Abstract
Drug concentrations associated with protection from HIV-1 acquisition have not been determined. This study evaluated drug concentrations among men who have sex with men in a substudy of the iPrEx trial,(1) a randomized placebo controlled trial of daily oral emtricitabine/tenofovir disoproxil fumarate pre-exposure prophylaxis (PrEP). Any detectable drug in blood plasma and viably cryopreserved peripheral blood mononuclear cells (vPBMCs) was less frequent in HIV-infected cases at the visit when HIV was first discovered compared with controls at the matched time point of the study (8% vs 44%, P<0.001) and in the 90 days prior to that visit (11% vs 51%, P<0.001). An intracellular tenofovir-diphosphate (TFV-DP) concentration of 16 fmol per million vPBMCs was associated with a 90% reduction in HIV acquisition relative to the placebo arm. Directly observed dosing in a separate study, STRAND, yielded TFV-DP concentrations that, when analyzed with this iPrEx model, corresponded with HIV-1 risk reduction of 76% for 2 doses per week, 96% for 4 doses per week, 99% for 7 doses per week. Prophylactic benefits were observed over a range of doses and drug concentrations, suggesting ways to optimize PrEP regimens for this population.
INTRODUCTION
UNAIDS estimates there are 2.6 million new HIV-1 infections per year, despite widespread awareness of the modes of transmission and the protective benefits of condom use.(2) Men who have sex with men carry a disproportionate burden of infection on all continents.(3) The iPrEx trial demonstrated that randomization to daily oral emtricitabine/tenofovir (TFV) disoproxil fumarate (FTC/TDF) PrEP decreased HIV-1 acquisition by 44% compared with placebo among such men, with greater reductions in HIV-1 risk associated with higher reported adherence and detectable drug in the blood. (1)
In a predefined pharmacology substudy of iPrEx, FTC, TFV in plasma and/or FTC-triphosphate (FTC-TP), TFV-DP in vPBMC was detected in 22 of 43 of seronegative subjects (51%) versus 3 of 34 HIV-infected subjects from the active arm (9%) (P<0.001). (1) Predicted efficacy in iPrEx increased from 44% to over 90% when detectable drug was accounted for. Thus, adherence to daily FTC-TDF was critical for PrEP efficacy in iPrEx, and the same has been found in other PrEP studies.(4–7) However, the quantitative relationship between drug concentration and/or level of adherence with PrEP efficacy has not been determined. The present study expands on the predefined pharmacology substudy in iPrEx and quantifies the concentrations of drugs associated with protection from HIV-1 acquisition, as well as the frequency of PrEP use required to achieve those concentrations.
RESULTS
Overview
Intracellular tenofovir-diphosphate, the active form of tenofovir, was analyzed in vPBMCs arising from two studies, STRAND and iPrEx. TFV-DP in vPBMC from the STRAND study were used to establish expected concentrations from 2, 4, or 7 doses/week of directly observed TDF therapy, and TFV-DP in vPBMC from iPrEx was used to estimate concentrations associated with decreased HIV acquisition. PrEP efficacy was then quantified for 2, 4, and 7 doses/week by analyzing TFV-DP concentrations from STRAND with the iPrEx HIV risk reduction model. The vPBMC collection, processing and storage for iPrEx and STRAND used identical laboratory protocols, and the drug concentration testing was performed in the same laboratory using identical laboratory methods.
Expected TFV-DP from STRAND
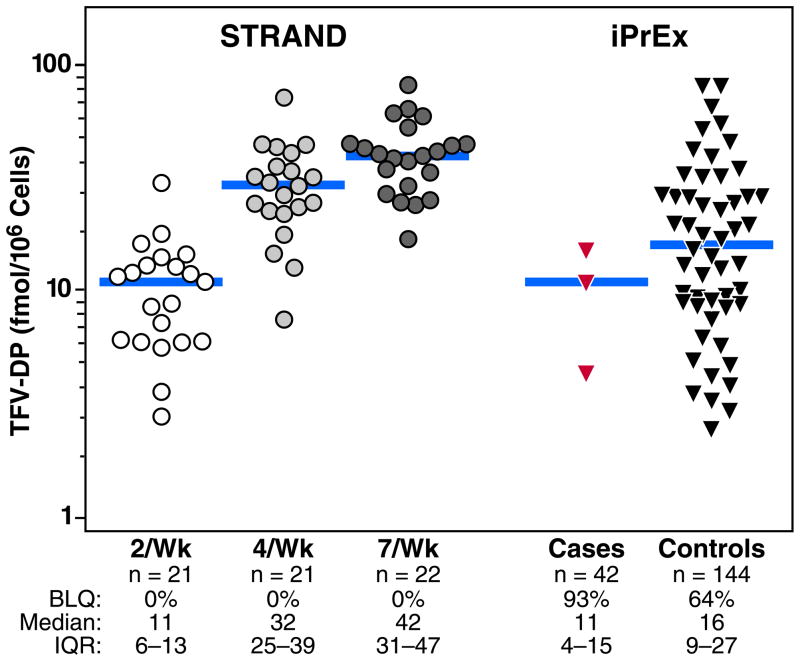
The STRAND study administered oral TDF to 24 HIV-negative adults (FTC was not included), each of whom received 6 weeks of 2, 4, or 7 doses per week. Dosing was directly observed Monday through Friday, including all the 2 and 4 doses per week, and participants provided confirmation of the date and time of doses taken on Saturday and Sunday by text message or telephone contact on the day of use. TFV-DP in vPBMC was measured at the end of the 6 weeks of each dosing regimen. The median (IQR) values were 11 fmol/M (6 to 13) fmol/M for 2 doses per week, 32 fmol/M (25 to 39) for 4 doses per week, and 42 fmol/M (31 to 47) (Figure 1). The median (IQR) times from the last dose to vPBMC collection were 24 hours (20 to 141), 25 hours (22 to 62), and 24 hours (21 to 25) hours, respectively. TFV-DP was quantifiable in all STRAND participants at all visits.
