Abstract
This work investigates the suitability of molecular weight cut-off membrane-based centrifugal filter devices (MWCO) for sub-microgram peptide enrichment passing through the membrane by introduction of methanol and a salt modifier. Using a neuropeptide standard, bradykinin, a reduction in sample loss of over two orders of magnitude is demonstrated with and without undigested protein present. Additionally, a bovine serum albumin (BSA) tryptic digestion was investigated and 27 tryptic peptides were identified using MALDI mass spectrometry whereas only two BSA tryptic peptides were identified after MWCO separation using H2O. The protocol presented here enhances recovery from MWCO separation for sub-μg peptide samples.
Keywords: Mass spectrometry, Peptides, Molecular weight cut-off fractionation, Sample preparation
INTRODUCTION
Molecular weight cut-off membrane-based centrifugal filter devices (MWCO) are commonly used to desalt and concentrate large molecular weight proteins [1]. Greeing and Simpson recently investigated various types of MWCO membranes for large amounts of starting material (~6 mg) and focused on optimal conditions for the sub 25kDa protein fraction [2]. The authors obtained recovery of proteins of interest at minimum 200 μg to 2.9 mg from multiple MWCO experiments and demonstrated that a 10% acetonitrile (ACN) and 90% H2O elution solvent produced optimal results [3]. In addition, Manza et. al. provided an alternative approach using NH4HCO3 with 5kDa MWCO for protein isolation, but instead focused on the retained proteins [4]. Alternatively, the flow through or elution from MWCOs can be collected to acquire a sample with higher molecular weight proteins removed, retaining the low molecular weight peptides. Multiple peptidomic studies have utilized MWCOs for peptide isolation during the first few steps of sample preparation [5, 6]. When sample amount is limited or peptide content is below 1 μg, sample loss is a significant concern when using MWCOs to isolate the peptidome for detection of endogenous peptides. Optimized protocols have been investigated using ACN [3, 7], salt [4, 8], SDS [5], or native sample [6, 9, 10]; these experiments primarily focused on large sample amounts and neglected thorough investigation of sub-microgram sample loss.
MWCOs separate large molecules from small molecules. The small molecule fraction may be rich with signaling peptides (SP), cytokines, and other small molecules involved in cell-cell signaling. Cell growth, cell survival, and hormonal signaling between organs are a few examples of what functions SP perform in the body and highlight the importance of investigation [11]. Individual SPs contribute to different aspects of behavior, such as pain (enkephalins) [12], feeding (neuropeptide Y) [13], and blood pressure (bradykinin) [14]. One of the analytical methods to enrich biologically important SP is via MWCO separation, and exploration of peptide content from readily available biological fluids, such as blood or cerebrospinal fluid (CSF) with relatively low peptide content. In a recent investigation the detection of neuropeptides and standards in crustacean hemolymph was improved when methanol and protease inhibitors were present before performing MWCO neuropeptide isolation. The impact of methanol on MWCO sample loss was not investigated in the study [15]. A large scale mass fingerprinting protocol of endogenous peptides from CSF for biomarker discovery in Alzheimer’s disease was performed with a combination of salts before MWCO fractionation, but the impact of adding salts was not investigated [16]. The most commonly used brand of MWCO in the publications and in peptidomic studies is Millipore. Therefore, Millipore MWCOs (using regenerated cellulose as the membrane) are used for this present study. The purpose of this work is to provide an optimized sample preparation technique for MWCO filtering to reduce sample loss and allow sub-μg detection of peptides using MALDI mass spectrometry.
MATERIALS AND METHODS
Materials and Chemicals
Water, acetonitrile, methanol (optima LC/MS grade) and sodium chloride (99.5%) were purchased from Fisher Scientific (Fair Lawn, NJ). The α-cyano-4-hydroxy-cinnamic acid (99%), formic acid (FA) (≥98%), and bovine serum albumin (≥96%) were purchased from Sigma-Aldrich (St. Louis, MO). Amicon Ultra 0.5 mL 10,000 MWCO centrifugal filters and ZipTips packed with C18 reversed-phase resin were purchased from Millipore (Billerica, MA). Trypsin digested bovine serum albumin (BSA) was purchased from Waters (Milford, MA). Bradykinin was purchased from American Peptide Company (Sunnyvale, CA).
