Zuercher et al., 2002). These events have the potential to mask the effects of stress on numbers of T cells in these tissues. However, we observed that mice that were restrained and infected still had a decreased number of CD8+ T cells in the NALT, MLN, and CLN (Figure 3), much like the restrained and non-infected mice. Using tetramer-based flow cytometric analysis, we detected a significant decrease in the number of gB498–505-specific T cells in the NALT of mice undergoing restraint. These results suggest that in mice experiencing stress at the time of infection, fewer antigen-specific CD8+ T cells are available to fight the infection at the site of initial exposure. Such immunosuppression may result in a poor response to intranasal infection in humans experiencing chronic stress at the time of either infection or vaccination. In contrast, there was no difference in the numbers of gB498–505-specific T cells between stressed and non-stressed mice in the CLN and MLN, despite the overall decrease in CD8+ T cells in each of these sites. Although these results were somewhat surprising, there are several possible explanations for these findings including hyperproliferation and expansion of CTL, increased trafficking of activated T cells to lymph nodes, and a heightened resistance of activated CD8+ T cells to apoptosis. These NALT-derived gB498–505-specific T cells are likely to be most important in eliminating virally-infected cells at the site of infection.
The function of CD8+ T cells is as important as their number at the site of infection. For example, interferon-γ (IFN-γ) augments the anti-viral immune response by activating neighboring macrophages and increasing NK cell lytic activity. Lysosome degranulation, as measured by expression of lysosomal-associated membrane protein-1 (CD107a), is a potent indicator of CTL function as well. Although we had seen a stress-induced reduction in the number of gB498–505-specific T cells only in the NALT (Figure 3A), we examined the MLN and CLN for alterations in CTL function as well. Despite this decrease in number of both CD8+ T cells and gB498–505-specific T cells in the NALT, no difference in CTL function of NALT-derived lymphocytes was observed (Figure 4A, 4B). This observation was a consequence of an increased percentage of functional gB498–505-specific CD8+ T lymphocytes at this site. Together, these results imply that stress is not impairing the immune response in the NALT; however, it is important to point out that we only examined CTL function on day 7 post-infection and have not yet investigated differences in antigenic load between stressed and non-stressed mice at this specific timepoint.
Exposure of mice to corticosterone significantly decreased the number of MLN-derived CD8+ T cells that either produced IFN-γ or degranulated following peptide stimulation, and was similar to the number that was observed in restrained mice. This finding is consistent with the finding that synthetic glucocorticoids interfere with cytokine production at the transcriptional level (Franchimont et al., 2000). In contrast to what was seen in mice that were infected and restrained, corticosterone did not alter the number of CLN-derived CD8+ cells that degranulated or produced IFN-γ. This finding may be explained by the continuously elevated levels of serum corticosterone that these mice experienced, unlike the peaks and valleys of corticosterone that are observed in restrained mice. It is likely that the continual corticosterone exposure and GR activation suppress IFN-γ to a greater extent than in the restrained mice. While this suppression is apparently overcome in mice undergoing restraint stress, the continuous supply of corticosterone in the drinking water may be more difficult to surmount.
The aforementioned functional studies revealed an unexpected difference between the function of MLN- and CLN-derived CD8+ T cells. One possible explanation is that exposure to stress impairs clearance of the infection and thus provides an increased level of antigen to naive CD8+ T cells in the CLN, but not the MLN. This increased antigen results in a sufficiently strong immune response that is able to overcome the stress-induced decrease in cell number in non-infected mice. As the CLN drain the nasal cavity and upper respiratory tract, we hypothesized that increased viral replication could account for this stronger immune response. Consistent with this hypothesis, we found significantly higher levels of infectious virus in the nasal cavities of restrained mice as compared to FWD control mice (Figure 5). The levels of infectious virus in mice whose drinking water contained corticosterone were even higher. These findings are consistent with other results (Figure 2), in which we show that providing mice with corticosterone results in greater immunosuppression within the NALT than does restraint stress. The findings may also explain why restraint stress did not alter CTL function within the NALT. For example, the increased viral load in stressed mice may be more effective in activating naive T cells, thereby compensating for immunosuppression caused by increased glucocorticoids.
