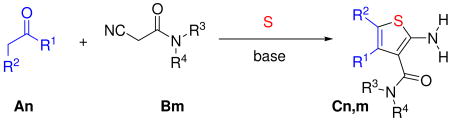
Abstract
Cyanoacetic acid derivatives are the starting materials for a plethora of multicomponent reaction (MCR) scaffolds. Here we describe valuable general protocols for the synthesis of arrays of 2-aminothiophene-3-carboxamides from cyanoacetamides, aldehydes or ketones and sulfur via a Gewald-3CR variation. In many cases the reactions involve a very convenient work up by simple precipitation in water and filtration. >40 New products are described. We foresee our protocol and the resulting derivatives to become very valuable to greatly expanding the MCR scaffold space of cyanoacetamide derivatives.
In 1966 the German chemist Prof. Karl Gewald discovered that methylene-active carbonyl compounds reacted with methylene-active nitriles and sulfur at ambient temperature to yield 2-aminothiophenes.1 This reaction constitutes a very versatile and useful multicomponent reaction (MCR).2,3,4 As often found with MCRs the reaction conditions are simple and do not foresee protection from atmosphere or moisture and are easily amenable to up-scaling (Fig. 1).
Figure 1.

Simple to perform large scale open vessel Gewald-3CR (courtesy of Prof. Ulrich Jordis, Vienna).
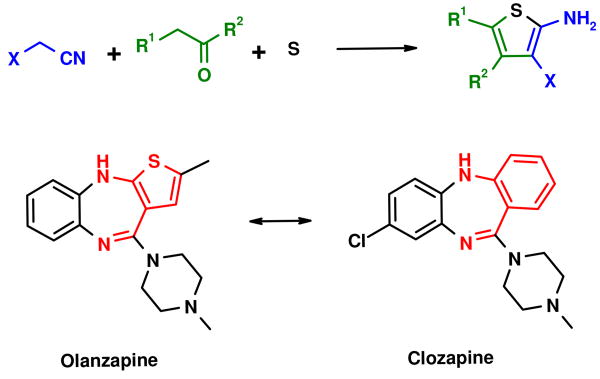
In addition this transformation is remarkable, since it consists one of the few organic reactions incorporating elemental sulfur at ambient temperature. Other examples are the Asinger-3CR and the Wilgeroth Kindler reaction.5,6 The importance of the Gewald reaction steams from the formation of 2-aminothiophene which is abundantly used in pharmaceutical industry to produce bioactive compounds.3,4 The 2-amino-3-carbonyl thiophene is bioisostere to the anthranilic acid which per se is an important bioactive scaffold.7 As opposed to anthranilic acid derivatives however, which are difficult to access and only a few are commercially available, the Gewald-3CR allows virtually infinite access to substituted bioisostere 2-amino-3-carboxythiophenes. A prominent example of the thiophene-phenol bioisosterie is the atypical antipsychotic blockbuster drug olanzepine for the treatment of schizophrenia and bipolar disorder (Scheme 1).8 The pharmacokinetic/dynamic properties of olanzapine are presumably even enhanced over the bioisosteric clozapine.
Scheme 1.
Gewald-3CR and phenol-thiophene based biosisosterie between the drugs olanzapine and clozapine.
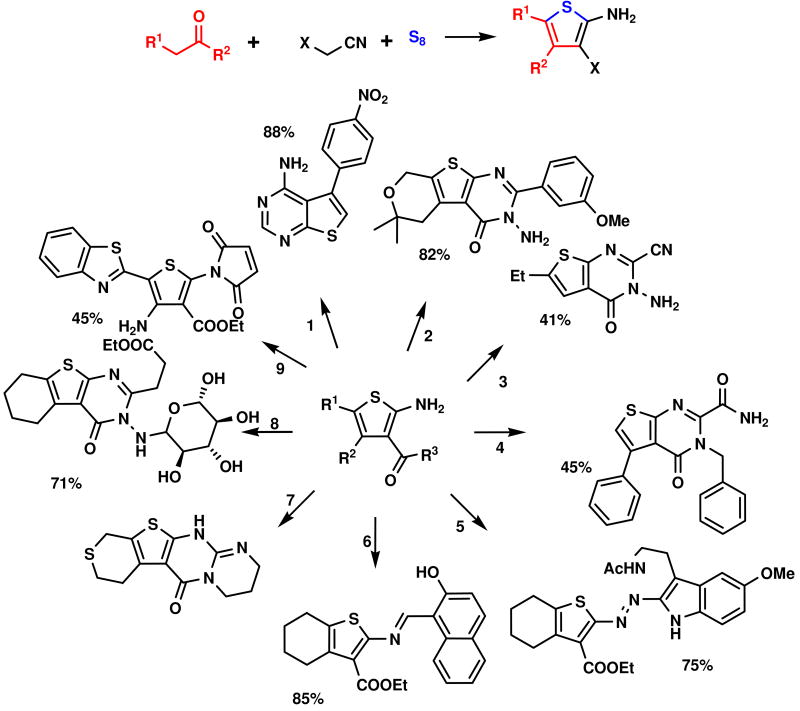
In addition Gewald-3CR primary products are the starting materials for many other compound classes such as kinase inhibitor thienopyrimidines 1,9 thienoquinazolines 2,10 pyrimidines 3 and 4, 11 diazocompounds 5, 12 Schiff bases 6, 13 tetracyclic thienopyrimidinones 7,14 and N-thieno-maleinicacid amides 915 as shown in Scheme 2, just to name a few.
