Abstract
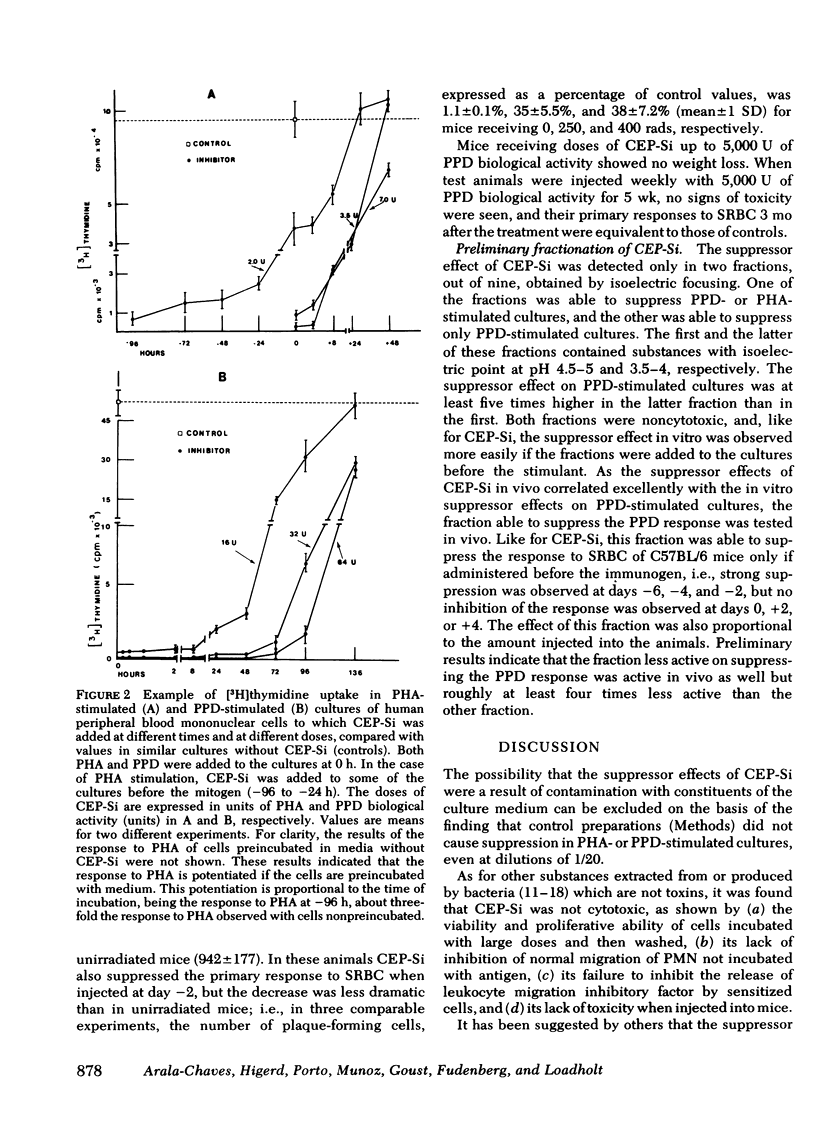
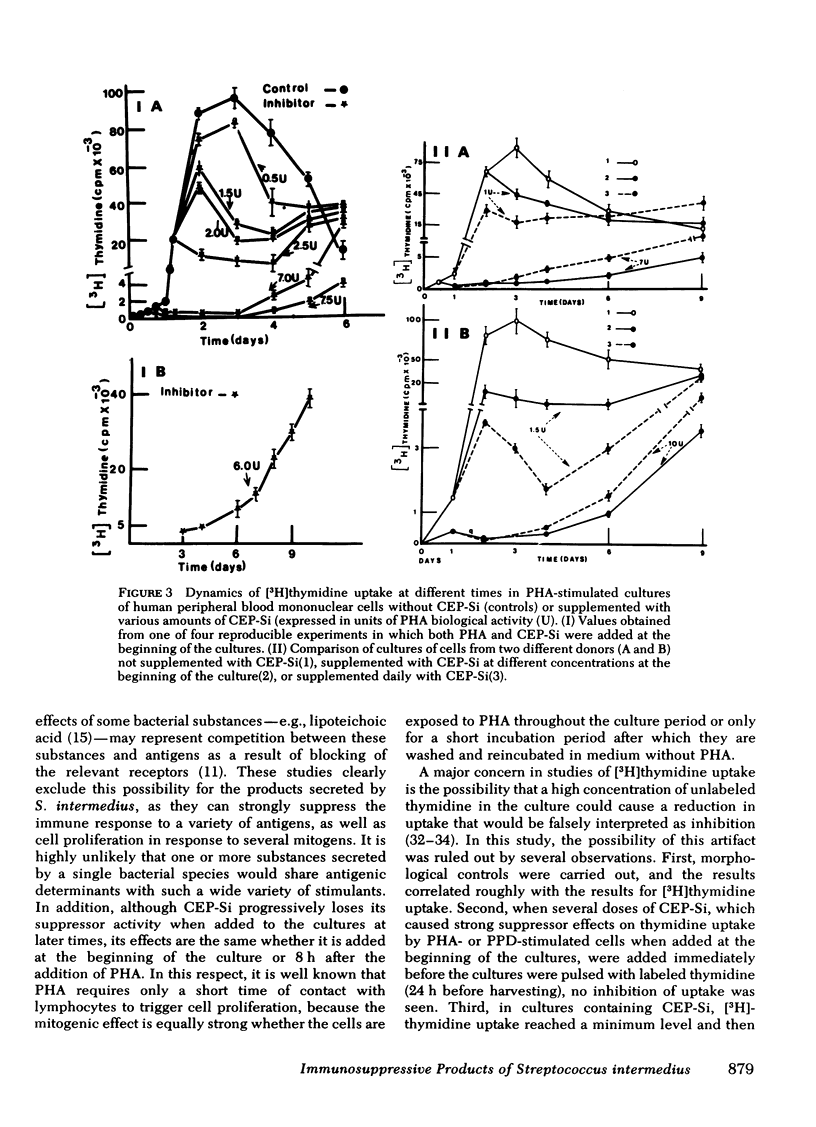
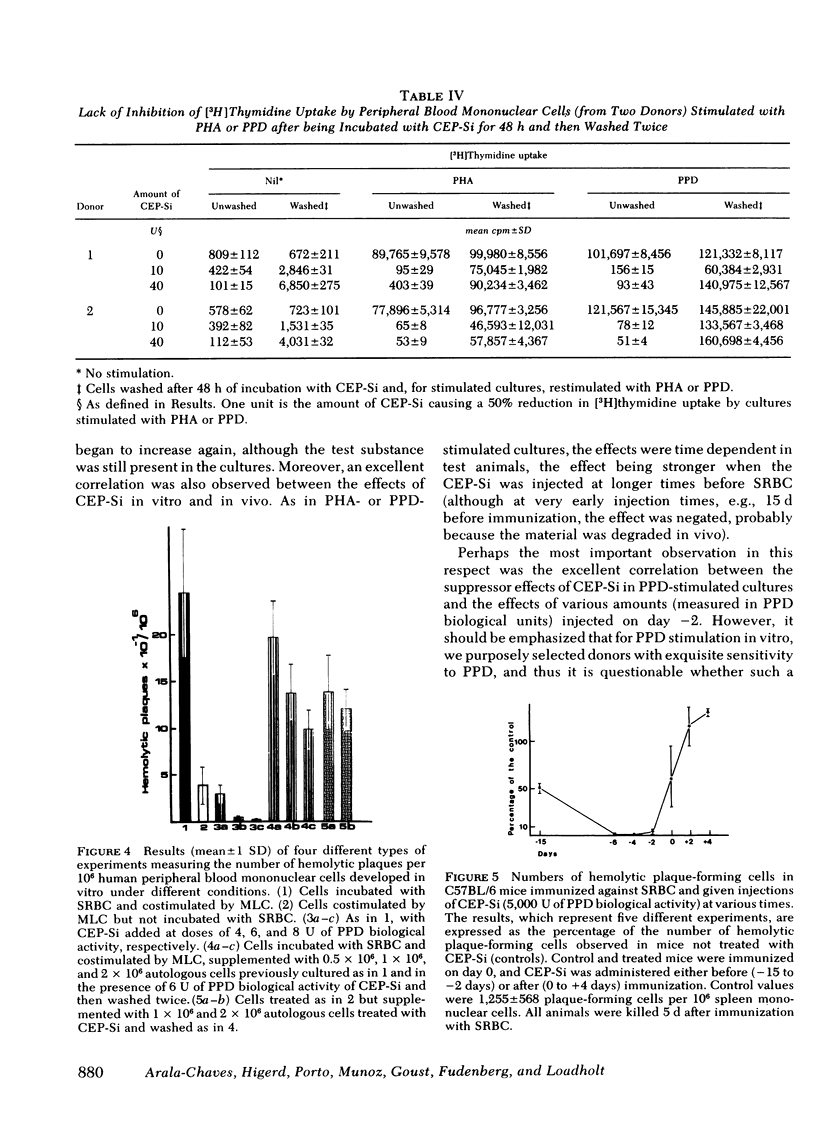
Products secreted by Streptococcus intermedius were studied for their effects on the immune response. Three different preparations of crude extracellular products from S. intermedius (CEP-Si) were found to have powerful suppressor activity in vitro as shown by inhibition of human lymphocyte proliferation (uptake of [3H]thymidine) and protein synthesis in response to a wide variety of stimulants, including mitogens and antigens, and suppression of plaque formation by human cells in response to sheep erythrocytes. CEP-Si was noncytotoxic, because cells incubated with high concentrations of CEP-Si and subsequently washed were viable and recovered their ability to respond to mitogens, and because leukocyte migration was not inhibited by CEP-Si, nor was the release of leukocyte migration inhibitory factor from sensitized lymphocytes. The possibility of antigen or mitogen competition was excluded. The effects of CEP-Si in vitro were time dependent and did not require the presence of monocytes. Cells pretreated with CEP-Si and then washed suppressed plaque formation by fresh autologous cells in highly stimulated cultures. CEP-Si injected into C57BL/6 mice also strongly suppressed their immune response to sheep erythrocytes, and the in vivo suppression was correlated with the effects of CEP-Si in vitro.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arala-Chaves M. P., Hope L., Korn J. H., Fudenberg H. Role of adherent cells in immune responses to phytohemagglutinin and concanavalin A. Eur J Immunol. 1978 Feb;8(2):77–81. doi: 10.1002/eji.1830080202. [DOI] [PubMed] [Google Scholar]
- Bentwich Z., Douglas S. D., Siegal F. P., Kunkel H. G. Human lymphocyte-sheep erythrocyte rosette formation: some characteristics of the interaction. Clin Immunol Immunopathol. 1973 Jul;1(4):511–522. doi: 10.1016/0090-1229(73)90007-x. [DOI] [PubMed] [Google Scholar]
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bro-Jorgensen K., Güttler F., Jorgensen P. N., Volkert M. T lymphocyte function as the principal target of lymphocytic choriomeningitis virus-induced immunosuppression. Infect Immun. 1975 Apr;11(4):622–629. doi: 10.1128/iai.11.4.622-629.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock W. E., Carlson E. M., Gershon R. K. The evolution of immunosuppressive cell populations in experimental mycobacterial infection. J Immunol. 1978 May;120(5):1709–1716. [PubMed] [Google Scholar]
