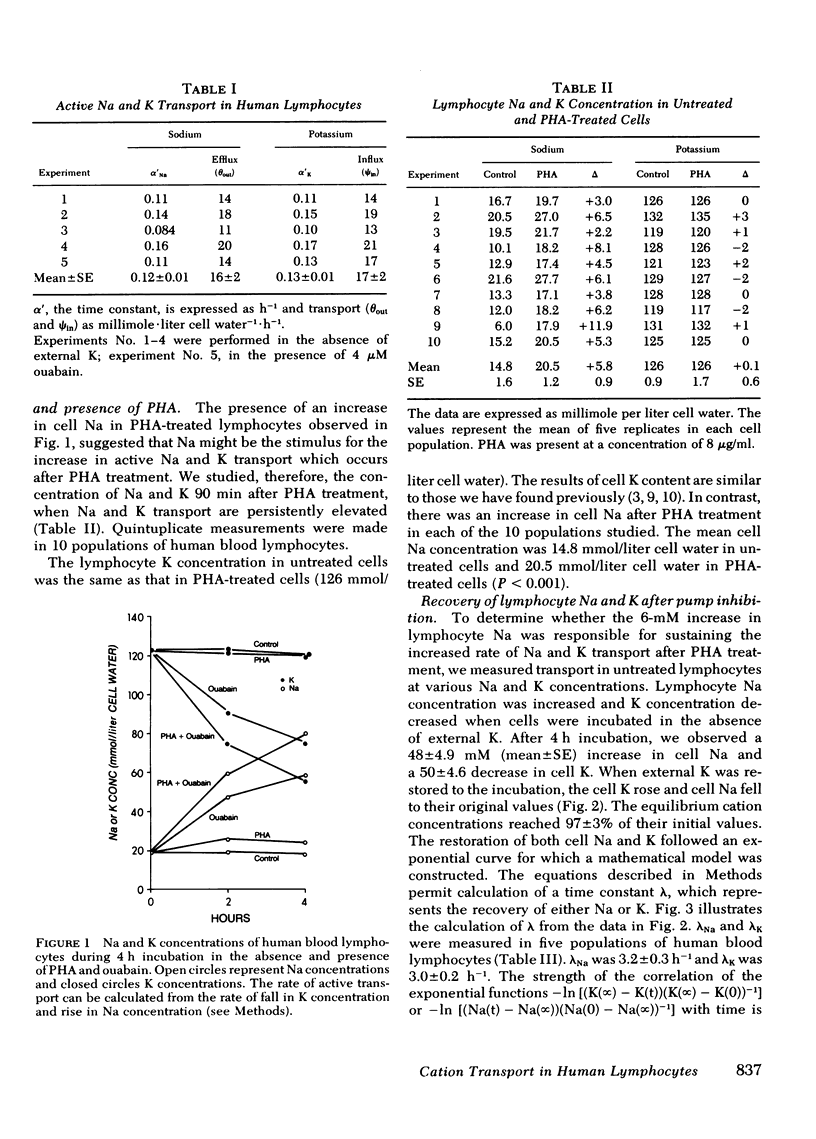
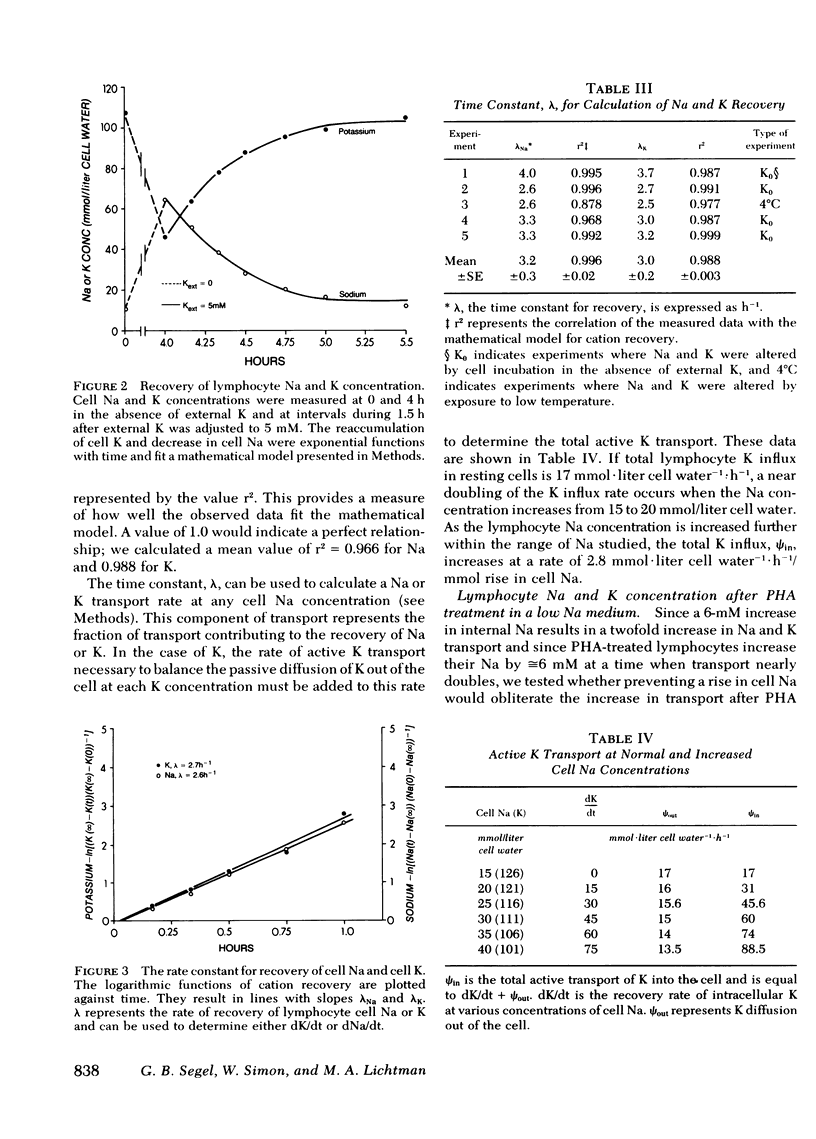
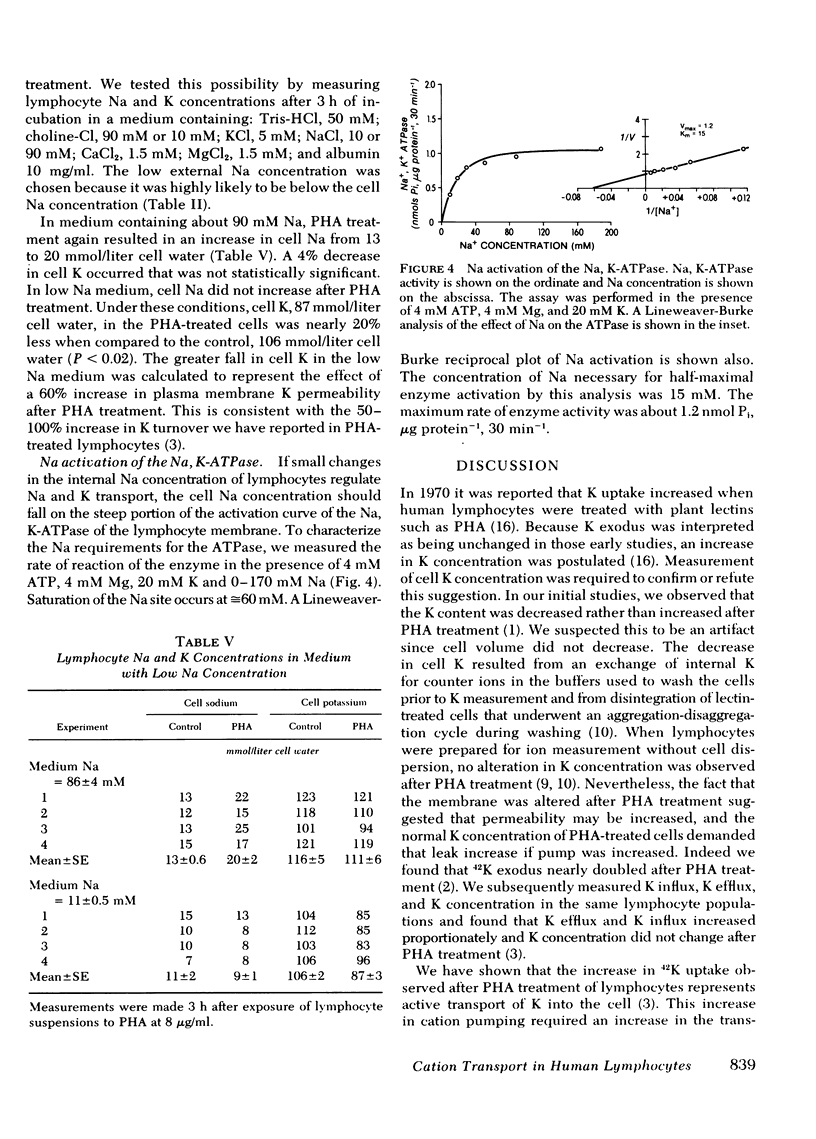
Abstract
Phytohemagglutinin (PHA) or concanavalin A treatment of lymphocytes causes an increase in membrane permeability so that the leak rates of Na and K increase 1.5- to 2-fold. Active Na and K transport increase proportionately in response to the increased membrane permeability. We have examined the role of lymphocyte Na concentration in sustaining the increased Na and K transport observed after PHA treatment. Cell Na concentration increases from 14.8 to 20.5 mmol/liter cell water in PHA-treated lymphocytes (P < 0.001). Four lines of evidence suggest that the 5-6 mmol/liter cell water increase in lymphocyte Na accounts for the increase in active Na and K transport in mitogen-treated lymphocytes. First, PHA does not increase directly the maximal Na, K-ATPase activity of isolated lymphocyte membrane vesicles. Second, when the Na concentration is increased by 6 mmol/liter cell water in unstimulated lymphocytes, Na and K transport increase nearly twofold. Third, the cell Na concentration (15 mmol/liter cell water) is near the Km for Na activation of the Na, K-ATPase in lymphocyte membranes. The ATPase activity thus, is capable of increasing as the cell Na rises above normal. Fourth, if lymphocytes are incubated in a medium containing a low Na concentration, K transport does not maintain the internal K concentration and the fall in cell K is accentuated in PHA-treated lymphocytes. These studies indicate that the adaptive acceleration of Na and K transport in mitogen-treated lymphocytes is mediated by a small increase in cell Na.