To mimic the stress- and corticosterone-mediated observed decrease in NALT-derived lymphocytes, we employed a surgical approach that results in a reduction of cells similar to that seen in mice experiencing restraint stress. Mice which underwent this surgery had increased levels of infectious virus in their nasal cavity (Figure 6B), thus supporting the importance of NALT-derived lymphocytes in the clearance of an intranasally-acquired HSV infection. As these mice were not experiencing psychological stress during infection yet still demonstrated an increase in viral titers (Figure 6B), these results suggest that the increased levels of infectious virus seen in NALT-intact restrained mice (Figure 5) is a consequence of the glucocorticoid-mediated decrease in NALT-derived lymphocytes.
Although the goal of current vaccination strategies is to generate a systemic protective immune response, the immunological memory at mucosal sites eventually wanes (Gallichan and Rosenthal, 1996). Even though memory cells residing at non-mucosal sites usually confer sufficient protection, it is obviously preferable to have memory cells at mucosal sites where pathogens are first encountered. It is well documented that encounters with pathogens at one mucosal site elicit immune responses at distant mucosal sites throughout the body (Mestecky, 1987; Mestecky et al., 1978). After an infection has been cleared, memory immune cells continue to guard these distant mucosal sites (Gallichan and Rosenthal, 1996; Rosenthal and Gallichan, 1997; Vanitha et al., 2007) and provide an ideal first line of defense against pathogens that are encountered at these sites. Intranasal vaccination against HSV-2 results in a protective immune response comprised of both T cells (Rosenthal and Gallichan, 1997; Wonnacott and Bonneau, 2002) and B cells (Milligan et al., 2004) within the vaginal epithelium and associated lymph nodes. These studies have demonstrated that the immunological memory provided by intranasal vaccines is sufficient to protect mice infected intravaginally with a lethal dose of HSV-2. As the efficacy of these vaccines must be assured prior to their clinical use, it is important to characterize the role that both psychological and physiological factors, including glucocorticoids, may play in modulating a vaccine-elicited immune response. Studies are in progress to determine the consequences of alterations in the NALT on the establishment and restimulation of a memory immune response following HSV infection within the vaginal mucosa.
The studies reported here are the first to examine the impact of stress on the NALT, and the consequences of a compromised first-line of immunological defense on local control of infection. Although mucosal vaccines are an especially promising vaccine strategy, our results suggest that both psychological and physiological factors may modulate the immune response following intranasal challenge and compromise the protection offered by mucosal vaccines.
Acknowledgements
The authors gratefully acknowledge Jennifer Mellinger for her superb technical assistance. We also thank Melanie Epler for her preparation of the gB498–505/class I H-2Kb tetramer.
This work was supported by PHS Grants R01 AI49719 (RHB) and R01 HD39262 (RHB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akagi T, Ueno M, Hiraishi K, Baba M, Akashi M. AIDS vaccine: Intranasal immunization using inactivated HIV-1-capturing core-corona type polymeric nanospheres. J Control Release. 2005;109:49–61. doi: 10.1016/j.jconrel.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- Anglen CS, Truckenmiller ME, Schell TD, Bonneau RH. The dual role of CD8+ T lymphocytes in the development of stress-induced herpes simplex encephalitis. J Neuroimmunol. 2003;140:13–27. doi: 10.1016/s0165-5728(03)00159-0. [DOI] [PubMed] [Google Scholar]