MALDI MS Instrumentation
An AutoFLEX III MALDI TOF/TOF mass spectrometer (Bruker Daltonics, Billerica, MA) was operated in positive ion reflectron mode. The MALDI MS instrument is equipped with a proprietary smart beam (Bruker Daltonics, Billerica, MA) laser operating at 200 Hz repetition rate. The instrument was internally calibrated over the mass range of analysis using a standard peptide mix. Two thousand laser shots were collected per sample spot, at an accelerating voltage of 19 kV and a constant laser power using random shot selection. The acquired data were analyzed using FlexAnalysis software (Bruker Daltonics, Billerica, MA). Mass spectrometry data acquisition was obtained by averaging 2000 laser shots covering the mass range m/z 500–2500.
Molecular weight cut off separation procedure
The MWCO separations were performed using Amicon Ultra 0.5 mL 10,000 MWCO centrifugal filters (Billerica, MA). Before MWCO separation three washing steps were performed to remove contaminates from the filter. The three washes were 500 μL of 50:50 H2O:MeOH followed by 500 μL H2O, and finally 400 μL of the solution used for MWCO separation. For 100% H2O solution, 1 μg of BSA or bradykinin were used for separation. All the other MWCO separation experiments used 500 ng BSA or 100 ng or less of bradykinin. The MWCO filter was then centrifuged at 14,000 rpm for 5 min at room temperature in an Eppendorf 5415 D microcentrifuge (Brinkmann Instruments Inc., Westbury, NY). The filtrate was concentrated in a Savant SC 110 SpeedVac concentrator (Thermo Electron Corporation, West Palm Beach, FL) and acidified. The resulting sample was desalted using C18 ZipTips from Millipore (Billerica, MA) by washing the ZipTip with three times 100% ACN, three aqueous washes of 0.1% FA, binding the peptides from the solution and one aqueous wash of 0.1% FA. Peptides were then eluted from the ZipTips using 15 μL of 50% ACN in 0.1% FA.
Matrix deposition
Equal volumes of 0.5 μL from the 15 μL sample solution (including standards not subject to MWCO filtering) and α-cyano-4-hydroxy-cinnamic acid (CHCA) matrix solution in 50% ACN were mixed using a dried-droplet method and spotted on a MALDI target. The resulting droplets were allowed to air dry prior to mass spectrometry acquisition.
RESULTS AND DISCUSSION
Analysis of bradykinin standard and two orders of magnitude increase in signal via improved MWCO separation/elution protocol
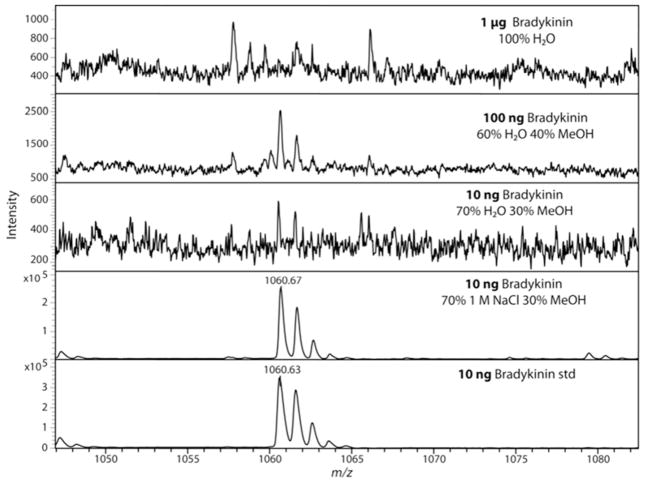
To assess the potential peptide sample loss in performing MWCO separation in the flow-through, bradykinin was selected as a neuropeptide standard. As shown in Figure 1, 1 μg of bradykinin standard does not produce a detectable signal by MALDI mass spectrometry analysis after 10 kDa MWCO separation in water (performed in triplicate). For comparison, 1 ng of bradykinin standard was diluted to 15 μL and produced an intense signal on the MALDI mass spectrometer, whereas 1 μg of bradykinin produced no detectable signal after MWCO filtration with water, suggesting significant sample loss occurs when target analyte is low in quantity (data not shown, performed in triplicate). Figure 1 shows that the addition of a salt, in this case NaCl, improves the limits of detection and decreases sample loss when 70/30 water/methanol was compared to 70/30 aqueous 1 M NaCl/methanol with reproducible results (N=4) giving a relative standard deviation (RSD) of 6% for peak intensity.. Two orders of magnitude are represented in Figure 1 showing no signal for 1 μg bradykinin, but showing intense signal for 10 ng bradykinin after MWCO separation using a 70/30 aqueous 1 M NaCl/methanol as elution solvent. At 10 ng of bradykinin the sample loss attributed to zip-tipping was estimated to be around 41% with triplicates calculated from the decrease in peak intensity. In comparison to the yield seen with the 10 ng bradykinin with 70/30 aqueous 1 M NaCl/methanol MWCO separation and zip-tipping showing an estimated sample loss of 63%, more loss can be attributed to sample clean-up than the MWCO filtration.
