Abstract
Breast malignancies often have high levels of COX-2. The COX-2 product prostaglandin E2 (PGE2) contributes to the high metastatic capacity of breast tumors. Our published data indicates that inhibiting either PGE2 production or PGE2-mediated signaling through the PGE2 receptor EP4 (one of four EP expressed on the malignant cell) reduces metastasis by a mechanism that requires Natural Killer (NK) cells. Tumor derived PGE2 and exogenous PGE2 are known to have direct inhibitory effects on NK cell functions, but less is known regarding which EP receptors mediate these effects. We now show that several NK functions (lysis, migration, cytokine production) are compromised in tumor-bearing mice and that tumor produced PGE2 interferes with NK cell functions. PGE2 inhibits the potential of NK cells to migrate, exert cytotoxic effects, and secrete IFNγ. The ability of PGE2 to inhibit NK cells from tumor bearing mice is by acting on EP2 and EP4 receptors. NK cells from tumor-bearing mice were more sensitive to inhibition by EP4 and EP2 agonists compared to endogenous NK cells from healthy mice. PGE2 was inhibitory to most NK functions of either normal or tumor-bearing mice. In contrast, there was a trend for enhanced TNFα production in response to PGE2 by NK cells from tumor-bearing mice. We also report that a recently described EP4 antagonist, frondoside A, inhibits breast tumor metastasis in an NK-dependent manner and protects IFNγ production by NK cells from PGE2 mediated suppression. Taken together these data show that NK functions are depressed in tumor-bearing hosts relative to normal NK cells and that PGE2 suppresses NK functions by acting on EP2 and EP4 receptors.
Keywords: PGE2, EP4, NK cells, Immunosuppression, Frondoside A
Introduction
NK cells have the ability to recognize and kill tumor cells. Once activated, NK cells have increased cytolytic and proliferative functions to mediate anti-tumor activity. Therefore, it is important for NK cells to mobilize from a resting state. It is well established that the tumor cell releases immunosuppressive agents such as PGE2 that inhibit NK effector function. Other laboratories have found that macrophages from tumor bearing mice produce PGE2 in vitro and suppress NK cell activity and this inhibition is relieved with the dual COX-1/COX-2 inhibitor, indomethacin [1]. Monocytes from patients with breast cancer contribute to increased levels of PGE2 produced in culture and this activity correlates with a decrease in NK cell activity [2].
Our lab has demonstrated that murine and human breast tumors secrete high levels of PGE2, which correlate with metastatic disease [3]. PGE2 is the principle COX-2 product in cancer and mediates actions through a family of G-protein coupled receptors, EP1-4. Despite structural and sequence similarities among the four receptors, they are coupled to different intracellular signaling pathways [4]. Ligand binding of EP1 activates phospholipase C (PLC), which generates second messenger release of Ca2+ from intracellular stores and stimulates phosphorylation by protein kinase C (PKC). EP2 and EP4 are coupled to protein kinase A/adenylyl cyclase resulting in increased cyclic AMP (cAMP). The EP4 receptor has also been associated with coupling to phosphatidylinositol 3-kinase activating extracellular signal-regulated kinase (ERK)1 and ERK 2. EP3 inhibits adenylyl cyclase and thus decreases cAMP levels. We have shown that by inhibiting PGE2 production with COX inhibitors or blocking signaling using EP4 receptor antagonists or expressing EP4 siRNA in tumor cells, that mammary tumor cell metastasis to the lung can be reduced [5-7]. This therapeutic mechanism is controlled by NK cells [6]. These studies point to a critical role for EP4 expressed on the tumor cell, but the function of EP4 on the NK cell had not been examined. Since previous work has shown that tumor derived PGE2 suppresses NK activity and NK cells are necessary for therapeutic control of metastasis by PGE2 inhibitors and EP4 antagonists, we wanted to understand which EP receptors participate in regulation of NK cell function during tumor progression. We also investigated the ability of a recently described novel EP4 antagonist to protect NK cells from PGE2-mediated immune suppression.
Materials and methods
Cell lines and mice
The Yac-1 (American Type Culture Collection, Manassas, VA, USA) murine T lymphoma cell line that is sensitive to NK mediated cytolysis was used as a tumor-target cell in cytotoxicity assays. Yac-1 cells were cultured at 37°C in 5% CO2 in RPMI 1640 plus media that contained 10mM HEPES, 1mM sodium pyruvate, 4500 mg/L glucose, 1500 mg/L sodium bicarbonate and supplemented with 10% FBS. Lines 410.4, 66.1, and 67 were derived from a single, spontaneously occurring mammary tumor in a Balb/cfC3H mouse. Lines 410.4 and 66.1 are highly tumorigenic and metastatic in syngeneic mice; line 67 is poorly tumorigenic and nonmetastatic. Mammary tumor cell lines are maintained in DMEM supplemented with 2mM sodium bicarbonate, 1mM penicillin/streptomycin, 1mM L-glutamine, 1mM minimal essential amino acids, and 10% FBS.
In vivo studies
For spontaneous metastases assays, 3×105 of line 410.4 or 66.1 tumor cells were injected subcutaneously proximal to the mammary gland of syngeneic Balb/cByJ female mice (Jackson Laboratory, Bar Harbor, ME, USA). When tumors became palpable, tumor growth was monitored by caliper measurement of two perpendicular tumor diameters. Preliminary time course studies revealed that NK-EP receptor expression was reduced at day 28 or later, therefore NK functions were examined at day 30-35 (average tumor diameter of 15mm). Mice were euthanized, the spleen removed and immediately placed in phosphate buffered saline (PBS) for spleen cell isolation. Experiments were performed according to IACUC approved protocols.
For experimental metastasis assays, 1×105 tumor cells were injected into the lateral tail vein of syngeneic immune competent Balb/cByJ or NK-depleted Balb/cByJ female mice and 21 days later, mice were euthanized and surface pulmonary metastases were quantified under a dissecting microscope. To deplete NK cells, mice were injected with rabbit asialoGM1 ganglioside antibody (20μL, Wako Bioproducts, Richmond, VA, USA) or normal rabbit serum 1 day prior to and 3 days after tumor cell injection.
Enriched NK cell isolation
An untouched population of NK cells from spleen cell suspension was isolated following manufacturer’s protocol using the NK Cell Isolation Kit (Miltenyi Biotec Inc., Auburn, CA, USA). The entire effluent fraction collected represented the enriched NK cells identified as DX5+CD3− cells.