- Asanuma H, Thompson AH, Iwasaki T, Sato Y, Inaba Y, Aizawa C, Kurata T, Tamura S. Isolation and characterization of mouse nasal-associated lymphoid tissue. J Immunol Methods. 1997;202:123–131. doi: 10.1016/s0022-1759(96)00243-8. [DOI] [PubMed] [Google Scholar]
- Bailey M, Engler H, Hunzeker J, Sheridan JF. The hypothalamic-pituitary-adrenal axis and viral infection. Viral Immunol. 2003;16:141–157. doi: 10.1089/088282403322017884. [DOI] [PubMed] [Google Scholar]
- Becker PD, Fiorentini S, Link C, Tosti G, Ebensen T, Caruso A, Guzman CA. The HIV-1 matrix protein p17 can be efficiently delivered by intranasal route in mice using the TLR 2/6 agonist MALP-2 as mucosal adjuvant. Vaccine. 2006;24:5269–5276. doi: 10.1016/j.vaccine.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Betts MR, Koup RA. Detection of T-cell degranulation: CD107a and b. Methods Cell Biol. 2004;75:497–512. doi: 10.1016/s0091-679x(04)75020-7. [DOI] [PubMed] [Google Scholar]
- Blaney JE, Jr, Nobusawa E, Brehm MA, Bonneau RH, Mylin LM, Fu TM, Kawaoka Y, Tevethia SS. Immunization with a single major histocompatibility complex class I-restricted cytotoxic T-lymphocyte recognition epitope of herpes simplex virus type 2 confers protective immunity. J Virol. 1998;72:9567–9574. doi: 10.1128/jvi.72.12.9567-9574.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blewitt RW, Abbott AC, Bird CC. Mode of cell death induced in human lymphoid cells by high and low doses of glucocorticoid. Br J Cancer. 1983;47:477–486. doi: 10.1038/bjc.1983.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau RH, Hunzeker JT. Stress-induced Modulation of the Immune Response to Herpes Simplex Virus Infections. In: Ader R, editor. Psychoneuroimmunology. 4th Edition. Vol. II. Elsevier, Inc.; 2007. pp. 1077–1096. [Google Scholar]
- Bonneau RH, Padgett DA, Sheridan JF. Twenty years of psychoneuroimmunology and viral infections in Brain, Behavior, and Immunity. Brain Behav Immun. 2007;21:273–280. doi: 10.1016/j.bbi.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Bonneau RH, Salvucci LA, Johnson DC, Tevethia SS. Epitope specificity of H-2Kb-restricted, HSV-1-, and HSV-2-cross-reactive cytotoxic T lymphocyte clones. Virology. 1993a;195:62–70. doi: 10.1006/viro.1993.1346. [DOI] [PubMed] [Google Scholar]
- Bonneau RH, Sheridan JF, Feng N, Glaser R. Stress-induced modulation of the primary cellular immune response to herpes simplex virus infection is mediated by both adrenal-dependent and independent mechanisms. J Neuroimmunol. 1993b;42:167–176. doi: 10.1016/0165-5728(93)90007-l. [DOI] [PubMed] [Google Scholar]
- Bonneau RH, Sheridan JF, Feng NG, Glaser R. Stress-induced suppression of herpes simplex virus (HSV)-specific cytotoxic T lymphocyte and natural killer cell activity and enhancement of acute pathogenesis following local HSV infection. Brain Behav Immun. 1991;5:170–192. doi: 10.1016/0889-1591(91)90015-3. [DOI] [PubMed] [Google Scholar]
- Brenner GJ, Moynihan JA. Stressor-induced alterations in immune response and viral clearance following infection with herpes simplex virus-type 1 in BALB/c and C57B1/6 mice. Brain Behav Immun. 1997;11:9–23. doi: 10.1006/brbi.1997.0480. [DOI] [PubMed] [Google Scholar]
- Bulloch K, Pomerantz W. Autonomic nervous system innervation of thymic-related lymphoid tissue in wildtype and nude mice. J Comp Neurol. 1984;228:57–68. doi: 10.1002/cne.902280107. [DOI] [PubMed] [Google Scholar]
- Buonaguro L, Devito C, Tornesello ML, Schroder U, Wahren B, Hinkula J, Buonaguro FM. DNA-VLP prime-boost intra-nasal immunization induces cellular and humoral anti-HIV-1 systemic and mucosal immunity with cross-clade neutralizing activity. Vaccine. 2007 doi: 10.1016/j.vaccine.2007.05.052. [DOI] [PubMed] [Google Scholar]
- Caron-Leslie LM, Schwartzman RA, Gaido ML, Compton MM, Cidlowski JA. Identification and characterization of glucocorticoid-regulated nuclease(s) in lymphoid cells undergoing apoptosis. J Steroid Biochem Mol Biol. 1991;40:661–671. doi: 10.1016/0960-0760(91)90288-g. [DOI] [PubMed] [Google Scholar]