Figure 1.
Representative MALDI mass spectra after MWCO separation of a bradykinin standard showing improvement over two orders of magnitude in detection limits. Each MWCO separation was performed at minimum in triplicate with representative spectrum selected for each with a calculated RSD from the peak heights. Three different amounts of bradykinin were tested to assess the magnitude of sample loss under different MWCO solvent conditions. The top panel shows 1 μg of bradykinin standard after MWCO separation with 100% H2O elution produced no signal. The addition of 40% or 30% MeOH produced very low bradykinin signals for both 100 ng (RSD of 28%) and 10 ng (S/N<3, no RSD calculated) respectively. In the bottom two spectra each showed very large intensity, but the 70/30 aqueous 1 M NaCl/methanol 10 ng bradykinin was processed with a 10kDa MWCO and zip-tipped and was reproducible with a RSD of 6%. The last spectrum was from 10 ng bradykinin (RSD of 3%) which was diluted to an equivalent volume as all the other experiments and directly spotted onto the MALDI plate.
A series of experiments were performed to determine if 70/30 aqueous 1 M NaCl/methanol was an optimized solution for peptide recovery using MWCO separation (data not shown). A 50/50 aqueous 1 M NaCl/methanol and 50/50 water/methanol elution were performed in duplicate, but signal intensity of the resulting bradykinin was poor and with similar low intensity as numerous polymer peaks were detected in the flow through. A lower salt concentration was used at aqueous 0.1 M NaCl which also produced greatly reduced signal, when compared to aqueous 1 M NaCl. To assess a lower level of sample recovery, the optimized 70/30 aqueous 1 M NaCl/methanol solution was added to 1 ng bradykinin for MWCO separation but no signal was obtained (data not shown). Using a neuropeptide standard, the addition of methanol and NaCl salt significantly improved the sample recovery in sub-μg amounts.
BSA tryptic peptide mixture analysis
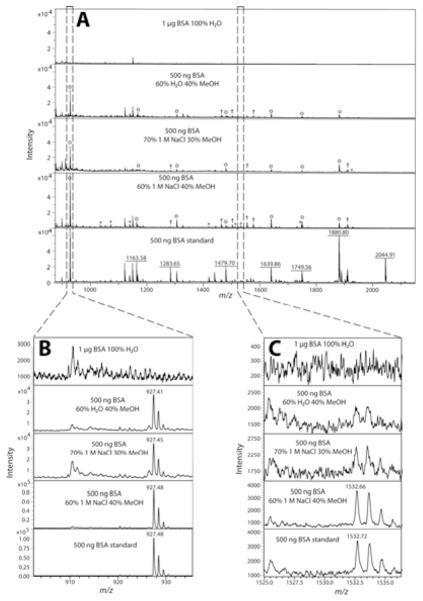
After demonstrating the importance of using an optimized solution for MWCO separation with an individual peptide, a more complex peptide mixture was investigated using a 500 ng BSA tryptic digest. Table 1 lists the BSA tryptic peptides identified in the MALDI MS analysis from different solution conditions processed by MWCO separation. As shown in Table 1, a directly spotted BSA tryptic peptide standard in the absence of any MWCO filtration enabled identification of 39 tryptic peptides by peptide mass fingerprinting. Once again, using 100% H2O for MWCO separation the starting amount was doubled to 1 μg (also done with 500 ng, data not shown), however, many tryptic peptides were not detected due to low signal intensities and non-optimal elution condition. Instead of H2O, a 1 M NaCl solution was used for MWCO separation elution, but only two tryptic peptides were identified (Table 1). The addition of 30% methanol into the sample before MWCO filtration produced the first increase in identified BSA tryptic peptides. The remaining data from Table 1 shows improved BSA tryptic peptide identifications as the sample (elution) conditions were further optimized. Figure 2 shows the actual mass spectra associated with the three most promising elution solutions along with 100% H2O.