Scheme 2.
A diversity of secondary reactions based on the initial Gewald-MCR.
The scope of the Gewald-3CR, however, in the past was hampered by the almost exclusive use of structurally simple not variable activated nitrile components, e.g. malonodinitrile, cyanoacetophenones, 16 cyanoacetic acid esters and primary cyanoacetamides. Rarely, cyanoacetamides have been used as potentially versatile component in the Gewald-3CR. In cases where cyanoacetamides have been used they were based on aromatic amides or hydrazides.17 In fact only very few references could be found using aliphatic amine derived cyanoacetamides in the Gewald-3CR.18 Thus the Gewald-3CR can be described as a MCR with rather low dimensionality similar to the Hantzsch dihydropyrimidine and the Biginelli reaction, where the main variable component is the oxo component.19 Examples of high dimensional MCRs are isocyanide-based MCRs, e.g. Ugi, Passerini and van Leusen reactions, were all of the components can be broadly varied.20 To solve the problem of low dimensionality we describe herein a major extension of current Gewald-3CR by introducing aliphatic cyanoacetamides as generally useful components and thus rendering the Gewald MCR with effectively only one diversity point in the past into a MCR comprising two highly variable inputs.
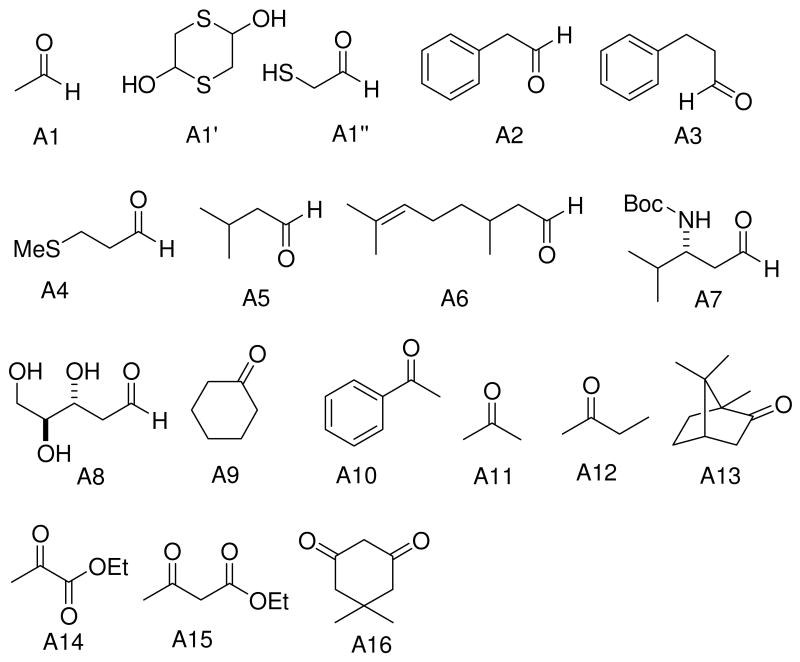
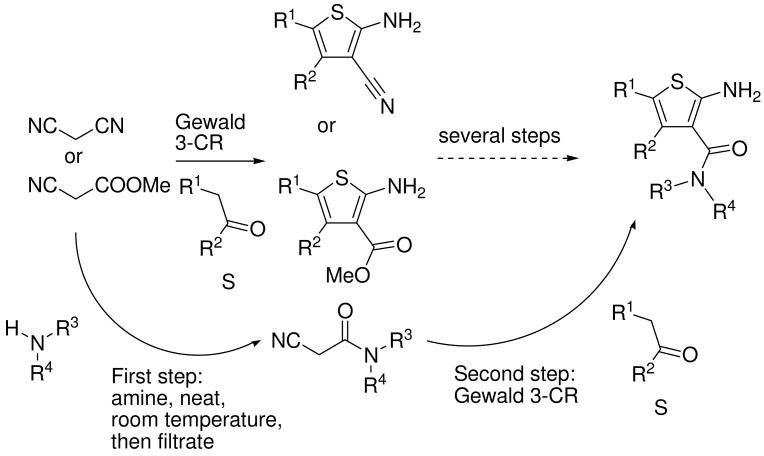
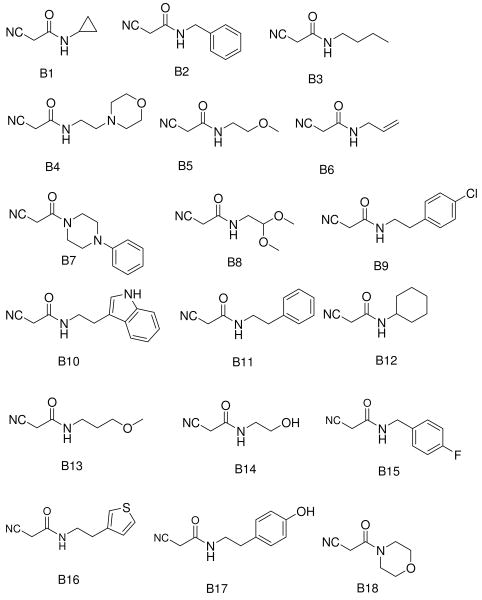
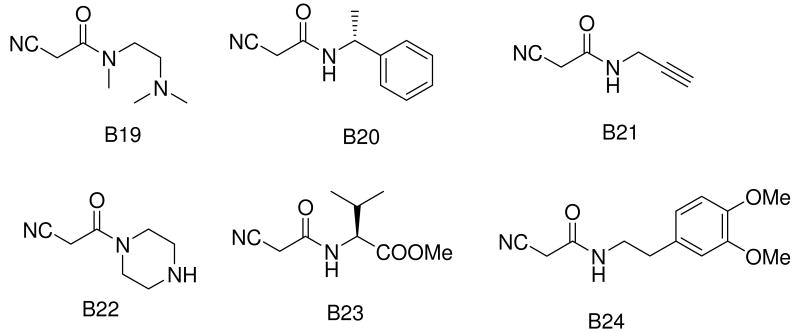
Recently, we communicated a versatile and experimentally very simple access to arrays of cyanoacetamides by solventless mixing methyl cyanoacetate and primary and secondary amines and filtration of the formed products.21 Having easy access to such large arrays we investigated the versatile MCR chemistry of this compound class.22 We intended to investigate the Gewald-3CR of cyanoacetamides and we herein report our results. First we reacted several cyanoacetamides with methylene active oxo components and sulfur in ethanol at 70°C for several hours. Upon cooling to room temperature no product precipitated and TLC and HPLC MS analysis indicated several reaction products and even some left starting materials. We then experimented with addition of water and temperature, and indeed by purring the reaction mixture into