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Averdunk R., Lauf P. K. Effects of mitogens on sodium-potassium transport, 3H-ouabain binding, and adenosine triphosphatase activity in lymphocytes. Exp Cell Res. 1975 Jul;93(2):331–342. doi: 10.1016/0014-4827(75)90458-9. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of leucocytes from human blood. A two-phase system for removal of red cells with methylcellulose as erythrocyte-aggregating agent. Scand J Clin Lab Invest Suppl. 1968;97:9–29. [PubMed] [Google Scholar]
- Garay R. P., Garrahan P. J. The interaction of sodium and potassium with the sodium pump in red cells. J Physiol. 1973 Jun;231(2):297–325. doi: 10.1113/jphysiol.1973.sp010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Rega A. F. Cation loading of red blood cells. J Physiol. 1967 Nov;193(2):459–466. doi: 10.1113/jphysiol.1967.sp008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton L. J., Kaplan J. G. Flux of 86Rb in activated human lymphocytes. Can J Biochem. 1977 Jul;55(7):774–778. doi: 10.1139/o77-113. [DOI] [PubMed] [Google Scholar]
- Iversen J. G. Unidirectional K+ fluxes in rat thymocytes stimulated by concanavalin A. J Cell Physiol. 1976 Oct;89(2):267–276. doi: 10.1002/jcp.1040890210. [DOI] [PubMed] [Google Scholar]