Table 1.
Identified BSA tryptic peptides from various MWCO separation conditions
| BSA tryptic peptide (MH+) | 100% H2O 1 μg |
100% 1 M NaCl |
70% H2O |
80% 1 M NaCl |
70% 1 M NaCl |
60% H2O |
60% 1 M NaCl |
|---|---|---|---|---|---|---|---|
| 508.3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 545.3 | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| 689.4 | ✓ | ✓ | ✓ | ✓ | |||
| 712.4 | ✓ | ✓ | ✓ | ✓ | |||
| 898.5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 927.5 | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| 1034.5 | ✓ | ||||||
| 1072.5 | ✓ | ✓ | |||||
| 1138.5 | ✓ | ✓ | |||||
| 1163.6 | ✓ | ✓ | ✓ | ||||
| 1249.6 | |||||||
| 1283.7 | ✓ | ✓ | |||||
| 1305.7 | ✓ | ✓ | ✓ | ✓ | |||
| 1399.7 | |||||||
| 1415.7 | |||||||
| 1419.7 | ✓ | ||||||
| 1439.8 | |||||||
| 1463.6 | ✓ | ✓ | ✓ | ||||
| 1479.8 | ✓ | ✓ | ✓ | ||||
| 1502.6 | ✓ | ✓ | |||||
| 1511.8 | ✓ | ||||||
| 1532.8 | ✓ | ||||||
| 1554.7 | ✓ | ✓ | |||||
| 1567.7 | |||||||
| 1576.8 | ✓ | ✓ | |||||
| 1639.9 | ✓ | ✓ | ✓ | ||||
| 1667.8 | ✓ | ||||||
| 1673.8 | |||||||
| 1724.8 | |||||||
| 1740.8 | |||||||
| 1747.7 | ✓ | ||||||
| 1749.7 | ✓ | ✓ | ✓ | ||||
| 1880.9 | ✓ | ✓ | ✓ | ||||
| 1889.0 | |||||||
| 1901.9 | ✓ | ✓ | |||||
| 1907.9 | ✓ | ||||||
| 2045.0 | |||||||
| 2113.9 | |||||||
| 2247.9 | |||||||
| Total: 39 | 2 | 2 | 6 | 8 | 15 | 15 | 27 |
The percentage listed represents the percent of aqueous present with the remaining being MeOH. The 100% H2O MWCO separation used 1 μg of BSA tryptic peptide standard, while all the other listed conditions used 500 ng. The column titled BSA tryptic peptide refers to a directly spotted BSA tryptic digest and the masses matched to tryptic peptides. A checkmark indicates a tryptic peptide was mass matched and present at the indicated m/z with a 13C isotope peak. The numbers of BSA tryptic peptides identified are listed at the bottom of the table.
Figure 2.