- Böyum A. Separation of leukocytes from blood and bone marrow. Introduction. Scand J Clin Lab Invest Suppl. 1968;97:7–7. [PubMed] [Google Scholar]
- Calderon J., Kiely J. M., Lefko J. L., Unanue E. R. The modulation of lymphocyte functions by molecules secreted by macrophages. I. Description and partial biochemical analysis. J Exp Med. 1975 Jul 1;142(1):151–164. doi: 10.1084/jem.142.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C. B., Milton J. D., Mowbray J. F., Butterworth A. E. Immunosuppressive properties of a ribonuclease-containing fraction from bacterial cultures. Immunology. 1972 Aug;23(2):171–182. [PMC free article] [PubMed] [Google Scholar]
- Clausen J. E. The agarose migration inhibition technique for in vitro demonstration of cell-mediated immunity in man. A review. Dan Med Bull. 1975 Jul;22(5):181–194. [PubMed] [Google Scholar]
- Colley D. G., Lewis F. A., Goodgame R. W. Immune responses during human schistosomiasis mansoni. IV. Induction of suppressor cell activity by schistosome antigen preparations and concanavalin A. J Immunol. 1978 Apr;120(4):1225–1232. [PubMed] [Google Scholar]
- Du Bois M. G., Huismans D. R., Schellekens P. T., Eijsvoogel V. P. Investigation and standardization of the conditions for micro-lymphocyte cultures. Tissue Antigens. 1973;3(5):402–409. doi: 10.1111/j.1399-0039.1973.tb00510.x. [DOI] [PubMed] [Google Scholar]
- Dutton R. W. Inhibitory and stimulatory effects of concanavalin A on the response of mouse spleen cell suspensions to antigen. I. Characterization of the inhibitory cell activity. J Exp Med. 1972 Dec 1;136(6):1445–1460. doi: 10.1084/jem.136.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton R. W. Inhibitory and stimulatory effects of concanavalin A on the response of mouse spleen cell suspensions to antigen. II. Evidence for separate stimulatory and inhibitory cells. J Exp Med. 1973 Dec 1;138(6):1496–1505. doi: 10.1084/jem.138.6.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb P., Feldmann M., Hogg N. Role of macrophages in the generation of T helper cells. IV. Nature of genetically related factor derived from macrophages incubated with soluble antigens. Eur J Immunol. 1976 May;6(5):365–372. doi: 10.1002/eji.1830060512. [DOI] [PubMed] [Google Scholar]