Fig 1.
Observed TFV-DP concentrations from STRAND and iPrEx. The values observed in the STRAND study are shown on the left for 2 doses/week (open circles), 4 doses/week (light-toned circles), and 7 doses/week (dark-toned circles). iPrEx values on the right included those from the visit with first evidence of HIV infection in cases (red triangles) and the matched study visit in HIV-negative controls (dark-toned triangles). The bars represent the medians. The numbers of participants tested, the proportion of vPBMC tested with detectable TFV-DP; the median concentrations among values in the detectable range; and the interquartile ranges are listed below the x-axis.
iPrEx analyses
The iPrEx study provided an opportunity to identify vPBMC concentrations associated with different levels of protection from HIV-1 acquisition. Plasma and/or vPBMC were tested for FTC-TDF in all 48 seroconverters at the visit when HIV-1 infection was first detected, either by plasma viral RNA, by serum antibodies, or both. The median time between the last HIV-negative test and the first evidence of HIV was 33 days (interquartile range of 28 to 48 days). Each HIV infected case was matched to 3 seronegative controls in the FTC-TDF arm by study site, and duration on study. One of the 3 controls was selected having reported high-risk sexual practices, to assure comparable HIV-1 exposure with cases, and the other two were selected randomly. The cases and controls were comparable with respect to HIV risk factors, level of schooling, and alcohol use (Table 1). Both plasma and PBMC were tested in 42/48 (88%) of cases at the visit when HIV-1 infection was first detected and 144/144 (100%) of controls at the matched time point on study. Plasma and/or PBMC were also tested from other longitudinal study visits in the cases and controls. Drug detection was defined as any quantifiable moiety among plasma TFV, FTC, and intracellular TFV-DP and FTC-TP.
Table 1.
Demographic and HIV risk characteristics at baseline among analysis groups in the iPrEx study
| Cases N=48 | Controls N=144 | p-value | |
|---|---|---|---|
| Baseline | mean (sd) | ||
| Age in years | 24 (6) | 27 (8) | 0.01 |
| Calculated CrCl ml/min | 125 (23) | 121 (22) | 0.30 |
| Male Partners In past 6 months | 12 (32) | 13 (46) | 0.78 |
| n (%) | |||
| Secondary School | 31 (66%) | 109 (76%) | 0.19 |
| Alcohol Use (≥5 drinks per day on days when drinking) | 24 (52%) | 74 (54%) | 0.87 |
| Unprotected Receptive Anal Intercourse | 34 (71%) | 109 (76%) | 0.57 |
| History of sexually transmitted infection in past 6 months | 15 (31%) | 52 (36%) | 0.60 |
| Herpes seropositive | 24 (50%) | 69 (48%) | 0.87 |
| At Time of HIV Detection in Cases | mean (sd) | ||
| Weeks on Study | 63 (35) | 65 (36) | 0.73 |
| n (%) | |||
| Unprotected Receptive Anal Intercourse | 19 (40%) | 74 (51%) | 0.18 |
Drug detection in plasma and vPBMC in iPrEx was concordant in >95% of all pairwise comparisons, indicating that plasma and vPBMC analysis yielded similar estimates of the proportion of groups having detectable drug.
Drug detection at the visit with first evidence of HIV-infection
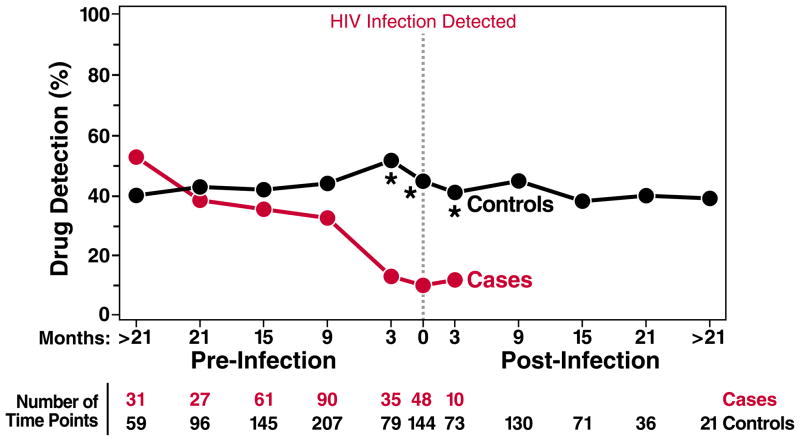
Any drug moiety was detected 5.5-fold less frequently (8% vs 44%; P<0.001) in HIV-positive cases at the visit when HIV infection was first detected compared with the matched time point in HIV-negative controls (Fig. 2). Lower drug detection among cases vs controls (11% vs. 51%; P<0.001) was also observed within the 90 days before the HIV infection visit or matched time point in controls. At time points more than 90 days before HIV was first detected, the proportion of cases and controls with any detectable drug was comparable (36% vs 41%; P=0.77), indicating that HIV infection occurred during periods of low drug exposure in the active arm of iPrEx. These drug detection rates suggest that a substantial fraction of iPrEx participants were dosing with fewer than 2 doses per week, given the 100% TFV-DP detection rate for 2, 4, and 7 doses per week in STRAND (fig 1).
Fig 2.