- Cohen JJ, Duke RC. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984;132:38–42. [PubMed] [Google Scholar]
- Connor TJ, Brewer C, Kelly JP, Harkin A. Acute stress suppresses proinflammatory cytokines TNF-alpha and IL-1 beta independent of a catecholamine-driven increase in IL-10 production. J Neuroimmunol. 2005;159:119–128. doi: 10.1016/j.jneuroim.2004.10.016. [DOI] [PubMed] [Google Scholar]
- de Souza AP, Haut LH, Silva R, Ferreira SI, Zanetti CR, Ertl HC, Pinto AR. Genital CD8+ T cell response to HIV-1 gag in mice immunized by mucosal routes with a recombinant simian adenovirus. Vaccine. 2007;25:109–116. doi: 10.1016/j.vaccine.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Emilie D, Galanaud P, Baulieu EE, Dormont J. Inhibition of in vitro immunosuppressive effects of glucocorticosteroids by a competitive antagonist RU-486. Immunol Lett. 1984;8:183–186. doi: 10.1016/0165-2478(84)90075-0. [DOI] [PubMed] [Google Scholar]
- Franchimont D, Galon J, Gadina M, Visconti R, Zhou Y, Aringer M, Frucht DM, Chrousos GP, O'Shea JJ. Inhibition of Th1 immune response by glucocorticoids: dexamethasone selectively inhibits IL-12-induced Stat4 phosphorylation in T lymphocytes. J Immunol. 2000;164:1768–1774. doi: 10.4049/jimmunol.164.4.1768. [DOI] [PubMed] [Google Scholar]
- Freeman ML, Sheridan BS, Bonneau RH, Hendricks RL. Psychological Stress Compromises CD8+ T Cell Control of Latent Herpes Simplex Virus Type 1 Infections. J Immunol. 2007;179:322–328. doi: 10.4049/jimmunol.179.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallichan WS, Rosenthal KL. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J Exp Med. 1996;184:1879–1890. doi: 10.1084/jem.184.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giron LT, Jr, Crutcher KA, Davis JN. Lymph nodes--a possible site for sympathetic neuronal regulation of immune responses. Ann Neurol. 1980;8:520–525. doi: 10.1002/ana.410080509. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, Malarkey WB, Sheridan JF. The influence of psychological stress on the immune response to vaccines. Ann N Y Acad Sci. 1998;840:649–655. doi: 10.1111/j.1749-6632.1998.tb09603.x. [DOI] [PubMed] [Google Scholar]
- Goujon E, Parnet P, Laye S, Combe C, Kelley KW, Dantzer R. Stress downregulates lipopolysaccharide-induced expression of proinflammatory cytokines in the spleen, pituitary, and brain of mice. Brain Behav Immun. 1995;9:292–303. doi: 10.1006/brbi.1995.1028. [DOI] [PubMed] [Google Scholar]
- Gruol DJ, Altschmied J. Synergistic induction of apoptosis with glucocorticoids and 3',5'-cyclic adenosine monophosphate reveals agonist activity by RU 486. Mol Endocrinol. 1993;7:104–113. doi: 10.1210/mend.7.1.8383286. [DOI] [PubMed] [Google Scholar]
- Hameleers DM, van der Ven I, Biewenga J, Sminia T. Mucosal and systemic antibody formation in the rat after intranasal administration of three different antigens. Immunol Cell Biol. 1991;69(Pt 2):119–125. doi: 10.1038/icb.1991.18. [DOI] [PubMed] [Google Scholar]
- Hanke T, Graham FL, Rosenthal KL, Johnson DC. Identification of an immunodominant cytotoxic T-lymphocyte recognition site in glycoprotein B of herpes simplex virus by using recombinant adenovirus vectors and synthetic peptides. J Virol. 1991;65:1177–1186. doi: 10.1128/jvi.65.3.1177-1186.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heritage PL, Brook MA, Underdown BJ, McDermott MR. Intranasal immunization with polymer-grafted microparticles activates the nasal-associated lymphoid tissue and draining lymph nodes. Immunology. 1998;93:249–256. doi: 10.1046/j.1365-2567.1998.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann G, Beck FM, Sheridan JF. Stress-induced glucocorticoid response modulates mononuclear cell trafficking during an experimental influenza viral infection. J Neuroimmunol. 1995;56:179–186. doi: 10.1016/0165-5728(94)00145-e. [DOI] [PubMed] [Google Scholar]