- Erb P., Feldmann M. Role of macrophages in in vitro induction of T-helper cells. Nature. 1975 Mar 27;254(5498):352–354. doi: 10.1038/254352a0. [DOI] [PubMed] [Google Scholar]
- Erb P., Feldmann M. The role of macrophages in the generation of T-helper cells. II. The genetic control of the macrophage-T-cell interaction for helper cell induction with soluble antigens. J Exp Med. 1975 Aug 1;142(2):460–472. doi: 10.1084/jem.142.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R., Booth C. G. Inhibition of 125IUdR incorporation by supernatants from macrophage and lymphocyte cultures: a cautionary note. Cell Immunol. 1976 Sep;26(1):120–126. doi: 10.1016/0008-8749(76)90354-3. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R. Activation of human B lymphocytes. I. Direct plaque-forming cell assay for the measurement of polyclonal activation and antigenic stimulation of human B lymphocytes. J Exp Med. 1976 Sep 1;144(3):674–684. doi: 10.1084/jem.144.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R. Polyclonal activation of bone-marrow-derived lymphocytes from human peripheral blood measured by a direct plaque-forming cell assay. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3676–3679. doi: 10.1073/pnas.73.10.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaumer H. R., Schwab J. H. Differential susceptibility of mouse lymphocytes to an immunosuppressant from group A streptococci. Cell Immunol. 1972 Aug;4(4):394–406. doi: 10.1016/0008-8749(72)90041-x. [DOI] [PubMed] [Google Scholar]
- Gershon R. K. T cell control of antibody production. Contemp Top Immunobiol. 1974;3:1–40. doi: 10.1007/978-1-4684-3045-5_1. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Activation of human B lymphocytes. III. Concanavalin A-induced generation of suppressor cells of the plaque-forming cell response of normal human B lymphocytes. J Immunol. 1977 Jun;118(6):2281–2287. [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Activation of human B lymphocytes. V. Kinetics and mechanisms of suppression of plaque-forming cell responses by concanavalin A-generated suppressor cells. J Immunol. 1978 Mar;120(3):700–708. [PubMed] [Google Scholar]
