Abstract
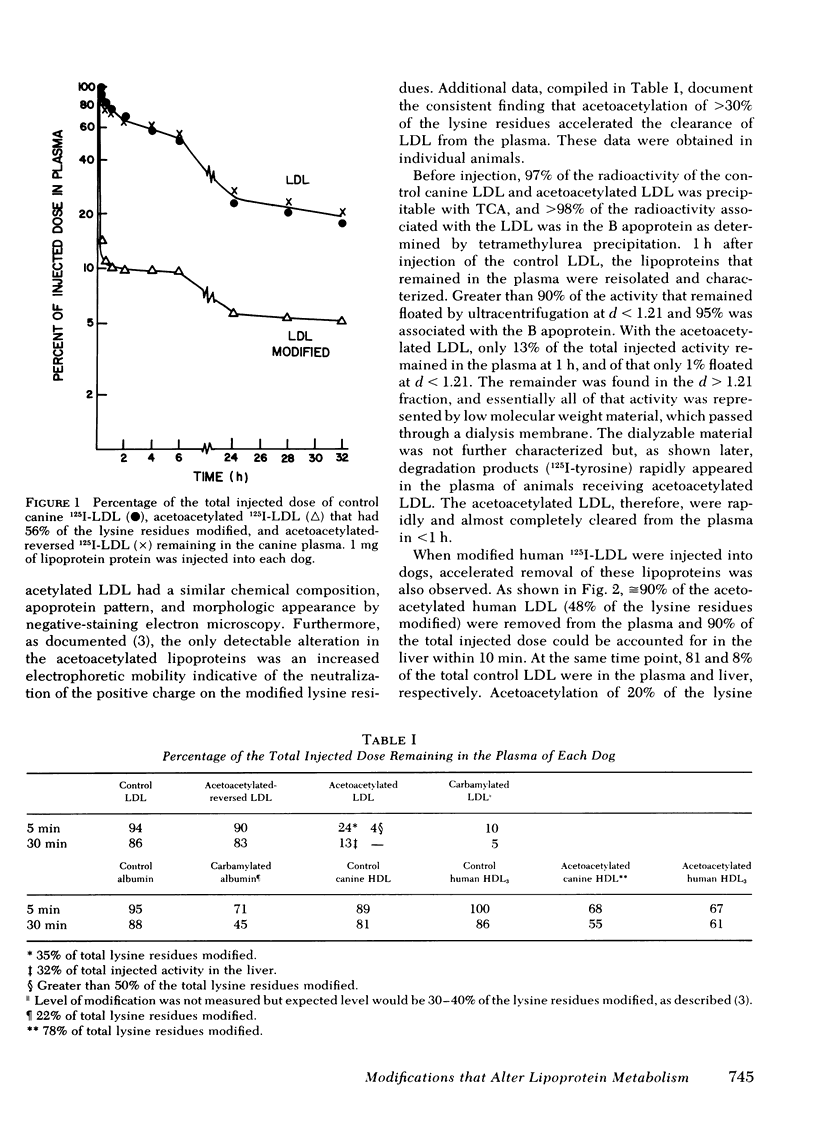
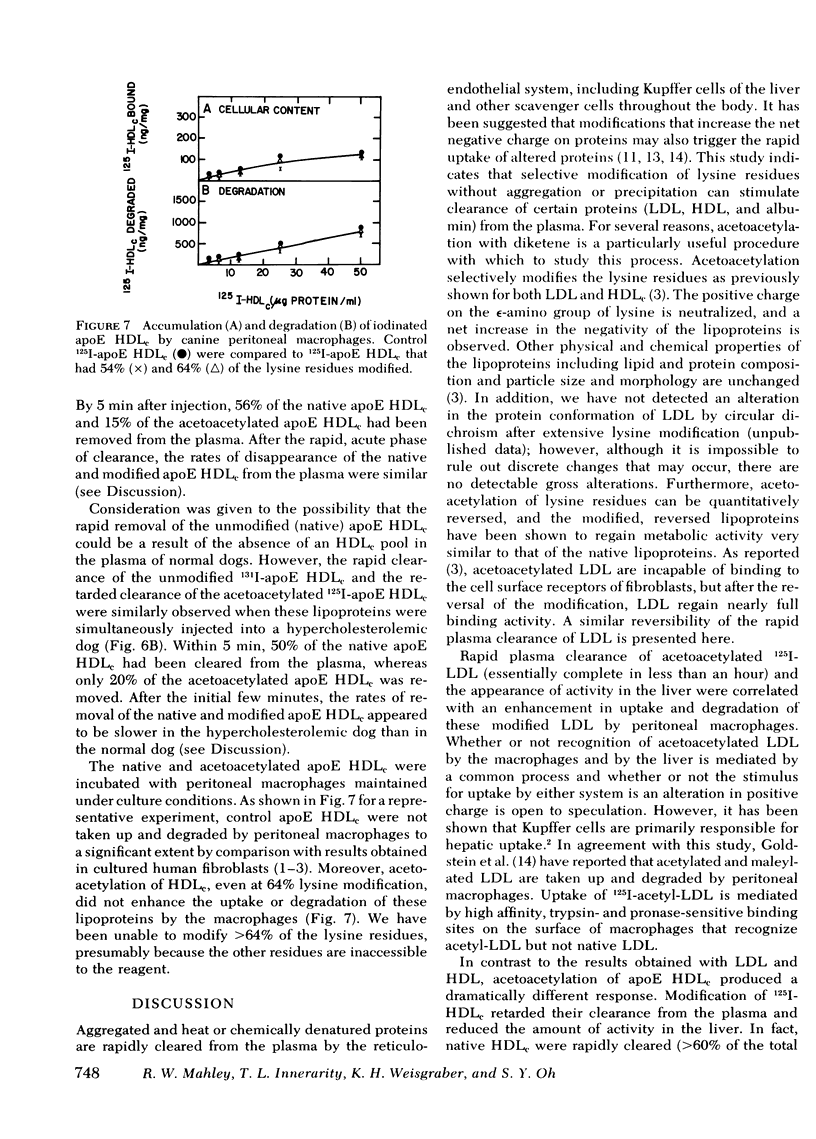
Chemical modification of lysine residues by acetoacetylation of the apoproteins of iodinated canine and human low density lipoproteins (LDL) and canine high density lipoproteins (HDL) resulted in a marked acceleration in the rate of removal of these lipoproteins from the plasma after intravenous injection into dogs. Clearance of the lipoproteins from the plasma correlated with their rapid appearance in the liver. Acetoacetylated canine 125I-LDL (30-60% of the lysine residues modified) were essentially completely removed from the plasma within an hour, and > 75% of the activity cleared within 5 min. Reversal of the acetoacetylation of the lysine residues of the LDL restored to these lipoproteins a rate of clearance essentially identical to that of control LDL. Identical results were obtained with modified human LDL injected into dogs. At 10 min, when ≅ 90% of the acetoacetylated human 125I-LDL had been removed from the plasma, 90% of the total injected activity could be accounted for in the liver. Furthermore, it was possible to demonstrate an enhancement in uptake and degradation of acetoacetylated LDL by canine peritoneal macrophages in vitro. The mechanism(s) responsible for the enhanced removal of the LDL and HDL in vivo and in vitro remains to be determined. By contrast, however, acetoacetylation of canine 125I-apoE HDLc did not accelerate their rate of removal from the plasma but, in fact, retarded their clearance. Control (native) apoE HDLc were removed from the plasma (64% within 20 min) and rapidly appeared in the liver (39% at 20 min). At the same time point, only 45% of the acetoacetylated apoE HDLc were cleared from the plasma and <10% appeared in the liver. Acetoacetylation of the apoE HDLc did not enhance their uptake or degradation by macrophages. The rapid clearance from the plasma of the native apoE HDLc in normal and hypercholesterolemic dogs suggests that the liver may be a normal site for the removal of the cholesteryl ester-rich apoE HDLc. The retardation in removal after acetoacetylation of apoE HDLc indicates that the uptake process may be mediated by a lysine-dependent recognition system.