Longitudinal drug detection relative to the time of first HIV-1 detection. The % of case and control time points with any drug detection in plasma or PBMC including TFV, FTC, TFV-DP, or FTC-TP, at the time of first laboratory evidence of HIV in cases or the matched time point in controls (dashed vertical line), and at pre- and post-HIV infection time points. The pre- and post-HIV infection time points were divided into those within 3 months (90 days) before or after HIV infection, and those within 6 month intervals distal to these time points. The number of time points with either plasma or vPBMC available for testing is listed for each time period.
TFV-DP concentrations and risk of HIV-acquisition
Of the iPrEx seronegative controls having quantifiable TFV-DP (36% of the total), the median concentration was 16 fmol/M (IQR 9 to 27) which was between the median concentrations observed for 2 and 4 doses per week in STRAND (Fig. 1). Only 18% of seronegative controls had TFV-DP concentrations in the range associated with daily dosing in STRAND. This suggests that TFV-DP concentrations below those associated with daily dosing were exerting antiviral effects to give the 44% overall efficacy in the group randomized to FTC/TDF versus placebo. Only 3 HIV infected cases had detectable TFV-DP at the time HIV was first detected (the concentrations at the time of infection are not known). These concentrations were in the range of those associated with 2 doses per week in STRAND and none were in the range of daily dosing.
The TFV-DP concentration in vPBMC associated with HIV-1 acquisition was estimated using a Cox proportional hazards model that assessed the risk of HIV infection in the iPrEx trial as a function of TFV-DP (or FTC-TP) concentrations in active arm participants. Exponential regression was also used in a second analysis. Multiple imputation(8, 9) was used to construct a dataset with drug concentrations at each visit when an HIV-1 test was performed. If a drug concentration was not available, imputation was employed using participant and visit level data (age, site, time on study, numbers of partners, creatinine clearance, alcohol use, secondary education, HSV, sexually transmitted disease, pharmacy drug dispensation records and unprotected receptive anal intercourse at baseline and followup) to estimate the missing concentration. This approach allowed HIV-1 risk to be estimated for the entire cohort, to estimate how drug exposure as a time dependent variable associated with HIV risk compared with the placebo arm. Drug concentrations below the limit of quantitation (BLQ) were set to 0 fmol/M.
The relationship between drug concentration and HIV infection risk was significant for both TFV-DP (p=0.016) and FTC-TP (p=0.004) among those assigned to receive oral FTC/TDF. Compared with the placebo HIV incidence, the TFV-DP concentrations associated with 50%, 90% and 99% reduced HIV-1 acquisition were 3 (95% CI: <1 to 7), 16 (95% CI: 3 to 28), and 33 (6 to 60) fmol/M vPBMC, respectively. For FTC-TP, the corresponding values were 0.82 (0.1 to 1.6), 3.7 (1.2 to 6.1), and 7.7 (2.6 to 12.9) pmol/M viable cells, respectively. Median (IQR) FTC-TP in cases at the visit when HIV was first detected and at the matching visit in controls were 1.8 (0.9 to 5.1) and 2.9 (1.6 to 5.2) pmol/M, respectively. However, neither TFV-DP nor FTC-TP was independent of the other in a model that included both, therefore further analyses focused upon TFV-DP so that TFV-DP concentrations from STRAND could be utilized.
Dosing and risk of HIV-acquisition
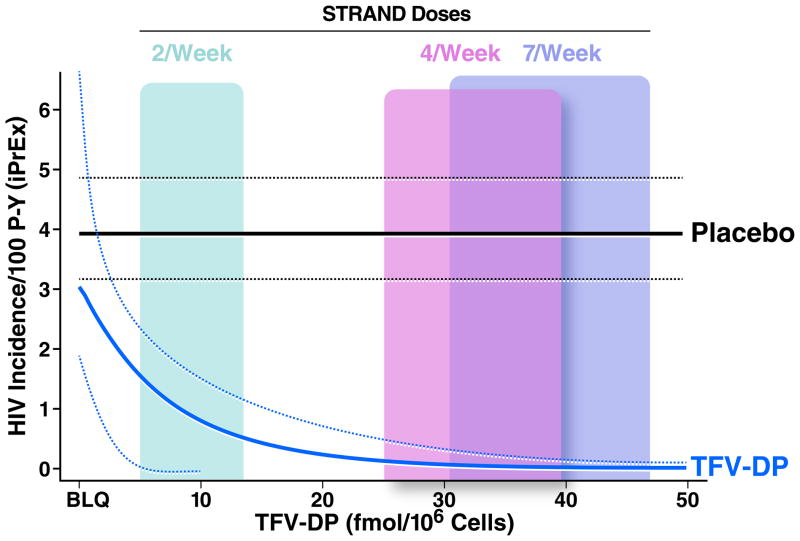
The exponential regression shown in Figure 3 demonstrated that the risk of HIV infection was comparable among participants in the active arm with undetectable TFV-DP compared to the placebo arm (Relative hazard = 0.78; 95% CI: 0.49 to 1.06; p=0.19). Similar to the Cox analysis above, a TFV-DP concentration of 16 fmol/M vPMBCs was associated with 90% HIV risk reduction (EC90) relative to placebo. For perspective, the concentrations from the STRAND study are with colored panels in Figure 3; the EC90 (16 fmol/M) was 38% of the median TFV-DP concentration observed in those taking 7 doses per week (42 fmol/M).
Fig. 3.
Estimated HIV incidence from exponential regression model of TFV-DP concentrations (fmol/M vPBMC) in iPrEx. The placebo HIV infection rate is shown as a horizontal line at 3.9 infections per 100 person years, with the relative rate in the active arm according to TFV-DP concentrations (x-axis). Dashed lines represent the 95% confidence intervals. The IQR of TFV-DP concentrations associated with directly observed dosing in STRAND are provided as colored panels overlying the curves.