Flow Cytometry
For EP receptor studies, enriched NK cells were reacted with monoclonal mouse DX5 (CD49b) and CD3ε (BD PharMingen, San Diego, CA, USA) conjugated antibodies. The cells were then stained with an unconjugated primary rabbit polyclonal antibody directed to EP1, EP2, EP3, or EP4 (Cayman Chemical Company, Ann Arbor, MI, USA) followed by fluorochrome conjugated secondary goat anti-rabbit serum and fixation in 4% paraformaldehyde for 15 minutes with final preparation in PBS. Data expressed as percent of cells positive for the marker minus percent positive in cells treated with IgG as control. All flow cytometry samples were processed at the Flow Cytometry Shared Services at the University of Maryland Greenebaum Cancer Center.
RT-PCR
RNA was extracted from enriched NK cells using NucleoSpin® RNA II kit (Macherey-Nagel, Bethlehem, PA, USA), and reverse transcribed and amplified using EP-specific or GAPDH control primers. The reaction mixture was then placed in a preheated (94°C) thermal cycler where the initial denaturing step was performed for 2 minutes followed by 36 cycles of denature 94°C for 1 minute, annealing at 56°C for 40 seconds, and extension at 72°C for 50 seconds. Upon completion, a final extension was performed at 72°C for 2 minutes and samples held at 4°C.
EP Receptor Reagents
NK cell EP receptors were pharmacologically targeted using selective agonists and antagonists from Cayman Chemical (Ann Arbor, MI, USA). SC19220 and SC52089 were used to competitively antagonize the EP1 receptor. The EP1/EP2 receptor was antagonized using AH6809 and EP4 receptor was antagonized with AH23848, GW627368X (GWX), or Frondoside A (FA). To stimulate the receptors, PGE2 (EP1-4), Sulprostone (EP1/EP3), Butaprost (EP2), and Prostaglandin E1 Alcohol (PGE1-OH, EP4) were used as agonists.
cAMP EIA
NK cells were untreated, treated with vehicle control (DMSO), PGE2 (0.1μM, 1μM, or 10μM), or antagonists to specific EP receptors (AH23848, GWX, AH6809, SC19220, and SC51089). NK cells were treated for 30 minutes and culture media was removed. NK cell pellets were lysed using reagent 1B provided in Amersham cAMP Biotrak Enzymeimmunoassay System (GE Healthcare, Piscataway, NJ, USA) and intracellular cAMP levels were determined according to manufacturer’s instructions.
Migration Assay
The Chemo Tx® System (Neuro Probe, Gaithersburg, MD, USA) with a 3μm pore size was used to estimate migration. NK cells, at time 0, were treated with or without EP receptor agonists (PGE2, Butaprost, Sulprostone, and PGE1-OH) and the chemotactic response to FBS was assessed. Agonist-treated NK cells were pipetted onto the filter top and incubated at 37°C, 5% CO2 for 3 hours. After incubation, cells were gently washed from the top side of the framed filter. Migrated cells were labeled by the addition of Calcein AM. After one-hour incubation, the microplate was placed in a fluorescence reader (485nm) to count migrated cells in the microplate wells. The results were calculated from a generated standard curve and data expressed as number of cells migrated.
NK Cell Mediated Cytotoxicity Assay
NK cell mediated cytotoxicity was measured using the CytoTox 96® Assay (Promega, Madison, WI, USA), which quantitatively measures the release of the stable enzyme lactate dehydrogenase (LDH) upon cell lysis. NK cells were cultured with Yac-1 lymphoma targets at several effector:target (E:T) ratios: 2:1, 5:1, 10:1. NK cells were pre-treated with various PGE2 concentrations (1.0μM and 10μM), Butaprost (1.0μM and 10μM), Sulprostone (1.0μM and 10μM), or PGE1-OH (1μM and 10μM). After pre-treatment with agonists, Yac-1 tumor targets were added and co-incubated for 18 hours. The supernatant was harvested and transferred to the enzymatic assay plate where the Substrate Mix was added, and the reaction halted with Stop Solution and absorbance determined at 490nm. To compute corrected absorbance values the following formula was used to obtain percent cytotoxicity for each effector:target cell ratio:
ELISA
NK cells were pretreated with either vehicle control (DMSO) or agonists (PGE2, Butaprost, Sulprostone, PGE1-OH) for 30 minutes. At the end of 30 minutes, cells were stimulated with 1000U/mL of IL2 and incubated at 37°C, 5% CO2. At 18 hours, cell culture conditioned media was collected. Mouse IFNγ ELISA Ready-SET-Go!® and Mouse TNFα ELISA Ready-SET-Go!® kits (eBioscience, San Diego, CA, USA) were used to measure cytokines in cell conditioned media and assays were performed according to the manufacturer’s instructions.
Statistical Analysis
The results are expressed as mean ± standard error of at least n=3. A one-way analysis of variance (ANOVA) was used to compare means of control (DMSO) and experimental groups. Overall test analysis was reported as significant at p-value < 0.05.