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Innerarity T. L., Mahley R. W. Enhanced binding by cultured human fibroblasts of apo-E-containing lipoproteins as compared with low density lipoproteins. Biochemistry. 1978 Apr 18;17(8):1440–1447. doi: 10.1021/bi00601a013. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Pitas R. E., Weisgraber K. H., Brown J. H., Gross E. Inhibition of lipoprotein binding to cell surface receptors of fibroblasts following selective modification of arginyl residues in arginine-rich and B apoproteins. J Biol Chem. 1977 Oct 25;252(20):7279–7287. [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Weisgraber K. H., Fry D. L. Canine hyperlipoproteinemia and atherosclerosis. Accumulation of lipid by aortic medial cells in vivo and in vitro. Am J Pathol. 1977 Apr;87(1):205–226. [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H. Canine lipoproteins and atherosclerosis. I. Isolation and characterization of plasma lipoproteins from control dogs. Circ Res. 1974 Nov;35(5):713–721. doi: 10.1161/01.res.35.5.713. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Innerarity T. L., Windmueller H. G. Accelerated clearance of low-density and high-density lipoproteins and retarded clearance of E apoprotein-containing lipoproteins from the plasma of rats after modification of lysine residues. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1746–1750. doi: 10.1073/pnas.76.4.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba T. M. Physiology and physiopathology of the reticuloendothelial system. Arch Intern Med. 1970 Dec;126(6):1031–1052. [PubMed] [Google Scholar]
- Stephenson E. H., Osterman J. V. Canine peritoneal macrophages: cultivation and infection with Ehrlichia canis. Am J Vet Res. 1977 Nov;38(11):1815–1819. [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Role of lysine residues of plasma lipoproteins in high affinity binding to cell surface receptors on human fibroblasts. J Biol Chem. 1978 Dec 25;253(24):9053–9062. [PubMed] [Google Scholar]
- Weisgraber K. H., Mahley R. W., Assmann G. The rat arginine-rich apoprotein and its redistribution following injection of iodinated lipoproteins into normal and hypercholesterolemic rats. Atherosclerosis. 1977 Oct;28(2):121–140. doi: 10.1016/0021-9150(77)90150-2. [DOI] [PubMed] [Google Scholar]