- Hersh E. M. Immunosuppressive enzymes. Transplant Proc. 1973 Sep;5(3):1211–1214. [PubMed] [Google Scholar]
- Hersh E. M. L-glutaminase: suppression of lymphocyte blastogenic responses in vitro. Science. 1971 May 14;172(3984):736–738. doi: 10.1126/science.172.3984.736. [DOI] [PubMed] [Google Scholar]
- Higerd T. B., Vesole D. H., Goust J. M. Inhibitory effects of extracellular products from oral bacteria on human fibroblasts and stimulated lymphocytes. Infect Immun. 1978 Aug;21(2):567–574. doi: 10.1128/iai.21.2.567-574.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoessli D. C., Jones A. P., Waksman B. H. Potentiation of the T lymphocyte response to mitogens IV. Serum-free production and testing of macrophage soluble products. Cell Immunol. 1977 May;30(2):310–320. doi: 10.1016/0008-8749(77)90074-0. [DOI] [PubMed] [Google Scholar]
- Hoffman P. M., Spitler L. E., Hsu M., Fundenberg H. H. Leukocyte migration inhibition in agarose. Cell Immunol. 1975 Jul;18(1):21–30. doi: 10.1016/0008-8749(75)90032-5. [DOI] [PubMed] [Google Scholar]
- Jerne N. K., Nordin A. A. Plaque Formation in Agar by Single Antibody-Producing Cells. Science. 1963 Apr 26;140(3565):405–405. doi: 10.1126/science.140.3565.405. [DOI] [PubMed] [Google Scholar]
- Klesius P. H., Swann A. I., Qualls D. F. Comparison of protein and DNA synthesis assays of bovine peripheral blood lymphocytes after antigenic and phytohemagglutinin stimulation. Clin Immunol Immunopathol. 1978 Jun;10(2):175–186. doi: 10.1016/0090-1229(78)90025-9. [DOI] [PubMed] [Google Scholar]
- Malakian A., Schwab J. H. Immunosuppressant from group A streptococci. Science. 1968 Feb 23;159(3817):880–881. doi: 10.1126/science.159.3817.880. [DOI] [PubMed] [Google Scholar]
- Miller G. A., Urban J., Jackson R. W. Effects of a streptococcal lipoteichoic acid on host responses in mice. Infect Immun. 1976 May;13(5):1408–1417. doi: 10.1128/iai.13.5.1408-1417.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musher D. M., Schell R. F., Jones R. H., Jones A. M. Lymphocyte transformation in syphilis: an in vitro correlate of immune suppression in vivo? Infect Immun. 1975 Jun;11(6):1261–1264. doi: 10.1128/iai.11.6.1261-1264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehler J. R., Herberman R. B., Campbell D. A., Jr, Djeu J. Y. Inhibition of rat mixed lymphocyte cultures by suppressor macrophages. Cell Immunol. 1977 Mar 15;29(2):238–250. doi: 10.1016/0008-8749(77)90319-7. [DOI] [PubMed] [Google Scholar]