water and cooling to 0 °C we notified after some time the formation of significant precipitate. Filtration of the precipitate and NMR and HPLC analysis of it reveals Gewald products with purity of >95% and good yields. In order to elaborate a general procedure we investigated several more reactions with different functional groups in the side chains (hydrophilic and hydrophobic, basic) and to our delight in all investigated cases we noticed the formation of considerable product precipitation. Again after filtration and drying we notified exceptional high purity in all cases. Next we were synthesizing an array of >40 Gewald compounds in parallel by the above procedure. Over a wide range of functional groups and substitutents we isolated the products in moderate to good to high yields but always in excellent purity. We used 16 different ketones and aldehydes (A1-A16, Fig. 2). To figure scope and limitations of the cyanoacetamide component we used 23 differentially substituted starting materials (B1-B24, Fig. 2).
Figure 2.
Aldehyde and ketone starting materials.
The purification of products differs depending on the solubility properties of the products. For some reactions, no solid could be filtered after water addition; for few compounds, purities were less than 90% by HPLC-MS and NMR analysis, these crude products were purified by silica gel column chromatography. The products and their yield are shown in table 1.
Table 1.
Gewald reaction to prepare 2-amino-thiophene-carboxamides (Cn,m).
| |||||
|---|---|---|---|---|---|
| yield | Structure | yield | Structure | yield | Structure |
| 45 % |
 C1,1 C1,1
|
56 % |
 C1,2 C1,2
|
60 % |
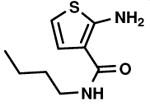 C1,3 C1,3
|
| 62 % |
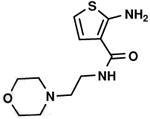 C1,4 C1,4
|
41 % |
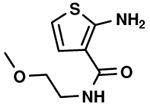 C1,5 C1,5
|
38 % |
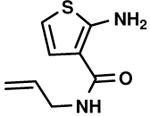 C1,6 C1,6
|
| 70 % |
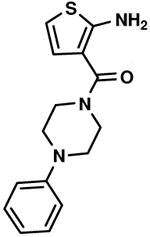 C1,7 C1,7
|
65 % |
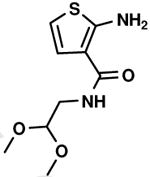 C1,8 C1,8
|
70 % |
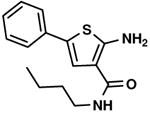 C2,3 C2,3
|
| 78 % |
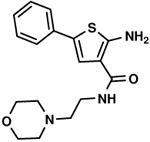 C2,4 C2,4
|
75 % |
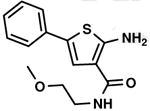 C2,5 C2,5
|
82 % |
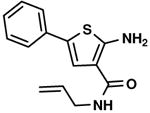 C2,6 C2,6
|
| 90 % |
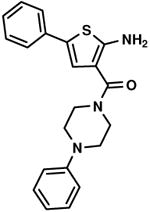 C2,7 C2,7