Representative MALDI mass spectra from MWCO separation of a BSA tryptic peptide standard showing sample loss. Stacked mass spectra from m/z range 875–2150, normalized to 7 × 104 intensity, representing the detection difference from a BSA tryptic peptide standard from different MWCO separation conditions (A). It should be noted that when the solvent for MWCO elution was 100% H2O, 1 μg of BSA tryptic peptides was processed instead of 500 ng. A zoomed in view of the most abundant BSA tryptic peptide detected, YLYEIAR, m/z 927.49 (B). Various percentages of MeOH produced significant signal, but addition of a salt (1 M NaCl) increases the signal, which is closest to a directly spotted BSA tryptic peptide standard. A zoomed in view of a representative low intensity BSA tryptic peptide detected, LKEC#C#DKPLLEK, m/z 1532.66 (C). The optimized solution to be used for MWCO filtration, 60/40 aqueous 1 M NaCl/methanol, was the only procedure that enabled the detection of the tryptic peptide in Figure 2C which was also detected in the directly spotted BSA tryptic peptide standard. All experiments were performed a minimum of two times with nearly identical results.
#) Carbamidomethyl amino acid modification
o) Tryptic peptide identified in three of the spectra in Figure 2A
†) Tryptic peptide identified in two of the spectra in Figure 2A
*) Tryptic peptide identified in a single spectrum in Figure 2A
The BSA tryptic peptide intensities are shown in Figure 2A, and the most intense tryptic peptide, YLYEIAR, m/z 927.49 was observed in the four different solutions shown in Figure 2B but not in the 100% H2O or 100% 1 M NaCl solutions (data not shown). Figure 2A has all the mass spectra normalized to 7 × 104 intensity to illustrate two points. First, MWCO filtering step still produced sample loss regardless of the solvent conditions chosen. Second, there is a noticeable increase in peptide peak intensity using the optimized solvent, 60/40 aqueous 1 M NaCl/methanol (Figure 2A). Figure 2C displays a zoomed in view of a detectable signal for a BSA tryptic peptide, LKEC#C#DKPLLEK, m/z 1532.66 (#: carbamidomethyl) observed only in the optimized solvent. The detection of the m/z 1532.66 peptide in Figure 2C highlights the potential gain in sample and detectable peptides by using an optimized salt/MeOH combination. A non-optimized salt/MeOH combination will still reduce sample loss, but further minimizing sample loss during sample preparation will always be desirable in any analytical protocol.
MWCO composition
The purpose of this application note is to provide evidence of sub-μg sample loss of peptide sample and a solution to overcome this limitation of using MWCO separation. The mechanism of why MeOH and NaCl addition to the sample solution provide a significant reduction in sample loss is beyond the scope of this application note. Regardless, Supplemental Table S1 is an expanded version of Table 1 showing the amino acid sequence, hydrophobicity calculated using GRAVY scores, and pI of the identified peptides in this study, but no discernible trend was obtained from the data. The membrane of commonly used MWCO in peptidomics and for this study is comprised of chemically treated (regenerated) cellulose, which is a polysaccharide containing β (1→4) linked D-glucose. Glucose has numerous free hydroxyl groups which could non-specifically adsorb peptides flowing through the MWCO. The addition of MeOH has the most significant effect on signal, which could be due to disrupting the interaction between peptides and hydroxyl groups from glucose. NaCl has less significant effect on sample recovery compared to MeOH, but a detectable reduction in sample loss is noted. This improvement in sample recovery could be analogous to the use of NaCl in immunodepletion to reduced non-specific binding, which is accomplished by adding 150 mM NaCl [17].
Analysis of bradykinin in the presence of undigested BSA
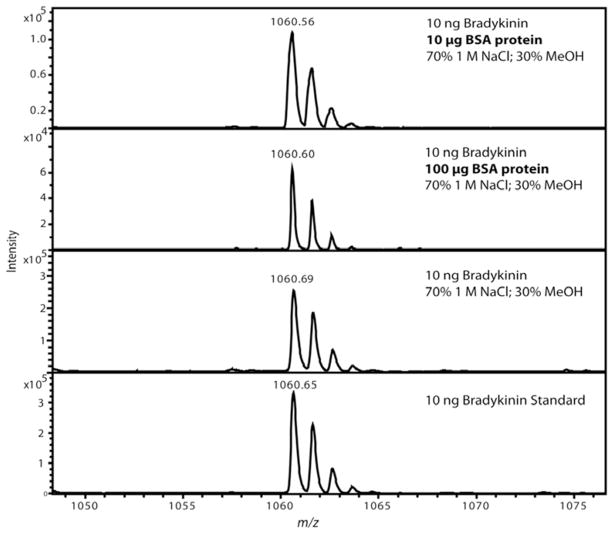
Commonly, when using MWCO for peptide isolation, proteins would be present in the samples, usually in larger amounts. Figure 3 shows the effect of adding BSA protein to a 10 ng bradykinin solution before MWCO fractionation on the resulting recovery of bradykinin. Using the optimized 70/30 aqueous 1 M NaCl/methanol solution we obtained strong, yet decreased signal of bradykinin with the addition of 10 μg BSA with a RSD of 10% and more severe signal reduction in the presence of 100 μg BSA with a RSD of 2% (N=2). It is not unexpected that more signal reduction due to sample loss would occur, especially in the 100 μg BSA sample because the BSA protein has a molar ratio of 160 BSA protein molecules to one bradykinin peptide. Figure 3 shows the usefulness of the MWCO method with samples containing large amounts of proteins.