- Hoppe HC, van Schalkwyk DA, Wiehart UI, Meredith SA, Egan J, Weber BW. Antimalarial quinolines and artemisinin inhibit endocytosis in Plasmodium falciparum. Antimicrob Agents Chemother. 2004;48:2370–2378. doi: 10.1128/AAC.48.7.2370-2378.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabaaij L, Grosheide PM, Heijtink RA, Duivenvoorden HJ, Ballieux RE, Vingerhoets AJ. Influence of perceived psychological stress and distress on antibody response to low dose rDNA hepatitis B vaccine. J Psychosom Res. 1993;37:361–369. doi: 10.1016/0022-3999(93)90138-6. [DOI] [PubMed] [Google Scholar]
- Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- Jin S, Zhang B, Weisz OA, Montelaro RC. Receptor-mediated entry by equine infectious anemia virus utilizes a pH-dependent endocytic pathway. J Virol. 2005;79:14489–14497. doi: 10.1128/JVI.79.23.14489-14497.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent SJ, Dale CJ, Ranasinghe C, Stratov I, De Rose R, Chea S, Montefiori DC, Thomson S, Ramshaw IA, Coupar BE, Boyle DB, Law M, Wilson KM, Ramsay AJ. Mucosally-administered human-simian immunodeficiency virus DNA and fowlpoxvirus-based recombinant vaccines reduce acute phase viral replication in macaques following vaginal challenge with CCR5-tropic SHIVSF162P3. Vaccine. 2005;23:5009–5021. doi: 10.1016/j.vaccine.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, Sheridan J. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93:3043–3047. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kip KE, Cohen F, Cole SR, Wilhelmus KR, Patrick DL, Blair RC, Beck RW. Recall bias in a prospective cohort study of acute time-varying exposures: example from the herpetic eye disease study. J Clin Epidemiol. 2001;54:482–487. doi: 10.1016/s0895-4356(00)00310-3. [DOI] [PubMed] [Google Scholar]
- Koopman G, Bogers WM, van Gils M, Koornstra W, Barnett S, Morein B, Lehner T, Heeney JL. Comparison of intranasal with targeted lymph node immunization using PR8-Flu ISCOM adjuvanted HIV antigens in macaques. J Med Virol. 2007;79:474–482. doi: 10.1002/jmv.20860. [DOI] [PubMed] [Google Scholar]
- Kusnecov AV, Grota LJ, Schmidt SG, Bonneau RH, Sheridan JF, Glaser R, Moynihan JA. Decreased herpes simplex viral immunity and enhanced pathogenesis following stressor administration in mice. J Neuroimmunol. 1992;38:129–137. doi: 10.1016/0165-5728(92)90097-5. [DOI] [PubMed] [Google Scholar]
- Lawrence CW, Ream RM, Braciale TJ. Frequency, specificity, and sites of expansion of CD8+ T cells during primary pulmonary influenza virus infection. J Immunol. 2005;174:5332–5340. doi: 10.4049/jimmunol.174.9.5332. [DOI] [PubMed] [Google Scholar]
- Leo NA, Bonneau RH. Mechanisms underlying chemical sympathectomy-induced suppression of herpes simplex virus-specific cytotoxic T lymphocyte activation and function. J Neuroimmunol. 2000;110:45–56. doi: 10.1016/s0165-5728(00)00336-2. [DOI] [PubMed] [Google Scholar]
- Leo NA, Callahan TA, Bonneau RH. Peripheral sympathetic denervation alters both the primary and memory cellular immune responses to herpes simplex virus infection. Neuroimmunomodulation. 1998;5:22–35. doi: 10.1159/000026323. [DOI] [PubMed] [Google Scholar]
- Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- Mestecky J, McGhee JR, Michalek SM, Arnold RR, Crago SS, Babb JL. Concept of the local and common mucosal immune response. Adv Exp Med Biol. 1978;107:185–192. doi: 10.1007/978-1-4684-3369-2_22. [DOI] [PubMed] [Google Scholar]