Results
Natural Killer Cells from Tumor-bearing Mice Have Enhanced PGE2 Production
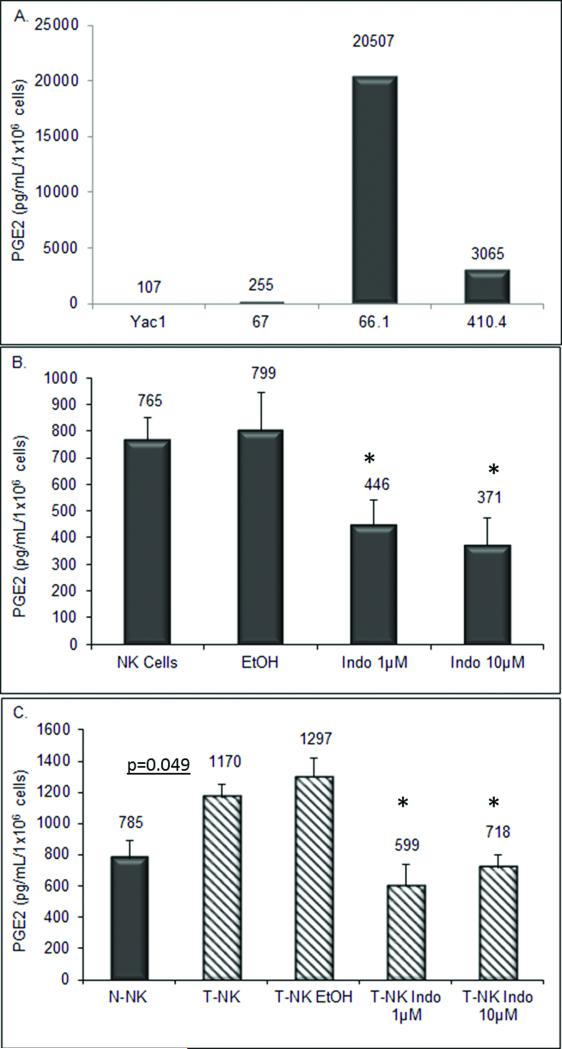
It is well known that the tumor milieu utilizes many mechanisms to escape NK cell mediated surveillance. One such mechanism is the immunosuppressive action of PGE2. We have previously shown that breast tumor cell lines produce PGE2 and increasing PGE2 correlates with metastatic potential as well as tumor development [3]. To confirm the stability of this property, PGE2 levels were measured in conditioned medium from three mammary tumor cell lines 66.1, 410.4, and 67, as well as the highly NK sensitive lymphoma line, Yac-1. PGE2 levels in the metastatic breast tumor lines 66.1 and 410.4 were 80 and 12 fold greater, respectively, than in conditioned medium from the non-metastatic line 67 (Figure 1a). The mouse lymphoma cell line Yac-1 produced very little PGE2; on a per cell basis, Yac-1 cells produced 107 pg PGE2/mL/106 cells versus line 67 cells that produced 255 pg PGE2/mL/106 cells and metastatic cell lines that produced more than 3,000 pg PGE2/mL/106 cells.
Fig. 1.
(a) PGE2 was measured in conditioned media from cultured murine mammary tumor cell lines 66.1, 410.4, 67, and the murine lymphoma line Yac-1. (b) Conditioned media was collected from NK cells, NK cells treated with vehicle control (EtOH) and COX-inhibitor (indomethacin) for 18 hours and PGE2 levels determined by ELISA assay. (c) Conditioned media was isolated from NK cells and T-NK cells, T-NK cells treated with vehicle control (EtOH) and COX-inhibitor (indomethacin). Results are from triplicate wells. Data reported as mean pg PGE2/mL/1×106 cells ± standard error.
T cells and macrophages produce PGE2, but it has not been reported that NK cells produce PGE2 [8]. Spontaneous release of PGE2 into medium was measured from NK cells isolated from normal mice and treated with vehicle control or with the inhibitor of PGE2 production, indomethacin. Figure 1b shows that an enriched NK cell population produces measurable amounts of PGE2. Normal NK cells produce more PGE2 on a per cell basis, in fact, than non-metastatic cell line 67 and lymphoma cell line Yac-1 but significantly less than metastatic breast cancer cell lines. This PGE2 production was partially inhibited by treatment with indomethacin (p<0.05). We also examined PGE2 production from NK cells isolated from tumor bearing mice. Tumor cells were implanted proximal to the abdominal mammary gland in mice and on day 30, splenic NK cells were isolated. NK cells from tumor bearing mice (T-NK) produced 1.5 fold more PGE2 than normal NK cells (N-NK1170 pg PGE2/mL/1×106 cells compared to 785 pg PGE2/mL/1×106 cells); which could be inhibited by indomethacin (Figure 1c).
NK Cell EP Receptor Expression is Suppressed in the Tumor Environment
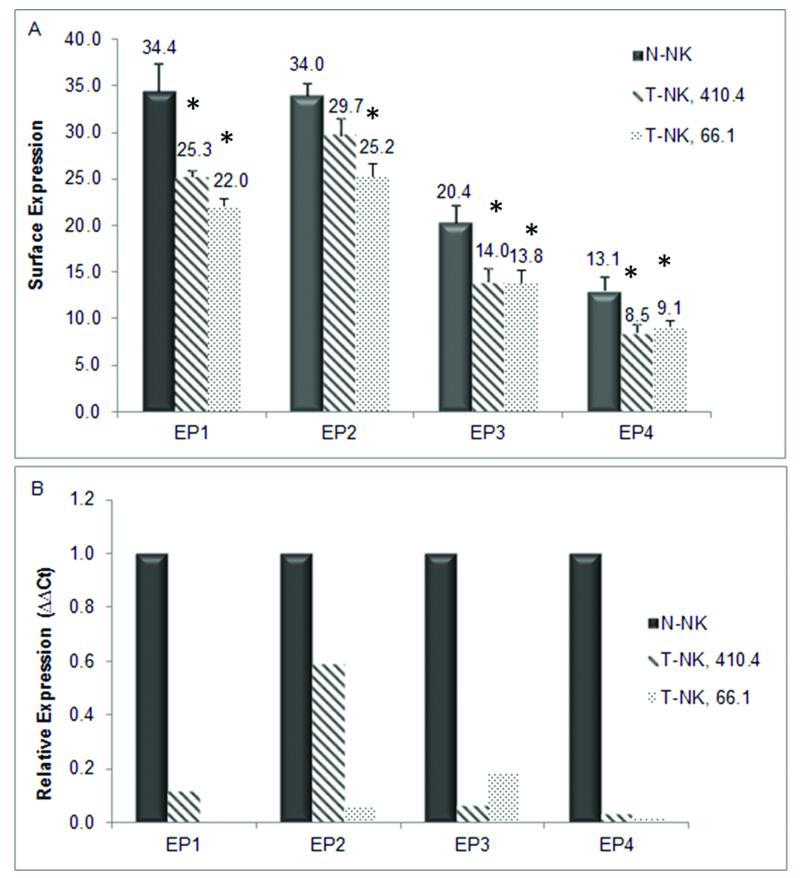
Endogenous NK cells from normal mice express all four EP receptors [9]. To begin to understand the mechanisms by which tumor-derived PGE2 acts on NK cells, we characterized the expression of EP receptors on NK cells from tumor bearing mice and compared them to endogenous NK cells from normal mice. 410.4 or 66.1 tumor cells were injected proximal to the abdominal mammary gland of Balb/cByJ female mice. At 30 days post-injection or when tumors reached 15mm in diameter, the mice were euthanized, lung weights and lung tumor colonies were quantified and splenic NK cells were isolated for further characterization. Mice injected with tumor cells had a 1.3 fold increase in lung weight compared to control mice reflecting the tumor burden in the lungs. Spleens from mice bearing 410.4 tumors were markedly heavier (average weight 0.7088g ± 0.0643) than spleens from normal mice (average weight 0.1039g ± 0.0049); spleens from 66.1 tumor-bearing mice weighed on average 0.1785g ± 0.0093, but were not statistically significantly different than normal spleens. NK cells isolated from spleens from tumor-bearing mice are henceforth referred to as T-NK. We examined the expression of EP on the surface of T-NK by flow cytometry and compared EP expression levels to those on N-NK cells [9]. N-NK cells express all EP receptors (Figure 2a). EP1 is detected on thirty-four percent of N-NK. Thirty-four percent, 20%, and 13% of N-NK are positive for EP2, EP3, and EP4, respectively, confirming our previous report which examined EP function on N-NK cells only [9]. Expression of each EP is reduced on NK cells from mice bearing 410.4 or 66.1 tumors in comparison to N-NK as indicated by the lower percentage of receptor positive cells in T-NK vs. N-NK. Like EP protein levels, EP expression at the mRNA levels is also markedly reduced in T-NK compared to N-NK and to a greater degree compared to protein expression (Figure 2b) indicating that the EP protein probably has a longer half-life than the mRNA.