Sensitivity analyses were conducted to evaluate the robustness of the estimated vPBMC EC90 TFV-DP. An analysis that adjusted for unprotected receptive anal intercourse (the main risk factor for HIV acquisition in this population), and other factors also used for multiple imputation, yielded an estimate of 15 (95% CI: 3 to 27) fmol/M. Allowing drug concentrations below the limit of quantitation to vary uniformly between 0 and 5 yielded an estimate of 20 (95% CI: 7 to 33) fmol/M. Using the averaged drug concentrations from the visit closest to HIV infection with values in the previous 90 days yielded an estimate of 23 (5 to 41) fmol/M. An analysis that brought drug detection at subsequent seroconversion time points to the time of the first detection of HIV-1 RNA yields an estimate of 19 (95% CI: 4 to 33). Adjustment of TFV-DP (from 4.19 to 13.4 fmol/M) in one person whose blood specimen was available 7 days after stopping oral FTC/TDF at seroconversion yielded an estimate of 20 (95% CI: 4 to 36). Estimating a TFV-DP concentration associated with a 90% HIV risk reduction in cases versus controls was also possible using conditional logistic regression without multiple imputation. This analysis yielded a similar estimate for the vPBMC EC90 of 16 fmol/M vPBMC (95% CI: 8 to 44). All estimates were comparable with the initial EC90 estimate of 16 fmol/M and well within the range of concentrations achieved with 4 to 7 tablets per week in STRAND.
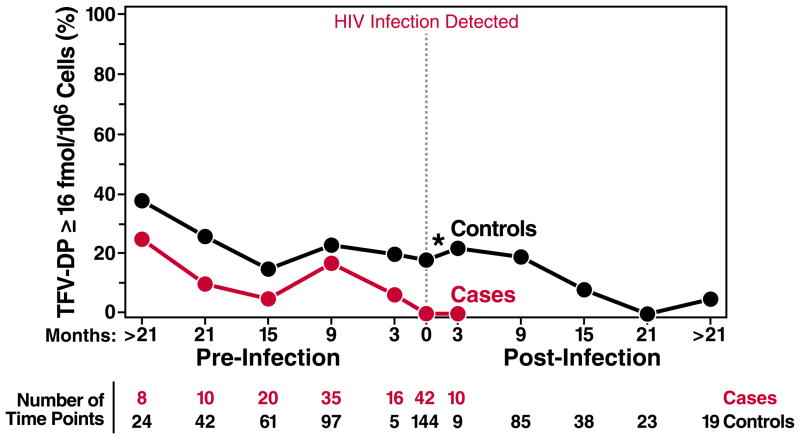
The fraction of case and control time points that exceeded the EC90 over time is shown in Figure 4. At the visit when HIV was first detected, no cases (0/42) had TFV-DP ≥ 16 fmol/M compared with 18% (26/144) of seronegative controls at the matched time point (P<0.001; Fig. 4). The proportion of iPrEx participant time points with TFV-DP at or above the EC90 decreased among cases (P=0.02) and controls (P<0.001) over time. Reported risk behavior associated with exposure to HIV-1 also decreased overtime in iPrEx.(1)
Fig 4.
The % of case and control time points with TFV-DP ≥ 16 fmol/M viable PBMC (estimated EC90). Dark-toned circles represent HIV-negative controls, and red circles represent HIV infected cases. The x-axis represents time relative to the visit with first evidence of HIV infection in the case and the matching time point in controls. Pre- and post-HIV infection time points were divided into those within 3 months (90 days) before or after HIV infection, and those within 6 month intervals distal to these time points. The number of time points with vPBMC tested for TFV-DP is listed for each time period. *indicates significant differences between cases and controls (P<0.05).
TFV-DP concentrations arising from 2, 4, and 7 directly observed doses per week in STRAND were analyzed with the exponential regression model from iPrEx described above. The estimated PrEP efficacy was 76% (95% CI: 56 to 96%) for 2 doses per week, 96% (95% CI: 90 to >99%) for 4 doses per week, 99% (95% CI: 96 to >99%) for 7 doses per week. The proportions of people who attained the TFV-DP EC90 were 14% for 2 tablets per week, 90% for 4 tablets per week, and 100% for 7 tablets per week.
DISCUSSION
Drug exposure in HIV-infected cases in iPrEx was critically low at the time of first laboratory evidence of HIV infection, providing a likely explanation for HIV acquisition in these participants. Other evidence indicated negligible drug exposure near the time of HIV infection in cases: plasma HIV-1 RNA levels were comparable in the placebo and active arms, and there was no evidence of TDF or FTC resistance among emergent infections in the active arm.(1) Drug detection in controls was higher than in cases, but was not commensurate with daily dosing in the majority. Only 44% of controls had any detectable drug moiety at the matching time point of the case. Only 18% of seronegative controls had TFV-DP concentrations above 16 fmol/M, a level achieved by 90% of STRAND participants taking 4 or more doses per week, and a level associated with a 90% HIV infection risk reduction.
The minimum protective drug concentrations in the blood, and the required numbers of tablets per week required to achieve those concentrations, may differ depending on the route and frequency of exposure to HIV.(10) For men who have sex with men, the tissue of greatest relevance to the acquisition of HIV-1 infection is the rectal mucosa. Oral dosing has been shown to deliver 20 to 100-fold higher TFV-DP in rectal compared with blood or vaginal/cervical cells/tissue.(11, 12) TFV-DP delivery to penile tissue, relevant for male insertive exposures, has not been determined to our knowledge. The uniquely high delivery of TFV-DP to rectal mucosa suggests that pharmacology findings relevant for MSM, such as in this study, may not directly extrapolate to parenteral, vaginal or penile exposures.