- POST R. L., JOLLY P. C. The linkage of sodium, potassium, and ammonium active transport across the human erythrocyte membrane. Biochim Biophys Acta. 1957 Jul;25(1):118–128. doi: 10.1016/0006-3002(57)90426-2. [DOI] [PubMed] [Google Scholar]
- Pommier G., Ripert G., Azoulay E., Depieds R. Effect of concanavalin A on membrane-bound enzymes from mouse lymphocytes. Biochim Biophys Acta. 1975 May 21;389(3):483–494. doi: 10.1016/0005-2736(75)90159-5. [DOI] [PubMed] [Google Scholar]
- Quastel M. R., Kaplan J. G. Early stimulation of potassium uptake in lymphocytes treated with PHA. Exp Cell Res. 1970 Nov;63(1):230–233. doi: 10.1016/0014-4827(70)90360-5. [DOI] [PubMed] [Google Scholar]
- Segel G. B., Androphy E. J., Lichtman M. A. Increased ouabain-sensitive glycolysis of lymphocytes treated with phytohemagglutinin: relationship to potassium transport. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):407–412. doi: 10.1002/jcp.1040970315. [DOI] [PubMed] [Google Scholar]
- Segel G. B., Gordon B. R., Lichtman M. A., Hollander M. M., Klemperer M. R. Exodus of 42K+ and 86Rb+ from rat thymic and human blood lymphocytes exposed to phytohemagglutinin. J Cell Physiol. 1976 Mar;87(3):337–343. doi: 10.1002/jcp.1040870309. [DOI] [PubMed] [Google Scholar]
- Segel G. B., Hollander M. M., Gordon B. R., Klemperer M. R., Lichtman M. A. A rapid phytohemagglutinin induced alteration in lymphocyte potassium permeability. J Cell Physiol. 1975 Oct;86(2 Pt 2 Suppl 1):327–335. doi: 10.1002/jcp.1040860404. [DOI] [PubMed] [Google Scholar]