Fig. 2.
N-NK cells and T-NK cells from 410.4 and 66.1 bearing mice were assessed by (a) Flow cytometry analysis and (b) quantitative PCR for EP receptor expression. Relative to N-NK, T-NK EP receptor expression *p<0.05.
PGE2 Induces Intracellular cAMP Response in NK Cells
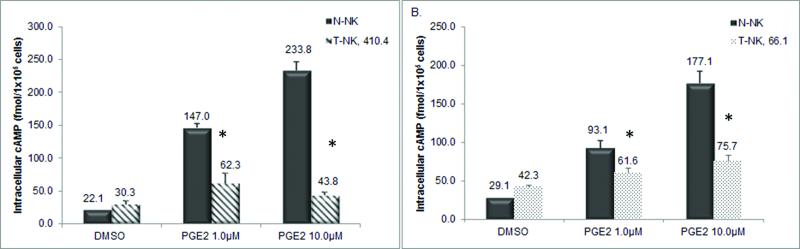
EP2 and EP4 receptors are G-protein coupled receptors that upon ligand-mediated activation elevate cAMP levels. We compared the effects of PGE2 on cAMP activity in T-NK to N-NK populations. Figure 3a and 3b confirms our previous findings that PGE2 (1.0 μM, 10.0μM) significantly increased cAMP levels in N-NK cells ranging from 6.7 to 10.6 fold. PGE2 (1.0 μM, 10.0μM) also induced an intracellular cAMP response in T-NK cells obtained from either 410.4 (Figure 3a) or 66.1 (Figure 3b) tumor bearing mice but only by 1.4 to 2 fold. Thus, the ability of PGE2 to induce cAMP in NK cells is significantly blunted in tumor bearing mice.
Fig. 3.
N-NK cAMP was compared to (a) T-NK 410.4 and (b) T-NK 66.1. Cells were stimulated in the presence of PGE2 for 30 minutes. Intracellular cAMP was assessed from cell lysates. Data represented as mean ± standard error fmol cAMP/1×105 cells in triplicate wells. Relative to N-NK, T-NK with PGE2 treatment *p<0.05.
IFNγ Secretion is Suppressed in NK Cells through EP2 and EP4 Receptors
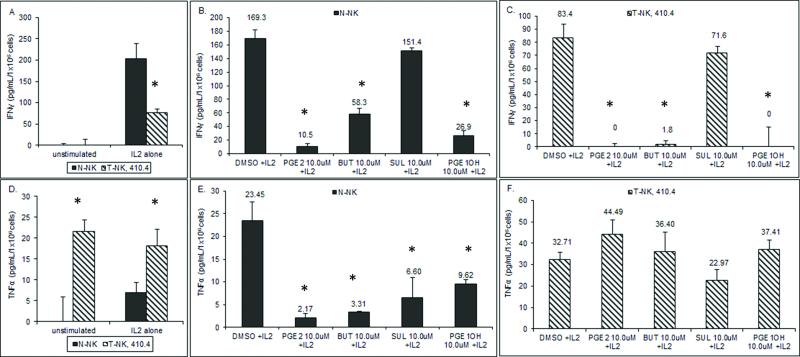
NK cells secrete IFNγ to enhance activities of NK and other effector cells. The effect of PGE2 on IFNγ secretion from N-NK and T-NK cells was examined. NK cells from normal and tumor bearing mice were pretreated with PGE2 and then stimulated with 1000U/mL of IL2 to induce cytokine production. T-NK cytokine secretion is reduced compared to N-NK (Figure 4a). N-NK secreted 203 pg/mL IFNγ after IL-2 stimulation whereas only 76 pg/mL IFNγ was secreted from T-NK. Pretreatment with PGE2 reduced the amount of IFNγ produced by N-NK cells (Figure 4b) and eliminated IFNγ production by T-NK cells (Figure 4c). To determine which EP receptor was involved in inhibition, NK cells were targeted with specific EP receptor agonists. The EP4 agonist (PGE1-OH) and EP2 agonist (Butaprost) significantly inhibited IFNγ production by N-NK (Figure 4b, 86 and 66 percent inhibition respectively, p<0.001), but the EP1/EP3 agonist Sulprostone only decreased IFNγ by 19 percent; this latter change was not statistically significant. IFNγ secretion in T-NK cells was significantly repressed with addition of 10μM of either an EP2 or EP4 agonist by 99 and 100 percent, respectively (Figure 4c, p<0.001). The EP1/EP3 agonist inhibited IFNγ secretion by 14 percent (p = n.s.). Thus in both N-NK and T-NK cells, PGE2 inhibits IFNγ production mainly through EP2 and EP4, but not through the EP1/EP3 receptors.
Fig. 4.
(a,d) N-NK and T-NK were analyzed for IFNγ or TNFα in the presence or absence of IL2 stimulation. (b,e) N-NK cells and (c,f) T-NK cells were pretreated with 10μM of PGE2, PGE1-OH, Butaprost (BUT), or Sulprostone (SUL). Cell culture supernatants were assayed for IFNγ or TNFα. Results are reported as mean pg/mL/1×106 cells ± standard error, n=3. Relative to DMSO, treatment *p<0.05.