- Milligan GN, Dudley-McClain KL, Chu CF, Young CG. Efficacy of genital T cell responses to herpes simplex virus type 2 resulting from immunization of the nasal mucosa. Virology. 2004;318:507–515. doi: 10.1016/j.virol.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Nair A, Bonneau RH. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol. 2006;171:72–85. doi: 10.1016/j.jneuroim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Nair A, Hunzeker J, Bonneau RH. Modulation of microglia and CD8(+) T cell activation during the development of stress-induced herpes simplex virus type-1 encephalitis. Brain Behav Immun. 2007;21:791–806. doi: 10.1016/j.bbi.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- Ortiz GC, Sheridan JF, Marucha PT. Stress-induced changes in pathophysiology and interferon gene expression during primary HSV-1 infection. Brain Behav Immun. 2003;17:329–338. doi: 10.1016/s0889-1591(03)00027-8. [DOI] [PubMed] [Google Scholar]
- Reading PC, Whitney PG, Barr DP, Smyth MJ, Brooks AG. NK cells contribute to the early clearance of HSV-1 from the lung but cannot control replication in the central nervous system following intranasal infection. Eur J Immunol. 2006;36:897–905. doi: 10.1002/eji.200535710. [DOI] [PubMed] [Google Scholar]
- Rosenthal KL, Gallichan WS. Challenges for vaccination against sexually-transmitted diseases: induction and long-term maintenance of mucosal immune responses in the female genital tract. Semin Immunol. 1997;9:303–314. doi: 10.1006/smim.1997.0086. [DOI] [PubMed] [Google Scholar]
- Spit BJ, Hendriksen EG, Bruijntjes JP, Kuper CF. Nasal lymphoid tissue in the rat. Cell Tissue Res. 1989;255:193–198. doi: 10.1007/BF00229081. [DOI] [PubMed] [Google Scholar]
- van der Ven I, Sminia T. The development and structure of mouse nasal-associated lymphoid tissue: an immuno- and enzyme-histochemical study. Reg Immunol. 1993;5:69–75. [PubMed] [Google Scholar]
- Vanitha DJ, Joo HM, Rouse BT, Sangster MY. Quantitative analysis of herpes simplex virus type 1-specific memory B cells generated by different routes of infection. Virology. 2007;360:136–142. doi: 10.1016/j.virol.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedhara K, Cox NK, Wilcock GK, Perks P, Hunt M, Anderson S, Lightman SL, Shanks NM. Chronic stress in elderly carers of dementia patients and antibody response to influenza vaccination. Lancet. 1999;353:627–631. doi: 10.1016/S0140-6736(98)06098-X. [DOI] [PubMed] [Google Scholar]
- Wiley JA, Tighe MP, Harmsen AG. Upper respiratory tract resistance to influenza infection is not prevented by the absence of either nasal-associated lymphoid tissue or cervical lymph nodes. J Immunol. 2005;175:3186–3196. doi: 10.4049/jimmunol.175.5.3186. [DOI] [PubMed] [Google Scholar]
- Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- Wonnacott KM, Bonneau RH. The effects of stress on memory cytotoxic T lymphocyte-mediated protection against herpes simplex virus infection at mucosal sites. Brain Behav Immun. 2002;16:104–117. doi: 10.1006/brbi.2001.0624. [DOI] [PubMed] [Google Scholar]
- Wu HY, Nikolova EB, Beagley KW, Eldridge JH, Russell MW. Development of antibody-secreting cells and antigen-specific T cells in cervical lymph nodes after intranasal immunization. Infect Immun. 1997;65:227–235. doi: 10.1128/iai.65.1.227-235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HY, Nikolova EB, Beagley KW, Russell MW. Induction of antibody-secreting cells and T-helper and memory cells in murine nasal lymphoid tissue. Immunology. 1996;88:493–500. doi: 10.1046/j.1365-2567.1996.d01-690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Zhang L, Nair A, Krady K, Corpe C, Bonneau RH, Simpson IA, Vannucci SJ. Estrogen stimulates microglia and brain recovery from hypoxia-ischemia in normoglycemic but not diabetic female mice. J Clin Invest. 2004;113:85–95. doi: 10.1172/JCI200418336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuercher AW, Coffin SE, Thurnheer MC, Fundova P, Cebra JJ. Nasal-associated lymphoid tissue is a mucosal inductive site for virus-specific humoral and cellular immune responses. J Immunol. 2002;168:1796–1803. doi: 10.4049/jimmunol.168.4.1796. [DOI] [PubMed] [Google Scholar]