- Segel G. B., Kovach G., Lichtman M. A. Sodium-potassium adenosine triphosphatase activity of human lymphocyte membrane vesicles: kinetic parameters, substrate specificity, and effects of phytohemagglutinin. J Cell Physiol. 1979 Jul;100(1):109–117. doi: 10.1002/jcp.1041000111. [DOI] [PubMed] [Google Scholar]
- Segel G. B., Lichtman M. A., Gordon B. R., MacPherson J. L., Nusbacher J. Plateletpheresis residues: a source of large quantities of human blood lymphocytes. Transfusion. 1976 Sep-Oct;16(5):455–459. doi: 10.1046/j.1537-2995.1976.16577039302.x. [DOI] [PubMed] [Google Scholar]
- Segel G. B., Lichtman M. A., Hollander M. M., Gordon B. R., Klemperer M. R. Human lymphocyte potassium content during the initiation of phytohemagglutinin-induced mitogenesis. J Cell Physiol. 1976 May;88(1):43–48. doi: 10.1002/jcp.1040880106. [DOI] [PubMed] [Google Scholar]
- Segel G. B., Lichtman M. A. Potasssium transport in human blood lymphocytes treated with phytohemagglutinin. J Clin Invest. 1976 Dec;58(6):1358–1369. doi: 10.1172/JCI108591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segel G. B., Lichtman M. A. The effect of method on the measurement of K+ concentration in PHA-treated human blood lymphocytes. Exp Cell Res. 1978 Mar 1;112(1):95–102. doi: 10.1016/0014-4827(78)90529-3. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Rozengurt E. Serum stimulates the Na+,K+ pump in quiescent fibroblasts by increasing Na+ entry. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5560–5564. doi: 10.1073/pnas.75.11.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]