PGE2 Suppresses TNFα in N-NK but not T-NK cells
Although TNFα was first identified as an anti-tumor cytokine that induced NK cells, emerging evidence suggests that TNFα acts as a tumor-promoting factor and is linked to migration and invasion of tumor cells [10]. We examined the production of TNFα from T-NK cells. Unstimulated T-NK cells produced TNFα (21.68 ± 2.73 pg/mL/1×106cells), which is in contrast to N-NK cells in which no TNFα was detected in the absence of IL2 (Figure 4d). In the presence of IL2, T-NK cells produced higher levels of TNFα than N-NK. We then examined the role of PGE2 and the EP receptors in regulating TNFα secretion by NK cells. PGE2 suppressed TNFα by N-NK (Figure 4e). In contrast, T-NK cells when treated with 1.0μM and 10.0μM PGE2 modestly increased TNFα secretion by 1.4 fold (Figure 4f). Like PGE2, the EP2 agonist Butaprost, EP1/EP3 agonist Sulprostone, and EP4 agonist PGE1-OH were able to inhibit secretion of TNFα by N-NK by 86%, 72%, and 58%, respectively (Figure 4e). In contrast, Butaprost and PGE1-OH modestly increased TNFα secretion by T-NK (Figure 4f). Sulprostone decreased TNFα secretion by 30 percent in T-NK cells. Thus, T-NK cells and N-NK cells are very different concerning cytokine production and in response to PGE2. N-NK cells are superior in terms of IFNγ production whereas T-NK cells produce higher levels of TNFα. Furthermore, PGE2 inhibits TNFα production by N-NK but this cytokine is induced by PGE2 in T-NK.
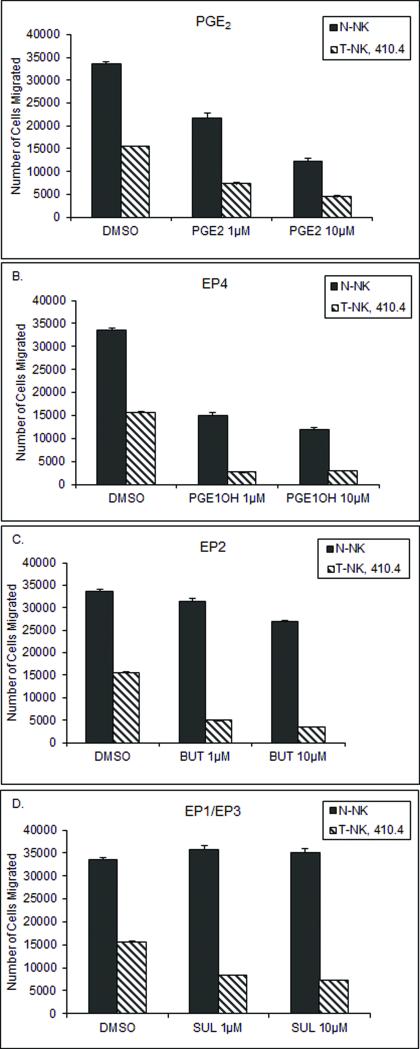
NK Cell Migration is Inhibited by PGE2
The ability of NK cells to migrate to tumor targets is important in tumor control. We compared the migratory ability of T-NK to N-NK and assessed the inhibitory effects of PGE2 on migration. PGE2 inhibited migration of N-NK cells by 48 or 70 percent (Figure 5a). T-NK cells migrate poorly in comparison to N-NK even in the absence of exogenous PGE2. T-NK from mice bearing tumor 410.4 could nevertheless be further inhibited by exogenous PGE2 (Figure 5a). To further delineate which EP receptor is involved in PGE2-mediated inhibition of NK cell migration, N-NK and T-NK cells were treated with EP receptor specific agonists and migration was assessed. Like PGE2, the EP4 agonist PGE1-OH significantly blocked N-NK or T-NK cell migration (Figure 5b). The EP2 agonist Butaprost did not significantly impede migration of N-NK, but did inhibit T-NK (Figure 5c); in contrast, the EP1/EP3 agonist Sulprostone modestly increased migration of N-NK cells, but inhibited T-NK cells (Figure 5d). The EP4 agonist PGE1-OH reduced T-NK cell migration by 81-83 percent; the EP2 receptor agonist Butaprost achieved 68-77 percent inhibition and migration was reduced by 46-53 percent by the EP1/EP3 agonist Sulprostone. Like T-NK, the migration of N-NK was also significantly inhibited by EP4 activation, but unlike T-NK, N-NK migration was not inhibited by an EP2 agonist. EP1 and EP3 activation actually promotes migration of N-NK. In contrast, T-NK are inhibited by activation of either EP2 or EP4 and modestly by EP1/EP3 agonists. Thus in N-NK, EP4 is dominant in suppressing migration whereas the broader inhibitory response in T-NK is mediated through all EP receptors.
Fig. 5.
N-NK or T-NK cells were pretreated with 1μM or 10μM of (a) PGE2, (b) PGE1-OH, (c) Butaprost (BUT), or (d) Sulprostone (SUL) and migration capacity was determined. The number of cells that crossed a 3μm pore membrane was counted based on fluorescence. Data reported as mean number of cells migrated ± standard error, n=3. Relative to DMSO, treatment p<0.05 and N-NK relative to T-NK, p<0.05.