|
72 % |
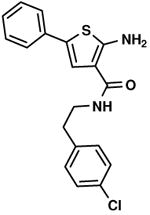 C2,9 C2,9
|
85 % |
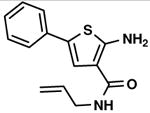 C2,10 C2,10
|
| 77 % |
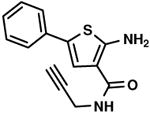 C2,21 C2,21
|
52 % |
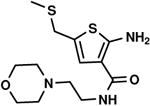 C4,4 C4,4
|
60 % |
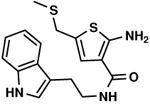 C4,10 C4,10
|
| 64 % |
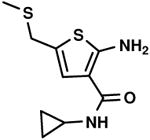 C4,1 C4,1
|
75 % |
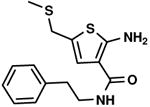 C4,11 C4,11
|
70 % |
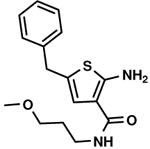 C3,13 C3,13
|
| 95 % |
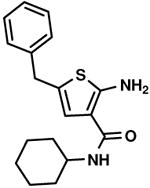 C3,12 C3,12
|
75 % |
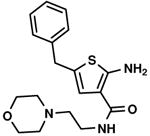 C3,4 C3,4
|
74 % |
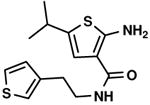 C5,16 C5,16
|
| 67 % |
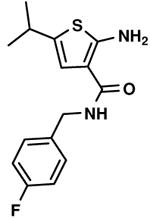 C5,15 C5,15
|
90 % |
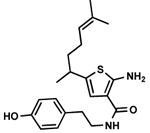 C6,17 C6,17
|
50 % |
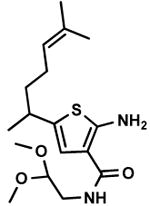 C6,8 C6,8
|
| 75 % |
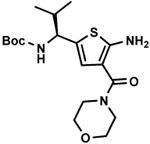 C7,18 C7,18
|
84 % |
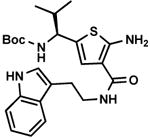 C7,10 C7,10
|
86 % |
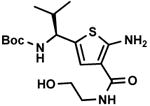 C7,14 C7,14
|
| 90 % |
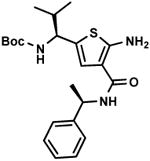 C7,20 C7,20
|
90 % |
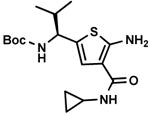 C7,1 C7,1
|
71 % |
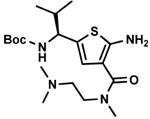 C7,19 C7,19
|
| 17 % |
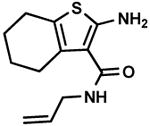 C9,6 C9,6
|
19 % |
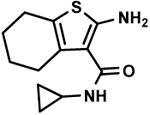 C9,1 C9,1
|
20 % |
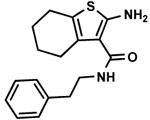 C9,11 C9,11
|
| 9 % |
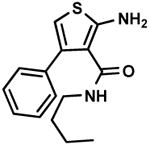 C10,3 C10,3
|
65 % |
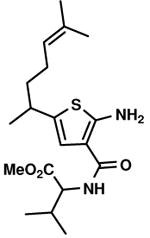 C6,23 C6,23
|
78 % |
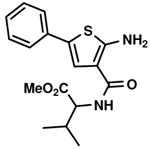 C2,23 C2,23
|
| 84 % |
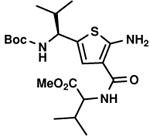 C7,23 C7,23
|
24 % |
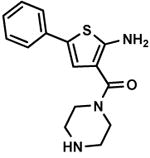 C2,22 C2,22
|
93 % |
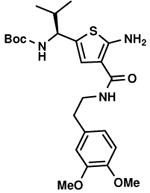 C7,24 C7,24
|
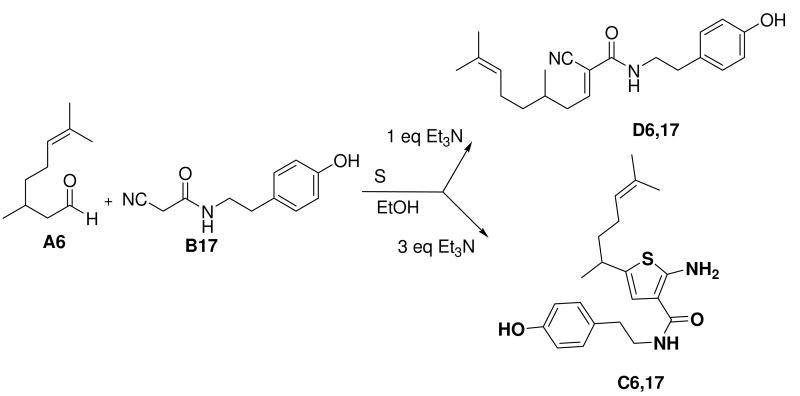
Some additional observations were made. For the phenolic starting material B17 the Gewald reaction with one equivalent of triethylamine base produced no target compound, but the condensation product (D6,17). Addition of two more equivalents of triethylamine, however produced the product (C6,17) in 82% yield (Scheme 3). The basic condition is critical for the cyclization step.
Scheme 3.
Different products depending on added base amounts.