Figure 3.
Representative MALDI mass spectra after MWCO separation of a bradykinin standard showing optimized solvent conditions prevented non-specific binding to bovine serum albumin (BSA). Each experiment was performed in duplicate with representative spectrum selected for each. Two different amounts of BSA protein were tested to assess the magnitude of sample loss caused by the presence of a protein. The top panel shows 10 μg of BSA protein and the second panel shows 100 μg of BSA protein added while only 10 ng of bradykinin was added. Detectable sample loss was observed when the BSA protein was added, but panel two shows that the amount of BSA protein was 1×104 greater (equivalent to 160 fold molar excess) than bradykinin. The last two spectra were controls using a MWCO with the optimized solution in panel 3 and panel 4 using 10 ng bradykinin which was diluted to an equivalent volume as all the other experiments and directly spotted onto the MALDI plate.
Recommendation/Conclusion
The present work provides solutions to reduce the sample loss incurred from the use of MWCO for sub-μg peptide isolation with or without non-digested proteins present in the sample. Despite its widespread utility, the MWCO fractionation step often produces significant sample loss that has not been addressed, which is particularly problematic for low-abundance peptide analysis with limited starting material. This application note aims to reduce sample loss after MWCO separation, specifically for sub-μg peptide isolation. If complex samples are processed with MWCO separation, the authors recommend eluting the sample with 60/40 aqueous 1 M NaCl/methanol solution as a starting point to minimize sample loss. This application note provides a viable alternative for sub-μg peptide MWCO separation, circumventing the need to increase the starting material just to address sample loss from using a MWCO membrane-based centrifugal filter device.
Acknowledgments
This work was supported in part by the National Science Foundation grant (CHE-0967784), University of Wisconsin Graduate School, and Wisconsin Alzheimer’s Disease Research Center Pilot Grant. L.L. acknowledges an H. I. Romnes Faculty Fellowship.
References
- 1.Georgiou HM, Rice GE, Baker MS. Proteomic analysis of human plasma: failure of centrifugal ultrafiltration to remove albumin and other high molecular weight proteins. Proteomics. 2001;1:1503–1506. doi: 10.1002/1615-9861(200111)1:12<1503::aid-prot1503>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 2.Greening DW, Simpson RJ. Low-molecular weight plasma proteome analysis using centrifugal ultrafiltration. Methods Mol Biol. 2011;728:109–124. doi: 10.1007/978-1-61779-068-3_6. [DOI] [PubMed] [Google Scholar]
- 3.Greening DW, Simpson RJ. A centrifugal ultrafiltration strategy for isolating the low-molecular weight (<or=25K) component of human plasma proteome. J Proteomics. 2010;73:637–648. doi: 10.1016/j.jprot.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Manza LL, Stamer SL, Ham AJ, Codreanu SG, Liebler DC. Sample preparation and digestion for proteomic analyses using spin filters. Proteomics. 2005;5:1742–1745. doi: 10.1002/pmic.200401063. [DOI] [PubMed] [Google Scholar]