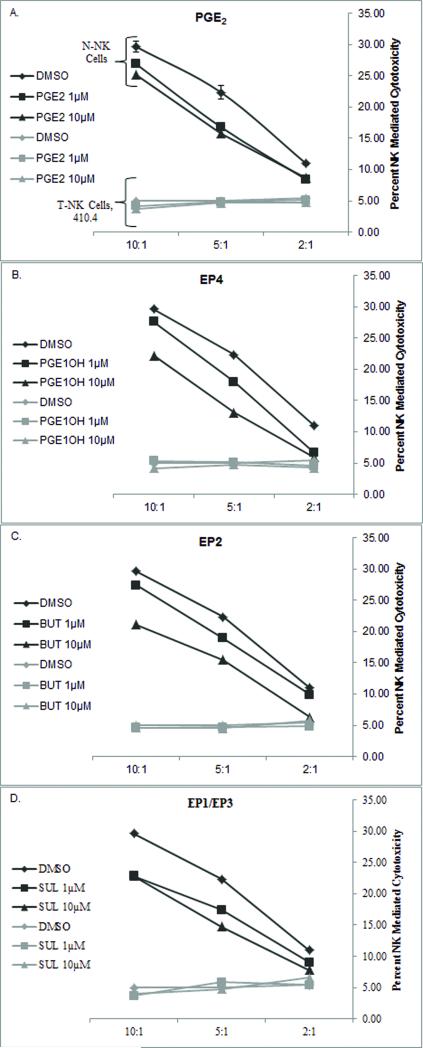
NK Cell Cytotoxic Activity is Compromised in the Tumor Environment
Although NK cells have the capacity to infiltrate and kill tumor targets, cytotoxic activity is compromised in the tumor environment. We showed that PGE2 is a major inhibitory factor produced by tumor cells. To determine the effects of the tumor on NK cytolytic actions, NK cell-mediated cytotoxicity from N-NK and T-NK cells was compared. T-NK cells lose the ability to lyse tumor targets compared to N-NK cells (Figure 6). In figure 6, lytic activity was compared at three effector to target ratios. In the absence of exogenous PGE2, T-NK from 410.4-bearing mice were significantly less lytic than N-NK (Figure 6a). Exogenous PGE2 modestly inhibited the cytolytic activity of N-NK cells, T-NK cell lytic activity was at baseline and no further suppression by PGE2 could be observed (Figure 6a). The EP4, EP2, and EP1/EP3 agonists mimicked the ability of PGE2 to inhibit lytic actions of N-NK cells (Figure 6b,c,d), but the minimal activity of T-NK cells was not further suppressed by PGE2 or receptor agonists.
Fig. 6.
N-NK cells or T-NK cells were assessed for the ability to lyse Yac-1 cell targets. N-NK and T-NK cells were pre-incubated with either PGE2, PGE1-OH (EP4), Butaprost (EP2), or Sulprostone (EP1/EP3). Three effector to target ratios were used: 2:1, 5:1, and 10:1. Data presented as percent NK mediated cytotoxicity ± standard error, n = 3. Key: Black = N-NK, Gray = T-NK
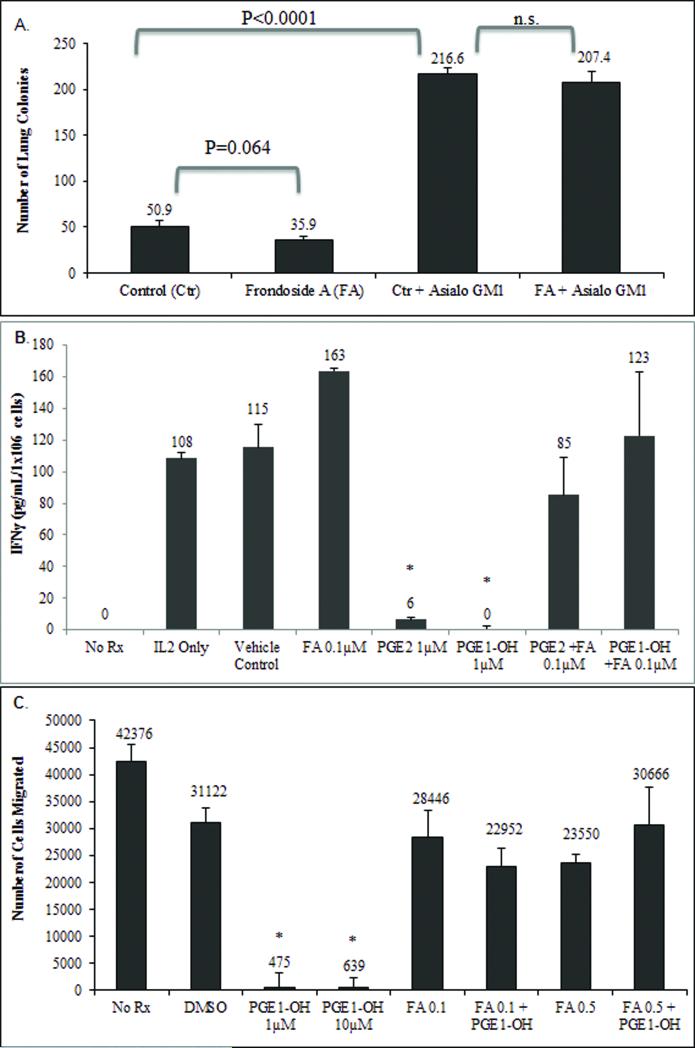
Novel EP4 Antagonist Frondoside A Inhibits Metastasis by an NK-dependent Mechanism
We have shown here that PGE2 exerts inhibitory effects most profoundly through the EP4 receptor in both N-NK and T-NK. This and our previous studies suggest that blocking EP4 activities inhibits metastasis and protects NK cells from PGE2-mediated immune suppression. There is a paucity of EP4 antagonists that can be used clinically. We recently described a novel EP4 antagonist, Frondoside A (FA) that has potent anti-metastatic activity [11]. We compared the ability of FA to control metastatic disease in immune competent Balb/cByJ mice and mice depleted of NK activity. Injection of vehicle-treated tumor cells into competent mice resulted in an average of 50.9 tumor colonies; FA pretreatment of tumor cells before injection reduced the mean number of metastases by 30 percent which was marginally significant (p=0.064, Figure 7a). Depletion of NK cells leads to loss of endogenous control of tumor dissemination leading to a 4.25 fold increase in lung metastasis, and in these mice, FA no longer inhibited metastasis. Thus, the ability of an EP antagonist to inhibit metastasis is by an NK-dependent mechanism.
Fig. 7.
(a) Line 66.1 tumor cells were treated with FA (1μM) or PBS control for 24h, washed and 1×105 viable cells injected intravenously into Balb/cByJ mice or Balb/cByJ mice depleted of NK cells with asialoGM1 antibody. Twenty-one days later, mice were euthanized and surface lung tumor colonies were enumerated. Mean ± SE of lung metastases in 10 mice/group. (b) N-NK cells were pretreated with PGE2, PGE1-OH, and FA (0.1μM) with and without EP receptor agonists and cell culture supernatants were assayed for IFNγ. Results are reported as mean pg/mL/1×106 cells ± standard error. (c) N-NK cells were pretreated with FA (0.1μM and 0.5μM) in the presence and absence of PGE2 and PGE1-OH and migration capacity was determine by the number of cells that crossed a 3μm pore membrane. Data reported as mean number of cells migrated ± standard error. Relative to DMSO, treatment *p<0.05.