Acetaldehyde (A1), which owns a low boiling point, is not a good starting materials for the Gewald 3-CR. However, the commercially available compound, 1,4-dithiane-2,5-diol (A1′), which is the dimmer of precondensation product 2-mercapto acetaldehyde (A1″) of acetaldehyde and sulfur, is a good substitute for acetaldehyde in the Gewald reaction.23
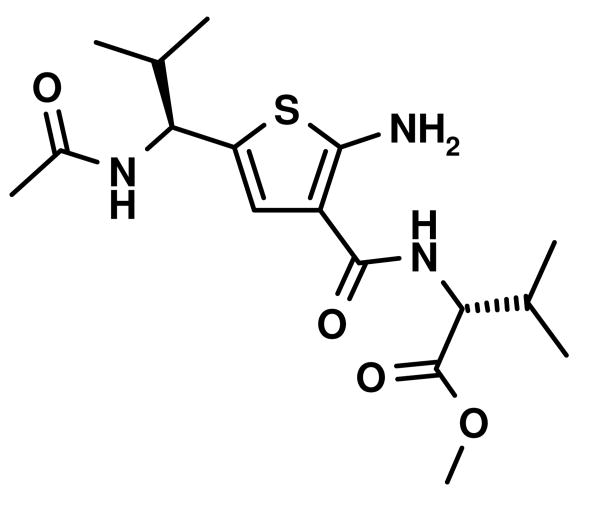
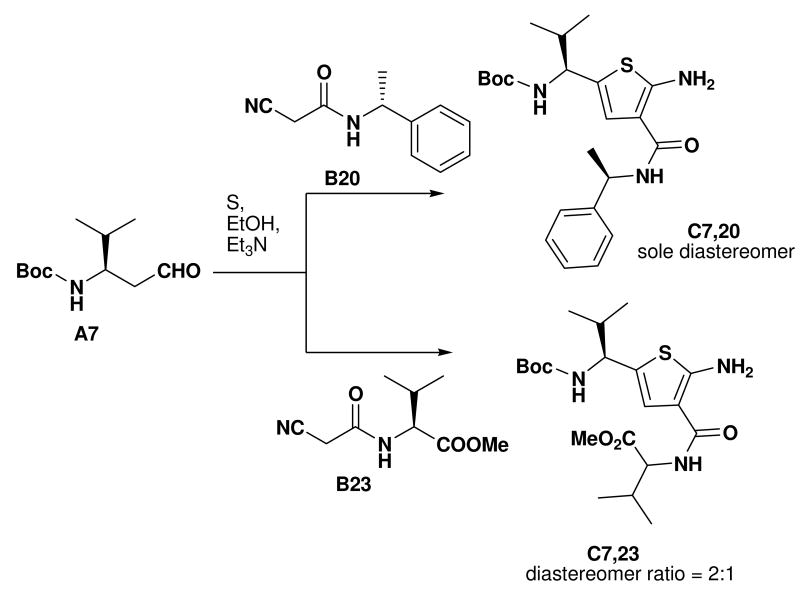
Other aldehyde with α-methylene moiety (A2-A7) also have good Gewald reactivity. The reaction is performing well by simply mixing each 1 equivalent of cyanoacetamides, sulfur, aldehyde or ketones and triethylamine as the ratio of 1: 1: 1: 1 in ethanol solvent and generally good yields are obtained. A Boc protected chiral β-amino aldehyde (A7) was treated into this reaction with different cyanoacetamides to produce corresponding products (C7-1,10,14,18,19,20,23) with good yield (70-90 %). It is interesting that two enantiomerically pure starting materials (A7 and B20) produce the thiophene C7,20 with not even traces of another diastereomer observed (Scheme 4). There is no significant racemization observed despite the fact that the reaction is base promoted. But in the reaction with another cyanoacetamide B23, which is derived from the methyl ester of valine amino acid, the corresponding product C7,23 contains two diastereomers at a ratio 2: 1; the result indicates that strong epimerization happens at the valine moiety under basic conditions. 2-Deoxy-D-ribose (A8), which contains three free hydroxyl groups, doesn't produce the target product under the conditions used here.
Fig. 4.
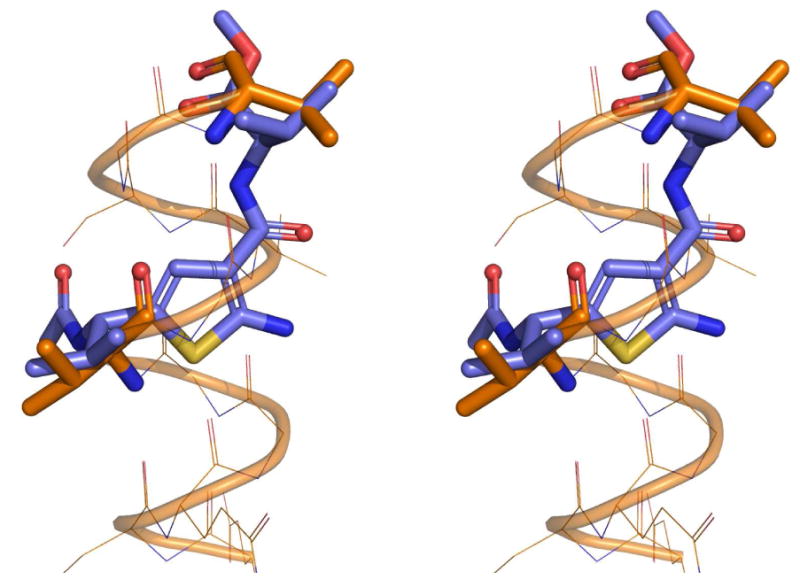
Stereoview of an overlap of an amphiphatic α-helix with two valine in i and i+6 positions (orange sticks) with a Gewald thiophene-derived dipeptide mimetic (blue sticks). The small molecule derived from aldehyde A7, cyanoacetamide B23, sulfur was deprotected and N-acylated and then energy minimized using MOLOC software.28 An important feature of the Gewald-thiophene derivatives of cyanoacetamides is the intramolecular hydrogen bonding of the 2-amino group with the 3-amide carbonyl, reducing the conformational freedom of the amide group considerably. In support of the above hypothetical structure, the intramolecular hydrogen bridge can be seen in most of the published x-ray structure analysis.4
The Gewald reaction can be applied in the synthesis of thiophenediazepine.24 For example the cyanoacetamides B23 could be prepared simply from methyl ester of (S)-valine chloride salt with methyl cyanoacetate in the presence of 2 equivalents of triethylamine without additional solvent. After Gewald reaction with aldehyde and sulfur, the desired thiophenes (C2,23, C6,23 and C7,23) are generated in good yield. These intermediates would provide a different way to thiophenediazepine-2,5-diones (E, Scheme 5). The detailed cyclization conditions are in progress in our lab and will be reported in the future.