- Pavia C. S., Folds J. D., Baseman J. B. Depression of lymphocyte response to concanavalin A in rabbits infected with Treponema pallidum (Nichols strain). Infect Immun. 1976 Jul;14(1):320–322. doi: 10.1128/iai.14.1.320-322.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelley R. P., Ruffier J. J., Warren K. S. Suppressive effect of a chronic helminth infection, schistosomiasis mansoni, on the in vitro responses of spleen and lymph node cells to the T cell mitogens phytohemagglutinin and concanavalin A. Infect Immun. 1976 Apr;13(4):1176–1183. doi: 10.1128/iai.13.4.1176-1183.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redelman D., Scott C. B., Sheppard H. W., Jr, Sell S. In vitro studies of the rabbit immune system. V. Suppressor T cells activated by concanavalin A block the proliferation, not the induction of antierythrocyte plaque-forming cells. J Exp Med. 1976 Apr 1;143(4):919–936. doi: 10.1084/jem.143.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich R. R., Pierce C. W. Biological expressions of lymphocyte activation. II. Generation of a population of thymus-derived suppressor lymphocytes. J Exp Med. 1973 Mar 1;137(3):649–659. doi: 10.1084/jem.137.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H. Suppression of the immune response by microorganisms. Bacteriol Rev. 1975 Jun;39(2):121–143. doi: 10.1128/br.39.2.121-143.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D., Paul S., Van Scoy R. E., Hermans P. E. Suppressor thymus-derived lymphocytes in fungal infection. J Clin Invest. 1976 Feb;57(2):319–328. doi: 10.1172/JCI108283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesikari T., Buimovici-Klein E. Lymphocyte responses to rubella antigen and phytohemagglutinin after administration of the RA 27/3 strain of live attenuated rubella vaccine. Infect Immun. 1975 Apr;11(4):748–753. doi: 10.1128/iai.11.4.748-753.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedanz W. P., Rank R. G. Regional immunosuppression induced by Plasmodium berghei yoelii infection in mice. Infect Immun. 1975 Jan;11(1):211–212. doi: 10.1128/iai.11.1.211-212.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