This study demonstrated that the EC90 in vPBMC (16 fmol/M) was 38% the median from daily dosing in STRAND (42 fmol/M). With this information, a rectal mononuclear cell EC90 can be estimated. The rectal mononuclear cell concentration observed with daily oral dosing was reported to be 1846 fmol/M (95% CI: 931 to 3659).(11) Assuming that the kinetics of TFV-DP in rectal mononuclear cells are similar to PBMC, the EC90 in rectal mononuclear cells would be 38% of this value, or approximately 700 fmol/M rectal cells (95% CI: 350 to 1400). This estimate makes several assumptions that require further validation, but nevertheless provides a starting point for a target cell concentration in tissue to translate into animal or ex vivo systems for validation.(13, 14) The threshold identified here is analogous to the TFV concentration threshold of 1000 ng/mL in vaginal fluid identified as protective in the CAPRISA 004 study and in ex vivo assays.(5, 14)
Drug concentrations were measured as close to the HIV infection as possible (at the time HIV infection was first discovered and within 90 days of this time point). Through use of multiple imputation, drug concentrations were assigned to all active arm participants at the time of HIV infection in placebo cases. However, the model could not account for variations in dosing patterns and relationship to timing of HIV exposure and transmission risk.
Confounding is also possible in this analysis, as there may be factors that link higher adherence with lower exposure to HIV. The finding that HIV acquisition among active arm participants with undetectable drug concentrations was not higher than the placebo rate argues against confounding (Fig. 3). In addition, the statistical analysis adjusted for several markers of HIV incidence, including numbers of sexual partners, unprotected anal intercourse, sexually transmitted diseases, age, level of schooling, and substance use.
vPBMC were available for drug analysis, whereas freshly processed and lysed PBMC are traditionally used for cell pharmacology studies.(15) Measurements of TFV-DP in vPBMC from STRAND were a median of 48% (IQR: 38% to 67%) that of freshly lysed PBMC also collected in that study. Processing vPBMC also introduces additional variability in TFV-DP/FTC-TP measures. Despite this added variation, levels of drug concentrations in blood were found to be strongly associated with reduced HIV risk in the active arm compared with placebo. This finding suggests that drug concentration monitoring could inform HIV acquisition risk in persons taking FTC/TDF for PrEP. Other specimens, such as hair, or dried blood spots, may afford more convenient long term measures of drug exposure which will be particularly useful if they can be correlated with protective drug concentrations in vPBMC.(16, 17)
This study identified a relationship between systemic drug exposure and reduction of HIV acquisition risk in one important population. Protective TFV-DP concentrations were readily achieved with 4 or more doses per week. This study focused on TFV-DP because independent relationships for FTC-TP could not be identified in iPrEx and STRAND did not include FTC-TP so expected concentrations for non-daily dosing are not known. FTC co-administration is not expected to affect intracellular concentrations of TFV-DP in PBMC.(18) Importantly the TFV-DP EC90 identified in iPrEx was in the presence of FTC-TP, indicating that reaching these protective TFV-DP concentrations with FTC-TDF dosing is relevant for PrEP efficacy in MSM. Nevertheless, future studies should aspire to evaluate the contribution of FTC-TP to PrEP efficacy.
Alternative dosing regimens, such as pre- and post-intercourse dosing, warrant controlled clinical trials to evaluate the acceptability, behavioral feasibility, and pharmacokinetics of non-daily regimens. The 95% confidence interval for the estimate of the TFV-DP EC90 was 3 to 28 fmol/M vPBMC. The lower bound (3 fmol/M vPBMC) would be achievable after a single dose,(19) and 28 fmol/M was just below the median achieved with 4 doses per week. While animal studies indicate that both pre- and post-exposure dosing are important,(20) more information is needed to define the timing and duration of drug exposure that is required to prevent infection. Dose optimization in the absence of a surrogate marker of protection would require prohibitively large trials, so better definition of pharmacological parameters associated with differing dosing strategies (eg: dose and dosing interval) and protection will be essential to move the PrEP field forward. Ultimately, recommendations for daily PrEP use may be more robust, as daily regimens encourage routinization, and afford drug concentrations that are expected to persist in the protective range even if some doses are missed. The low fraction of iPrEx participants with TFV-DP in the range of daily dosing suggests that demonstration projects should optimize ways to promote daily dosing.
In conclusion, a target TFV-DP concentration for MSM was identified. This threshold enables further studies in MSM that evaluate new ways of promoting the consistent use of PrEP, the key determinant of efficacy.
MATERIALS AND METHODS
iPrEx Trial
The design, conduct, and outcomes of the iPrEx trial have been published previously.(1) Briefly, the iPrEx initiative was a randomized, double-blinded, controlled trial of daily FTC-TDF versus placebo in HIV-negative men and transgender women 18 years or older who have sex with men, meeting behavioral criteria that put them at risk for sexual acquisition of HIV. Two thousand four hundred ninety nine participants were randomized and followed monthly through a median of 87 weeks of therapy (IQR 61 to 125). Eighty-three infections were observed in the placebo arm versus 48 infections in the FTC-TDF arm (efficacy, 42% (95% CI 18% to 60%).(21)
Plasma specimens were stored every 12 weeks and viably cryopreserved PBMC specimens were stored every 24 weeks and at the time of study discontinuation or seroconversion. Each participant from the active arm who contracted HIV during the study was included in this pharmacology substudy (cases). For each visit week when HIV was detected in cases, samples were selected from 3 HIV negative controls from the active arm at the same site; 2 randomly, and 1 from among those reporting unprotected receptive anal intercourse (URAI) in a recall period prior to the specimen collection. The latter control was chosen to enrich the control sample for people exposed to HIV, to better match the HIV infected cases. Specimens were tested from the time of first evidence of HIV infection in the cases, and the nearest visit week in controls, as well as longitudinally at other available time points during the treatment period.