Scheme 5.
Amidation-Gewald-cyclization sequence to thiophenediazepine.
Ketone starting materials produced far poorer result compared to aldehyde staring materials. Cyclohexanone (A9) only produced less than 20 % yield with three cyanoacetamides (B1, B6 and B11) under the conditions used. Corresponding reaction with methyl cyanoacetate had obtained much higher yield. Acetophenone (A10), produced target product C10,3 in less than 10 % yield and it is difficult to purify the product. Other ketones, including acetone (A11), 2-butone (A12), (R)-camphor (A13), dimedone (A16), ethyl acyacetate (A14), ethyl pyruvate (A15) do not yield the Gewald products at all under the herein described reaction conditions; despite starting materials was observed after the reactions were stopped. In many references therefore the use of precondensed Knoevenagel intermediates is described.25
Discussion
We herein describe a convenient way to synthesize arrays of Gewald thiophene-3-amides. The convenience of this procedure is based on two points: first the reaction can be performed in many cases in a way that the Gewald products precipitate and this leads to high quality products without the need to run time and cost intensive purifications. Second the starting material class of cyanoacetamides was recently described by us to be accessible in a very convenient way by simply mixing methyl cyanoacetate with an appropriate primary or secondary amine, thus leading to potentially large arrays of starting materials for subsequent cyanoacetamide dependant MCRs. This extension of the Gewald-3CR is significant since in the past mostly simple and non variable malondinitrile, cyanoacetic acid and their esters or hydrazides where used. Thus Gewald reaction variability in the past was mostly confined to the variation of the α -methylene oxo component and the secondary reactions of their primary Gewald products. The herein described and generally shown reaction of sulfur, and cyanoacetamides, however renders the Gewald-3CR a truly variable MCR with two points of diversity.
There are two routes to prepare 2-aminothiophene-3-carboxamides (Scheme 6). The first route uses the classical Gewald products 2-amino-3-cyanothiophene or 2-aminothiophene-3-carboxylic methyl esters resulting from malonodinitrile or methyl cyanoacetate, and further transformations after several additional steps lead to 2-aminothiophene-3-carboxamides. Thus amide-substituted Gewald products have been described from the corresponding esters by the sequence saponification, activation and coupling or other methods however in poor yields and lengthy sequences.26 This route involves more steps and is less efficient, the yields-although potentially higher per step-at the end are always lower after several reaction steps. Additionally these reactions often involve harsh conditions. 27 The second herein described route starts from cyanoacetamides, which are easily and conveniently prepared from methyl cyanoacetate in a single step and on a gram scale if needed. In a one-step three-component Gewald reaction, 2-aminothiophene-3-carboxamides can be produced in high diversity. The advantage of the second route seems obvious.
Scheme 6.
Different route to 2-aminothiophene-3-carboxamides.
The scope of this reaction is large (Table 1). Different cyanoacetamides work fine in the Gewald-3CR and many function groups were tolerated, including alkenyl (B6), alkynyl (B21), acetal (B8), alcohol hydroxyl (B14), phenol (B17), indole (B10), ester (B23), secondary amino (B22) and tertiary amino (B4, B7 and B19). Regarding the α-methylene oxo component we observed that aldehydes are superior substrates to ketones and generally react faster, more complete and giving higher yields. The more hydrophobic the resulting Gewald product the greater the isolated yield. This is a direct consequence of the work-up procedure by aqueous precipitation. Amino acid side chains have been introduced at several positions of the Gewald scaffold thus rendering this chemistry potentially useful for the synthesis of peptidomimetics (C4,m methionine; C3,m and Cn,2 phenylalanine; C5,m and Cn,23 valine; Cn,10 tryptophan). Compound C7,23, for example can be considered as a peptidomimetic containing the two valine amino acids encompassed on a stiff Gewald heterocycles. To show the potential to act as peptidomimetic we computationally optimized the 3D-structure and overlapped it with an α -helix. As can be seen in Figure 4 there is a good overlap implying that such molecule might act as α -helix mimetic. The α -helix mimetics recently have become very important lead structures to (ant-)agonize protein protein interactions, e.g. in p53/Hdm2, p53/Hdm4 or Bcl2 family members.
Combinatorial chemistry is an important discipline in organic chemistry and highly useful to pharmaceutical industry since the majority of drug discovery projects currently relies on high throughput screening of large compound collection. Gewald reactions have been described to be purification intensive in the past. Improvements to overcome this issue and to increase synthesis speed have been solid phase bound synthesis.29 However these methods have attached issues, such as low compound generation and the use of often special linker systems which compromise the chemistry. Other generally used methods include mass triggered automatic preparative HPLC or the faster and more efficient SC-CO2-HPLC. The use of automatic HPLC purification systems however is expensive and needs special equipment. The best organic chemistry work-up procedures can avoid chromatography and can purify the products by extraction and/or crystallization. These procedures are of particular value in the context of parallel synthesis since herein all efforts are multiplied. Therefore we believe that our described often chromatography free Gewald-3CR procedure based on simple precipitation and filtration is advancement in Gewald chemistry. Equally important is the general use of cyanoacetic acid amides as highly variable class of compounds in the Gewald-3CR which renders this reaction a true MCR with two highly variable inputs instead of mostly one in the past. Thus a skilled lab worker using very simple equipment can synthesize hundreds of Gewald products in a short time frame on a multi mg scale with very high purity including the synthesis of the required cyanoacetamide building blocks.20 In summary the herein described modified Gewald-3CR procedure allows for the robust, fast, resource saving and efficient construction of large arrays of highly substituted 3-amido-2-aminothiophenes.