Frondoside A Reverses PGE2-Mediated Cytokine and Migration Inhibition
To determine how the EP4 antagonist FA and NK cells interact, we determined the effect of FA on NK cell cytokine secretion and migration. N-NK cells were pre-treated with the EP4 antagonist FA (0.1μM or 0.5μM) and EP agonists PGE2 or PGE1-OH. After pre-treatment, N-NK cells were stimulated with 1000U/mL of IL2 to induce cytokine production. As expected, PGE2 and the EP4 agonist PGE1-OH suppressed the secretion of IFNγ from N-NK cells, by 95 and 100 percent respectively (Figure 7b). FA restored the capacity of N-NK cells to secrete IFNγ in the presence of PGE2 or PGE1-OH. We also determined the effect of FA on N-NK cell migration. N-NK cells were treated with FA 0.1μM or 0.5μM with and without the EP4 agonist PGE1-OH and the degree of N-NK cell migration was determined 3h later. PGE1-OH alone markedly inhibited NK cell migration (Figure 7c). FA is able to reverse this PGE1-OH-mediated inhibition.
Discussion
We examined the effects of the tumor-milieu and specifically PGE2 on NK cell function and identified which EP receptors regulated NK cell functions. T-NK cells have reduced EP1-4 expression compared to N-NK cells. Elkashab and Lala [12] examined whether the presence of tumor caused changes in the affinity of PGE2 binding sites (receptors) on splenic lymphocytes. They completed their study before it was known that multiple EP receptors exist. Their findings revealed that there was no difference in 3H-PGE2 binding affinity between normal and tumor bearing lymphocytes, but did reveal possible evidence of lower maximum 3H-PGE2 binding in lymphocytes from tumor bearing hosts. Although Elkashab’s initial hypothesis was that the findings could be explained by a reduction in the number of PGE2 binding sites on splenic lymphocytes, this theory was ruled out by Scatchard analysis. They concluded that the reduced 3H-PGE2 binding was due to partial occupation of receptor sites by tumor-produced PGE2. Our results agree with that study in that we detected less EP receptor expression in T-NK compared to N-NK cells. We expanded these findings to show that expression of each individual EP receptor was reduced at the protein level. G protein coupled receptors are regulated by ligand-induced internalization and degradation. The presence of excess agonist on G-protein coupled receptors is known to cause the apparent decline in receptor number [13]. Gray and Roth report that prolonged exposure to ligand causes GPCRs to be internalized and bound for sorting to be either recycled or down-regulated. The loss of EP receptor expression in T-NK may be due, in part, to chronic exposure to excess tumor derived PGE2 resulting in the internalization of the EP receptors and subsequent degradation. In addition, we also showed that EP mRNA is profoundly reduced in T-NK indicating that the reduced surface expression is not only due to receptor occupancy, turnover, or degradation. Our data suggest that negative regulation of EP transcription and translation also contribute to the loss of EP expression on NK cells from tumor bearing mice.
The reduced EP2 and EP4 receptor expression we observed was accompanied by a dampened cAMP response to EP2 and EP4 activation. PGE2 stimulated cAMP in N-NK cells in a dose dependent manner but this response was blunted in T-NK cells. Since our studies also show that PGE2 is inhibitory to many NK functions, we hypothesized that T-NK down regulate EP in response to either chronic ligand stimulation and/or as a mechanism to escape immune suppression. In spite of EP downregulation however, we show that T-NK cells are still sensitive to further immunosuppressive actions of exogenous PGE2.
It is evident that the tumor releases factors that inhibit NK cell migratory ability. NK cells from tumor bearing mice have partially lost the innate ability to migrate, but this reduced response is still sensitive to further inhibition by PGE2. The treatment of T-NK cells with EP receptor agonists to each receptor revealed that NK cell migration was negatively regulated through all EP receptors. The EP4 and EP2 receptors appear to inhibit migration of both N-NK and T-NK, but the role of EP1/EP3 appears to diverge in the two populations. In N-NK cells the EP1/EP3 agonist induced migration, but the same agonists inhibited migration of T-NK cells. This differential response may be related to different receptor expression levels in T-NK and N-NK cells.
Although it is evident that NK cells have the potential to recognize and eliminate tumor targets, an imbalance of signals transmitted by inhibitory and activating receptors must be in place before NK cell mediated killing ensues. Tumor-derived PGE2 represents a major barrier to the success of NK cell mediated killing. T-NK cells were 83 percent less cytolytic than N-NK cells at a 10:1 effector to target ratio. PGE2 and the EP2 receptor agonist, Butaprost, inhibited N-NK mediated cytotoxicity, but the further inhibition of cytotoxicity exhibited by T-NK was difficult to detect due to the profound loss of intrinsic killing. In human NK cells from normal donors, PGE2 prevents NK cell activating receptors (NKG2D, NCR, CD16) from inducing NK cell mediated cytotoxicity and this mechanism is through EP2 and EP4 receptors [14]. While our study did not investigate the actions of PGE2 on activated (LAK) cells, it is possible that tumor-derived PGE2 and exogenous PGE2 would decrease activating receptors and increase inhibitory receptors preventing the switch of NK cells from a resting to an activated state. Other investigators have studied the effects of PGE2 on NK cells in the activated state. Su et al [15] showed that LAK cell (activated NK cells generated from adherent splenocytes cultured in IL2) cytotoxic activities were inhibited by PGE2 through the EP2 receptor. This was similar to our findings in endogenous NK cells in that we implicate the EP2 receptor, but we also demonstrate EP4 receptor involvement.
In addition to diminished capacity to migrate and to lyse tumor target cells, the intrinsic ability of T-NK cells to secrete IFNγ was compromised and was further inhibited by the addition of exogenous PGE2. In both N-NK and T-NK, IFNγ secretion is negatively regulated through EP2 and EP4. Likewise, EP2 and EP4 activation inhibited TNFα production by N-NK. In contrast, PGE2 induced TNFα secretion from T-NK cells. Consistent with this data, Zeidler et al [16] found that TNFα was induced in co-cultured monocytes and tumor cells (shown to produce PGE2). In data reported here, PGE2, Butaprost (EP2), and PGE1-OH (EP4) increased TNFα secretion by T-NK cells. Thus, in the tumor context regulation of TNFα by PGE2 is very different than in N-NK.