STRAND Study
The STRAND study was an open label, randomized, 6 sequence, 3-period, single-site, cross-over trial in 24 HIV-negative adults (12 men, 12 women) that tested the effects of three different oral TDF dosing regimens on TFV concentrations in hair, plasma, and PBMC. The 6 sequences were different orders of the 3 dosing strategies: 7 doses/week, 4 doses/week, and 2 doses/week. The 2 doses/week were dosed on Tuesday and Wednesday and 4 doses/week on Monday, Tuesday, Thursday, and Friday. Each dosing period lasted 6 weeks. All doses scheduled Monday through Friday were directly observed by study staff and the doses on the weekend were confirmed by telephone or text message. Stored viably cryopreserved PBMC (using the same method as in iPrEx) were collected at the end of each 6 week dosing period. Freshly processed/lysed PBMC were available from a subset of participants. The study was funded by the US National Institutes of Health and was approved by the UCSF Committee on Human Research.
Analytical pharmacology
An LC/MS/MS assay was validated for the determination of TFV and FTC in human plasma. (22) The method includes protein precipitation and stable isotopic internal standards. The linearity of the concentration curves were in the range 10 ng/mL to 1500 ng/mL for both analytes (250 μL plasma extracted). The lower limit of quantification was 10 ng/mL for both analytes.
The viable PBMC processing procedure involved a quick thaw in a 37°C water bath and mixing by inversion (1 minute). Cells were immediately transferred to a 15 ml centrifuge tube that already contained 10 ml of pre-warmed (37°C) PBS followed by gentle mixing by inversion. Cells were then pelleted and the supernatant discarded. If RBC contamination was visible, the cells were treated with a RBC lysing buffer. Cells were then counted with an automated hemocytometer. Viability and total cell count were recorded; cells were kept on crushed ice through the process. Cells were washed and lysed. Median (IQR) viability at the time HIV was discovered was 66% (56% to 76%) overall, 71% (56% to 79%) in cases and 64% (56% to 73%) in controls.
A validated liquid chromatography tandem mass spectrometry (LC–MS/MS) assay was used for the determination of TFV-DP and FTC-TP from lysed intracellular matrix.(15) The method utilized a strong anion exchange isolation of mono-(MP), di-(DP), and tri-phosphates (TP) from intracellular matrix. The TP fraction was then dephosphorylated to the parent moiety yielding a molar equivalent to the original nucleotide analog intracellular concentration. The analytical portion included desalting/concentration by solid phase extraction and detection by LC–MS/MS. The quantifiable linear range for TFV-DP was 2.5–2000 fmol/sample, and that for FTC was 0.1–200 pmol/sample. Stable labeled isotopic internal standards facilitated accuracy and precision in various cell matrices. (15) Two million total cells were typically extracted, constituting the “sample”, and results were corrected for cell viability and normalized to fmol or pmol per million (M) viable cells.
Stored viable PBMC samples such as in this study have been used for the measurement of intracellular TFV-DP in previous studies (see appendix in reference 1).(1) These studies demonstrated that values in viably cryopreserved PBMC (median approximately 40 fmol/M viable cells) were lower than those measured in pharmacology studies among HIV-infected subjects where PBMC samples were processed and lysed immediately (median approximately 90 fmol/M cells).(23, 24)
To evaluate viable cells in more detail, an internal study called iOptimum enrolled 10 HIV-infected participants for a single blood draw to assess the TFV-DP and FTC-TP in viable cryopreserved cells. Self-reported adherence in the prior 30 days was 63 to 100% and viral loads were largely suppressed (range, <40 to 240 cp/mL), suggesting good adherence to treatment. Aliquots of viable cells were stored in liquid nitrogen for 4, 12 and 24 weeks. Freshly lysed cells were also collected. Both TFV-DP and FTC-TP were quantifiable in all viable PBMC samples, and above the lower limit of quantitation by several fold. The median (IQR) ratio of viable PBMC/PBMC at weeks 4 and 12 in liquid nitrogen storage were 0.3 (0.22 to 0.38). The median (IQR) value for 24 weeks in liquid nitrogen was 0.56 (0.38 to 0.83), which was similar to STRAND 0.48 (0.38 to 0.67). Median (IQR) time in liquid nitrogen for iPrEx and STRAND samples were 70 (46 to 108) weeks and 57 (52 to 65) weeks, respectively. We have observed no loss in viable cell concentrations for storage times in liquid nitrogen through 119 weeks (2.3 years).(1)
Statistical Methods
All analyses were performed using Stata 12.1.(25)
Model for the Effect of Intracellular Drug Concentration
This analysis fits a (stratified by site) Cox proportional hazards model to the entire cohort with a model
| (1) |
hk(t) is the hazard of HIV infection at the kth site and rx = 1 if the participant is assigned to FTC/TDF and 0 if the participant is assigned to placebo. The variable Z(t) is a time-dependent covariate for a quantitative drug concentration (e.g., TFV-DP) where Z(t) is set to be a drug concentration of 0 if the participant is on the placebo group. We examined quadratic and logarithmic transformations of the concentration Z(t) but neither improved the fit.