Supplementary Material
Figure 3.
Cyanoacetoamide starting materials.
Scheme 4.
Gewald-3CR of chiral starting materials.
Acknowledgments
We thank Prof. Ulrich Jordis (Technical University Vienna, Austria) for Figure 1 of a large scale Gewald reaction (3.5 liter volume, 1 mol). This research has been partially supported by grant GM087617 from the National Institute of Health.
Footnotes
Supporting information available: Proton and carbon NMR, HR MS characterization and experimental procedures.
References
- 1.Gewald K, Schinke E, Bottcher H. Chem Ber. 1966;99:94–100. [Google Scholar]
- 2.Mayer R, Gewald K. Angew Chem Inter Ed. 1967;6:294–306. [Google Scholar]
- 3.Sabnis RW, Rangnekar DW, Sonawane ND. J Heterocyclic Chem. 1999;36:333–345. [Google Scholar]
- 4.Huang Y, Doemling A. Mol Div. 2009 in press. [Google Scholar]
- 5.(a) Asinger F. Angew Chem. 1956;68:413–413. [Google Scholar]; (b) Asinger F, Thiel M, Pallas E. Liebigs Ann Chem. 1957;602:37–49. [Google Scholar]; (c) Asinger F, Offermanns H. Angew Chem. 1967;79:953–956. [Google Scholar]; (d) Asinger F, Leuchtenberg W, Offermanns H. Chem Zeitung. 1974;98:610–615. [Google Scholar]; (e) Asinger F, Gluzek KH. Monats Chem. 1983;114:47–63. [Google Scholar]
- 6.(a) Willgerodt C. Ber Dtsch Chem Ges. 1887;20:2467–2469. [Google Scholar]; (b) Willgerodt C, Merck FH. J Prakt Chem. 1909;80:192–195. [Google Scholar]; (c) Kindler K. Liebigs Ann Chem. 1923;431:193, 222–224. [Google Scholar]
- 7.Wiklund P, Bergman J. Current Organic Synthesis. 2006;3:379–402. [Google Scholar]
- 8.Meltzer HY, Fibiger HC. Neuropsychopharmacology. 1996;14:83–85. doi: 10.1016/0893-133X(95)00197-L. [DOI] [PubMed] [Google Scholar]
- 9.Barnes DM, Haight AR, Hameury T, McLaughlin MA, Mei JZ, Tedrow JS, Toma JDR. Tetrahedron. 2006;62:11311–11319. [Google Scholar]
- 10.Mkrtchyan AP, Noravyan AS, Petrosyan VM. Chemistry of Heterocyclic Compounds. 2002;38:238–241. [Google Scholar]
- 11.Zavarzin IV, Smirnova NG, Chernoburova EI, Yarovenko VN, Krayushkin MM. Russian Chemical Bulletin. 2004;53:1257–1260. [Google Scholar]
- 12.Doss SH, Mohareb RM, Elmegeed GA, Luoca NA. Pharmazie. 2003;58:607–613. doi: 10.1002/chin.200350216. [DOI] [PubMed] [Google Scholar]
- 13.Roman G, Andrei M. Glasnikna Hemicaritei Tehnolozitena Makedonija. 2001;20:131–136. [Google Scholar]
- 14.Frohlich J, Shaifullah Chowdhury AZM, Hametner C. ARKIVOC. 2001:163–172. [Google Scholar]
- 15.Elkholy YM. Phosphorus, Sulfur and Silicon and the Related Elements. 2002;177:115–122. [Google Scholar]
- 16.See for example: Baraldi PG, Zaid AN, Lampronti I, Fruttarolo F, Pavani MG, Tabrizi MA, Shryock JC, Leung E, Romagnoli R. Bioorg Med Chem Lett. 2000;10:1953–1957. doi: 10.1016/s0960-894x(00)00379-6.