- 5.Theodorescu D, Fliser D, Wittke S, Mischak H, Krebs R, Walden M, Ross M, Eltze E, Bettendorf O, Wulfmg C, Semjonow A. Pilot study of capillary electrophoresis coupled to mass spectrometry as a tool to define potential prostate cancer biomarkers in urine. Electrophoresis. 2005;26:2797–2808. doi: 10.1002/elps.200400208. [DOI] [PubMed] [Google Scholar]
- 6.Antwi K, Hostetter G, Demeure MJ, Katchman BA, Decker GA, Ruiz Y, Sielaff TD, Koep LJ, Lake DF. Analysis of the plasma peptidome from pancreas cancer patients connects a peptide in plasma to overexpression of the parent protein in tumors. J Proteome Res. 2009;8:4722–4731. doi: 10.1021/pr900414f. [DOI] [PubMed] [Google Scholar]
- 7.Aristoteli LP, Molloy MP, Baker MS. Evaluation of endogenous plasma peptide extraction methods for mass spectrometric biomarker discovery. J Proteome Res. 2007;6:571–581. doi: 10.1021/pr0602996. [DOI] [PubMed] [Google Scholar]
- 8.Zougman A, Pilch B, Podtelejnikov A, Kiehntopf M, Schnabel C, Kumar C, Mann M. Integrated analysis of the cerebrospinal fluid peptidome and proteome. J Proteome Res. 2008;7:386–399. doi: 10.1021/pr070501k. [DOI] [PubMed] [Google Scholar]
- 9.Yuan X, Desiderio DM. Human cerebrospinal fluid peptidomics. J Mass Spectrom. 2005;40:176–181. doi: 10.1002/jms.737. [DOI] [PubMed] [Google Scholar]
- 10.Zheng X, Baker H, Hancock WS. Analysis of the low molecular weight serum peptidome using ultrafiltration and a hybrid ion trap-Fourier transform mass spectrometer. J Chromatogr A. 2006;1120:173–184. doi: 10.1016/j.chroma.2006.01.098. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Sweedler JV. Peptides in the brain: mass spectrometry-based measurement approaches and challenges. Annu Rev Anal Chem (Palo Alto Calif) 2008;1:451–483. doi: 10.1146/annurev.anchem.1.031207.113053. [DOI] [PubMed] [Google Scholar]
- 12.Stefano GB, Fricchione G, Goumon Y, Esch T. Pain, immunity, opiate and opioid compounds and health. Med Sci Monit. 2005;11:MS47–53. [PubMed] [Google Scholar]
- 13.Jensen J. Regulatory peptides and control of food intake in non-mammalian vertebrates. Comp Biochem Physiol A Mol Integr Physiol. 2001;128:471–479. doi: 10.1016/s1095-6433(00)00329-9. [DOI] [PubMed] [Google Scholar]
- 14.Kuoppala A, Lindstedt KA, Saarinen J, Kovanen PT, Kokkonen JO. Inactivation of bradykinin by angiotensin-converting enzyme and by carboxypeptidase N in human plasma. Am J Physiol Heart Circ Physiol. 2000;278:H1069–1074. doi: 10.1152/ajpheart.2000.278.4.H1069. [DOI] [PubMed] [Google Scholar]
- 15.Chen R, Ma M, Hui L, Zhang J, Li L. Measurement of neuropeptides in crustacean hemolymph via MALDI mass spectrometry. J Am Soc Mass Spectrom. 2009;20:708–718. doi: 10.1016/j.jasms.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jahn H, Wittke S, Zurbig P, Raedler TJ, Arlt S, Kellmann M, Mullen W, Eichenlaub M, Mischak H, Wiedemann K. Peptide fingerprinting of Alzheimer’s disease in cerebrospinal fluid: identification and prospective evaluation of new synaptic biomarkers. PLoS One. 2011;6:e26540. doi: 10.1371/journal.pone.0026540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cellar NA, Karnoup AS, Albers DR, Langhorst ML, Young SA. Immunodepletion of high abundance proteins coupled on-line with reversed-phase liquid chromatography: a two-dimensional LC sample enrichment and fractionation technique for mammalian proteomics. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:79–85. doi: 10.1016/j.jchromb.2008.11.020. [DOI] [PubMed] [Google Scholar]