The primary analyses have used models stratified by clinic site to avoid confounding the association between drug concentrations and HIV acquisition. Stratification was incorporated into the design of the nested case control study by matching controls by study site. Stratification can be highly efficient relative to an unstratified model even if confounding by site is weak.(26)
The model (1) uses the placebo group as the baseline hazard function permitting comparison the hazard of HIV acquisition at a given drug concentration to the placebo group. For instance, the concentration Z(t) which is associated with a d% reduction is risk relative to placebo is
The concentration associated with a d% reduction in risk relative to a 0/BLQ concentration on the FTC/TDF arm is
Here, β1, is the log hazard ratio of a 0/BLQ level on the FTC/TDF arm (relative to placebo), β2 quantifies the change in risk with the concentrations of drug and h0k(t) is the baseline hazard of HIV seroconversion on the placebo arm.
We also fit
| (2) |
where this exponential model is parameterized to yield the annualized incidence of HIV. The fit of this model to the data yielded similar results to the fit of model (1) and was used as the basis of the annualized HIV incidence graphed in Figure 3.
Note that the models (1) and (2) require a complete set of drug concentrations for participants in the active arm. We must confront two type of missing data. First, only a randomly selected subset of time points had drug concentrations tested. Second, intracellular drug (TFV-DP, FTC-TP) was only tested every 6 months and at the time of HIV seroconversion. Hence, even if a control has drug concentration tested, the information is sparse and may not correspond to an ideal time match to their HIV case.
We used two strategies for fitting model (1) with missing data in the drug concentrations: multiple imputation and conditional logistic regression. Conditional logistic regression functions only as a sensitivity analysis since it is unable to use the placebo group as a reference and does not handle the uncertainty in drug concentrations in the controls due to the long periods of time between measurement of drug concentrations.
Multiple Imputation
The concentration of drug for the HIV-infected cases at the time of HIV infection was taken as the intracellular concentration observed at the first laboratory evidence of HIV infection. Six participants lacked an intracellular specimen at the first laboratory evidence of HIV infection -- all had a plasma specimen at the first evidence of infection and all were BLQ (below the limit of quantitation) for TFV and FTC. In the case control study of 355 plasma specimens which are BLQ for TFV and FTC, 96% were BLQ for TFV-DP and 95% were BLQ for FTC-TP. Hence, the TFV-DP and FTC-TP for these 6 seroconverters were set as BLQ.
For controls, we attempted to infer the drug concentration at the identical day of follow up as seroconverters from the same site. We used the closest intracellular drug concentration within 45 days as the drug concentration if one was available and considered that as a measured drug concentration. If there was no tested drug concentration within 45 days, then drug concentrations were imputed. Varying the 45 day window had little effect on the results.
If the visit had plasma but not intracellular concentrations (vPBMC), we used whether the plasma had detectable or not in our imputation model. We performed multiple imputations (9) of TFV-DP, FTC-TP and any detection of drug in plasma or PBMC using medication possession ratio (defined as the number of tablets dispensed at the prior visit divided by days since the last visit at which medication was dispensed), study week, unprotected receptive anal intercourse (URAI) at baseline, URAI at follow-up (most recent report on or after the time point), baseline herpes simplex virus (HSV) status, number of male sexual partners at screening, participant report of a sexual transmitted infection in the six months prior to enrollment, secondary education, age at enrollment (in years) and baseline (estimated) creatinine clearance (by Cockcroft-Gault equation). Plasma and PBMC concentrations have monotone missingness hence chained imputation was used to create joint imputations. The imputation model for detection of drug in plasma was based on logistic regression and imputations for TFV-DP and FTC-TP were based on predictive means matching.(8) Predictive means matching used a regression model to identify observed values in the data which form the most plausible value for the imputations, yielding non-parametric imputations that must follow the observed distribution of the drug concentrations. This method seamlessly imputed concentrations of TFV-DP and FTC-TP that were below the limit of quantitation. This permitted us to form imputations in a first stage of analysis and to vary the strategy for quantifying BLQ values in a second stage. We performed 20 imputations per observation.
Conditional Logistic Regression
Analyses using conditional logistic regression mimics a (stratified) Cox proportional hazards model among participants on the active arm of the form
| (3) |
where k is the clinic site and Z(t) is a time-dependent covariate for drug concentration. Prentice and Breslow demonstrated that by measuring covariates in cases and a sample of (time matched) controls, it was possible to fit (3) using a conditional logistic regression model. (27) Hence, the coefficient in the conditional logistic regression is a valid estimator of the log hazard ratio β3 which is the risk reduction compared to a drug concentration of 0 on the active arm. As a sensitivity analysis, we compared the results of our estimated protective concentrations by calculating the intracellular drug concentrations associated with a d% reduction in risk relative to a 0/BLQ concentration on the FTC/TDF arm as
Inferred Protection
Based on estimates for model 1 fit using multiple imputation, we estimated the protective effect of dosing regimens used in STRAND. For this, we combined the parameter estimates from model (1) with the observed TFV-DP from the 2, 4, and 7 tablets per week regimens in STRAND. The estimated relative risk reduction was approximated by
where (Z1D,,........., ZnDD) were the nD observed TFV-DP concentrations for D tablet per week regimen and the parameter estimates were taken from fitting model 1 using multiple imputation with associated 0.95 level Wald-based confidence intervals.
Acknowledgments
We wish to thank the study participants and personnel/staff involved with the clinical trials.
Funding: NIH R21MH085598 (A.L), UO1 AI084735 (P.L.A.), RO1 AI062333 (R.M.G), and UO1 AI064002 (R.M.G), UL1 RR024131.