- 17.(a) Bogolubsky AV, Ryabukhin SV, Plaskon AS, Stetsenko SV, Volochnyuk DM, Tolmachev AA. J Comb Chem. 2008;10:858–862. doi: 10.1021/cc800074t. [DOI] [PubMed] [Google Scholar]; (b) Hazra K, Saravanan J, Mohan S. Asian J Chem. 2007;19:3541–3544. [Google Scholar]; (c) Hesse S, Perspicace E, Kirsch G. Tetrahedron Lett. 2007;48:5261–5264. [Google Scholar]; (d) Kovalenko SM, Vlasov SV, Chernykh VP. Heteroatom Chemistry. 2007;18:341–346. [Google Scholar]; (e) Kovalenko SN, Vlasov SV, Chernykh VP. Zhurnal Organichnoi ta Farmatsevtichnoi Khimii. 2006;4:43–46. [Google Scholar]; (f) Nikolakopoulos G, Figler H, Linden J, Scammells PJ. Bioorg Med Chem. 2006;14:2358–2365. doi: 10.1016/j.bmc.2005.11.018. [DOI] [PubMed] [Google Scholar]; (g) Huang W, Li J, Tang J, Liu H, Shen J, Jiang H. Syn Comm. 2005;35:1351–1357. [Google Scholar]; (h) Treu M, Karner T, Kousek R, Berger H, Mayer M, McConnell DB, Stadler A. J Comb Chem. 2008;10:863–868. doi: 10.1021/cc800081b. [DOI] [PubMed] [Google Scholar]
- 18.(a) Patra BR, Mohan S, Saravanan J. Asian Chem. 2007;19:4368–4372. [Google Scholar]; (b) Al-Mousawi SM, Abdelhamid IA, Moustafa MS. ARKIVOC. 2007:213–221. [Google Scholar]; (c) Arhin F, Belanger O, Ciblat S, Dehbi M, Delorme D, Dietrich E, Dixit D, Lafontaine Y, Lehoux D, Liu J, McKay GA, Moeck G, Reddy R, Rose Y, Srikumar R, Tanaka KSE, Williams DM, Gros P, Pelletier J, Parr TR, Jr, Far AR. Bioorg Med Chem. 2006;14:5812–5832. doi: 10.1016/j.bmc.2006.05.035. [DOI] [PubMed] [Google Scholar]; (d) Weber KH, Daniel H. Liebigs Annalen der Chemie. 1979:328–33. [Google Scholar]
- 19.(a) Hantzsch A. Chem Ber. 1881;14:1637–1638. [Google Scholar]; (b) Biginelli P. Chem Ber. 1891;24:1317–1319. 2962–2965. [Google Scholar]
- 20.Dömling A. Chem Rev. 2006;126:17–89. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]
- 21.Wang K, Nguyen K, Huang Y, Dömling A. J Comb Chem. 2009;11:920–927. doi: 10.1021/cc9000778. [DOI] [PubMed] [Google Scholar]
- 22.For example, the 3-CR synthesis of 2-amino-1H-pyrrole-3-carboxamide, see Wang K, Dömling A. Chem Biology Drug Design. 2009 doi: 10.1111/j.1747-0285.2009.00942.x. submitted.
- 23.(a) See ref. 18d; (b) Nagaoka H, Hara H, Mase T. Heterocycles. 1990;31:1241–1244. [Google Scholar]; (c) Walser A, Flynn T, Mason C, Crowley H, Maresca C, Yaremko B, O'Donnell M. J Med Chem. 1991;34:1209–1221. doi: 10.1021/jm00107a048. [DOI] [PubMed] [Google Scholar]
- 24.The Gewald-Ugi-Deprotection-Cyclisation (GUDC) strategy for preparing thiophenediazepine, see Huang YJ, Dömling A. unpublished result.Walser A, Flynn T, Mason C. J Heterocyclic Chem. 1991;28:1121–1125.Romagnoli R, Baraldi PG, Carrion MD, Cara CL, Cruz-Lopez O, Iaconinoto MA, Preti D, Shryock JC, Moorman AR, Vincenzi F, Varani K, Andrea Borea P. J Med Chem. 2008;51:5875–5879. doi: 10.1021/jm800586p.
- 25.(a) Mohareb RM, Ho JZ, Alfarouk FO. J Chinese Chem Soc. 2007;54:1053–1066. [Google Scholar]; (b) Kim MH, Park CH, Chun KW, Oh BK, Joe BY, Choi JH, Kwon HM, Huh SC, Won R, Kim KH, Kim SM. WO 2007102679. PCT Int Appl. 2007;147:365516. Chem. Abstr.
- 26.(a) Brouillette Y, Sujol G, Martinez J, Lisowski V. Synthesis. 2009:389–394. [Google Scholar]; (b) Pinkerton AB, Lee TT, Hoffman TZ, Wang Y, Kahraman M, Cook TG, Severance D, Gahman TC, Noble SA, Shiau AK, Davis RL. Bioorg Med Chem Lett. 2007;17:3562–3569. doi: 10.1016/j.bmcl.2007.04.076. [DOI] [PubMed] [Google Scholar]; (c) Nikolakopoulos G, Figler H, Linden J, Scammells PJ. Bioorg Med Chem. 2006;14:2358–2365. doi: 10.1016/j.bmc.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 27.(a) Orner BP, Ernst JT, Hamilton AD. J Am Chem Soc. 2001;123:5382–5383. doi: 10.1021/ja0025548. [DOI] [PubMed] [Google Scholar]; (b) Antuch W, Menon S, Chen QZ, Lu Y, Sakamuri S, Beck B, Schauer-Vukasinovic V, Agarwal S, Hess S, Dömling A. Bioorg Med Chem Lett. 2006;16:1740–1743. doi: 10.1016/j.bmcl.2005.11.102. [DOI] [PubMed] [Google Scholar]; (c) Haridas V. Europ J Org Chem. 2009;30:5112–5128. [Google Scholar]
- 28.Gerber PR, Muller K. J Comput Aided Mol Design. 1995;9:251–268. doi: 10.1007/BF00124456. [DOI] [PubMed] [Google Scholar]
- 29.(a) Castanedo GM, Sutherlin DP. Tetrahedron Lett. 2001;42:7181–7184. [Google Scholar]; (b) Hoener APF, Henkel B, Gauvin JC. Synlett. 2003:63–66. [Google Scholar]; (c) Zhang HQ, Yang GC, Chen JN, Chen ZX. Synthesis. 2004:3055–3059. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.