In summary, we have shown T-NK cells have reduced EP receptor expression compared to N-NK cells. Although there is loss of EP expression, the ability to induce adenylyl cyclase activation, while diminished, indicates that EP2 and EP4 are still functionally coupled to adenylyl cyclase in T-NK. Unlike N-NK cells where PGE2 predominately regulated inhibitory functions through the EP4 receptor, PGE2 inhibited T-NK cells through both EP2 and EP4 receptors. Whether this indicates a broadening of the response to include EP2 or whether EP receptors are more sensitive to activation on T-NK remains to be determined. Unlike EP2 and EP4, EP1 and EP3 are coupled to different signaling pathways. This may explain why the later two receptors do not mediate the suppressive effects of PGE2 on NK cells. Taken together, this shows that reduced EP receptor expression during tumorigenesis is not enough to overcome the inhibitory effects of PGE2 and further emphasizes the need to develop antagonists of EP4 and possibly EP2 to prevent PGE2-mediated immune suppression. Our previous work as well as the current study show that EP4 antagonists are dependent on NK cells to inhibit metastasis as well as to enhance NK lytic activity [5-7]. Direct antagonism of EP4 on the malignant cell inhibits metastasis. The current study, showing that T-NK functions are suppressed through EP4 emphasizes the rationale for identifying effective EP4 antagonists that can both protect NK cells and directly reduce tumor metastasis. These studies indicate that Frondoside A may be one potential EP4 antagonist that should be investigated further.
Acknowledgements
Studies were supported by the National Institutes of Health (U.S.) (AMF), Department of Defense (U.S.) (AMF, DMH), the U.S. Department of Veterans Affairs (AMF) and the Maine Technology Institute, Gardiner, ME (PDC).
Disclosure of Funding National Institutes of Health (NIH), Department of Defense, U.S. Department of Veterans Affairs
Footnotes
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parhar R, Lala P. Prostaglandin E2-mediated inactivation of various killer lineage cells by tumor-bearing host macrophages. Journal of Leukocyte Biology. 1988;44:474–484. doi: 10.1002/jlb.44.6.474. [DOI] [PubMed] [Google Scholar]
- 2.Baxevanis C, Reclos G, Gritzapis A, Dedousis G, Missitzis I, Papamichail M. Elevated prostaglandin E2 production by monocytes is responsible for the depressed levels of natural killer and lymphokine-activated killer cell function in patients with breast cancer. Cancer. 1993;72(2):491–501. doi: 10.1002/1097-0142(19930715)72:2<491::aid-cncr2820720227>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Kundu N, Yang Q, Dorsey R, Fulton A. Increased cyclooxygenase-2 (COX-2) expression and activity in a murine model of metastatic breast cancer. International Journal of Cancer. 2001;94:681–686. doi: 10.1002/ijc.1397. [DOI] [PubMed] [Google Scholar]
- 4.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid Receptors. Physiological Reviews. 1999;79(4):1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 5.Kundu N, Ma X, Holt D, Goloubeva O, Ostrand-Rosenberg S, Fulton A. Antagonism of the prostaglandin E receptor EP4 inhibits metastasis and enhances NK function. Breast Cancer Research and Treatment. 2009;117(2):235–242. doi: 10.1007/s10549-008-0180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kundu N, Walser T, Ma X, Fulton A. Cyclooxygenase inhibitors modulate NK activities that control metastatic disease. Cancer Immunology, Immunotherapy. 2005;54(10):981–987. doi: 10.1007/s00262-005-0669-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma X, Kundu N, Rifat S, Walser T, Fulton A. Prostaglandin E receptor EP4 antagonism inhibits breast cancer metastasis. Cancer Research. 2006;66(6):2923–2927. doi: 10.1158/0008-5472.CAN-05-4348. [DOI] [PubMed] [Google Scholar]
- 8.Goldyne M, Stobo J. Prostaglandin E2 as a modulator of macrophage-T lymphocyte interactions. Journal of Investigative Dermatology. 1980;74:297–300. doi: 10.1111/1523-1747.ep12543495. [DOI] [PubMed] [Google Scholar]
- 9.Holt D, Ma X, Kundu N, Fulton A. Prostaglandin E2 (PGE2) suppresses natural killer cell function through the PGE2 receptor EP4. Cancer Immunology, Immunotherapy. 2011 Jun 17; doi: 10.1007/s00262-011-1064-9. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Y, Zhou B. TNF-alpha/NF-KappaB/Snail pathway in cancer cell migration and invasion. British Journal of Cancer. 2010;102(4):639–644. doi: 10.1038/sj.bjc.6605530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma X, Kundu N, Collin P, Goloubeva O, Fulton A. Frondoside A inhibits breast cancer metastasis and antagonizes prostaglandin E receptors EP4 and EP2. Breast Cancer Research and Treatment. 2011 Jul 15; doi: 10.1007/s10549-011-1675-z. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elkashab M, Lala P. PGE2 receptors on murine splenic lymphocytes: effects of tumor bearing. Immunology Letters. 1991;30:7–16. doi: 10.1016/0165-2478(91)90082-l. [DOI] [PubMed] [Google Scholar]
- 13.Gray J, Roth B. A Last GASP for GPCRs? Science. 2002;297:529–531. doi: 10.1126/science.1075453. [DOI] [PubMed] [Google Scholar]
- 14.Martinet L, Jean C, Dietrich G, Fournie J, Poupot R. PGE2 inhibits natural killer and γδ T cell cytotoxicity triggered by NKR and TCR through a cAMP-mediated PKA type I-dependent signaling. Biochemical Pharmacology. 2010;80:838–845. doi: 10.1016/j.bcp.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Su Y, Huang X, Raskovalova T, Zacharia L, Lokshin A, Jackson E, Gorelik E. Cooperation of adenosine and prostaglandin E2 (PGE2) in amplification of cAMP-PKA signaling and immunosuppression. Cancer Immunology, Immunotherapy. 2008;57:1611–1623. doi: 10.1007/s00262-008-0494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeidler R, Csanady M, Gires O, Lang S, Schmitt B, Wollenberg B. Tumor cell-derived prostaglandin E2 inhibits monocyte function by interfering with CCR5 and Mac-1. FASEB Journal. 2000;14:661–668. doi: 10.1096/fasebj.14.5.661. [DOI] [PubMed] [Google Scholar]