Footnotes
Author contributions: PL Anderson helped design the pharmacology studies, oversee the analytical pharmacology work, and analyze the data. DV Glidden helped design the analyses and perform the statistical modeling. A Liu designed and conducted STRAND and assisted with the design and conduct of iPrEx. S. Buchbinder, J Lama, JV Guanira, O Montoya-Herrera, VG Veloso, S Chariyalertsak, K Mayer, LG Bekker, EG Kallás assisted with the design and conduct of iPrEx. LR Bushman oversaw the analytical pharmacology work. V McMahan coordinated the iPrEx study. RM Grant designed and conducted iPrEx, helped design the pharmacology studies, and analyze the data. All authors assisted with writing the manuscript.
Competing interests: Study drugs were donated by Gilead Sciences.
References
- 1.Grant RM, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. The New England journal of medicine. 2010 Dec 30;363:2587. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS. 2010 [Google Scholar]
- 3.Baral S, Sifakis F, Cleghorn F, Beyrer C. Elevated risk for HIV infection among men who have sex with men in low- and middle-income countries 2000–2006: a systematic review. PLoS Med. 2007 Dec;4:e339. doi: 10.1371/journal.pmed.0040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnell D, et al. paper presented at the 19th Conference on Retroviruses and Opportunistic Infections; Seattle, WA, USA. March 5–8 2012. [Google Scholar]
- 5.Karim SS, Kashuba AD, Werner L, Karim QA. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: implications for HIV prevention in women. Lancet. 2011 Jul 16;378:279. doi: 10.1016/S0140-6736(11)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thigpen MC, et al. 6th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Rome, IT. 2011. [Google Scholar]
- 7.Van Damme L, et al. paper presented at the 19th Conference on Retroviruses and Opportunistic Infections; Seattle, WA, USA. March 5–8 2012. [Google Scholar]
- 8.Little RJA. Missing-Data Adjustments in Large Surveys. Journal of Business & Economic Statistics. 1988;6:287. [Google Scholar]
- 9.Schafer JL. Multiple imputation: a primer. Statistical Methods in Medical Research. 1999 Feb 1;8:3. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 10.Kashuba AD, Patterson KB, Dumond JB, Cohen MS. Pre-exposure prophylaxis for HIV prevention: how to predict success. Lancet. 2011 Dec 6; doi: 10.1016/S0140-6736(11)61852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson PL, et al. 19th Conference on Retroviruses and Opportunistic Infection; Seattle, WA. 2012. [Google Scholar]
- 12.Patterson KB, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011 Dec 7;3:112re4. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anton P, et al. 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA, USA. 2011. [PubMed] [Google Scholar]
- 14.Keller MJ, et al. A randomized trial to assess anti-HIV activity in female genital tract secretions and soluble mucosal immunity following application of 1% tenofovir gel. PLoS ONE. 2011;6:e16475. doi: 10.1371/journal.pone.0016475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bushman LR, et al. Determination of nucleoside analog mono-, di-, and tri-phosphates in cellular matrix by solid phase extraction and ultra-sensitive LC-MS/MS detection. Journal of pharmaceutical and biomedical analysis. 2011 Sep 10;56:390. doi: 10.1016/j.jpba.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castillo-Mancilla J, et al. paper presented at the 19th Conference on Retroviruses and Opportunistic Infections; Seattle, WA. March 5–8 2012. [Google Scholar]
- 17.Liu A, et al. 17th Conference on Reteroviruses and Opportunistic Infections; San Francisco. 2010. [Google Scholar]
- 18.Borroto-Esoda K, Vela JE, Myrick F, Ray AS, Miller MD. In vitro evaluation of the anti-HIV activity and metabolic interactions of tenofovir and emtricitabine. Antivir Ther. 2006;11:377. [PubMed] [Google Scholar]
- 19.Anderson PL, et al. paper presented at the 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA. Feb 27–Mar 2 2011. [Google Scholar]
- 20.García-Lerma JG, et al. Intermittent prophylaxis with oral truvada protects macaques from rectal SHIV infection. Sci Transl Med. 2010 Jan 13;2:14ra4. doi: 10.1126/scitranslmed.3000391. [DOI] [PubMed] [Google Scholar]
- 21.Grant R, et al. 6th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Rome, IT. 2011. [Google Scholar]
- 22.Delahunty T, Bushman L, Robbins B, Fletcher CV. The simultaneous assay of tenofovir and emtricitabine in plasma using LC/MS/MS and isotopically labeled internal standards. Journal of Chromatography B. 2009;877:1907. doi: 10.1016/j.jchromb.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baheti G, Kiser JJ, Havens PL, Fletcher CV. Plasma and intracellular population pharmacokinetic analysis of tenofovir in HIV-1-infected patients. Antimicrob Agents Chemother. 2011 Nov;55:5294. doi: 10.1128/AAC.05317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiser JJ, et al. Pharmacokinetics of antiretroviral regimens containing tenofovir disoproxil fumarate and atazanavir-ritonavir in adolescents and young adults with human immunodeficiency virus infection. Antimicrob Agents Chemother. 2008 Feb;52:631. doi: 10.1128/AAC.00761-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.StataCorp. StataCorp LP, College Station, TX. 2011. [Google Scholar]
- 26.Glidden DV, Vittinghoff E. Modelling clustered survival data from multicentre clinical trials. Statistics in Medicine. 2004;23:369. doi: 10.1002/sim.1599. [DOI] [PubMed] [Google Scholar]
- 27.PRENTICE RL, BRESLOW NE. Retrospective studies and failure time models. Biometrika. 1978 Apr 1;65:153. [Google Scholar]