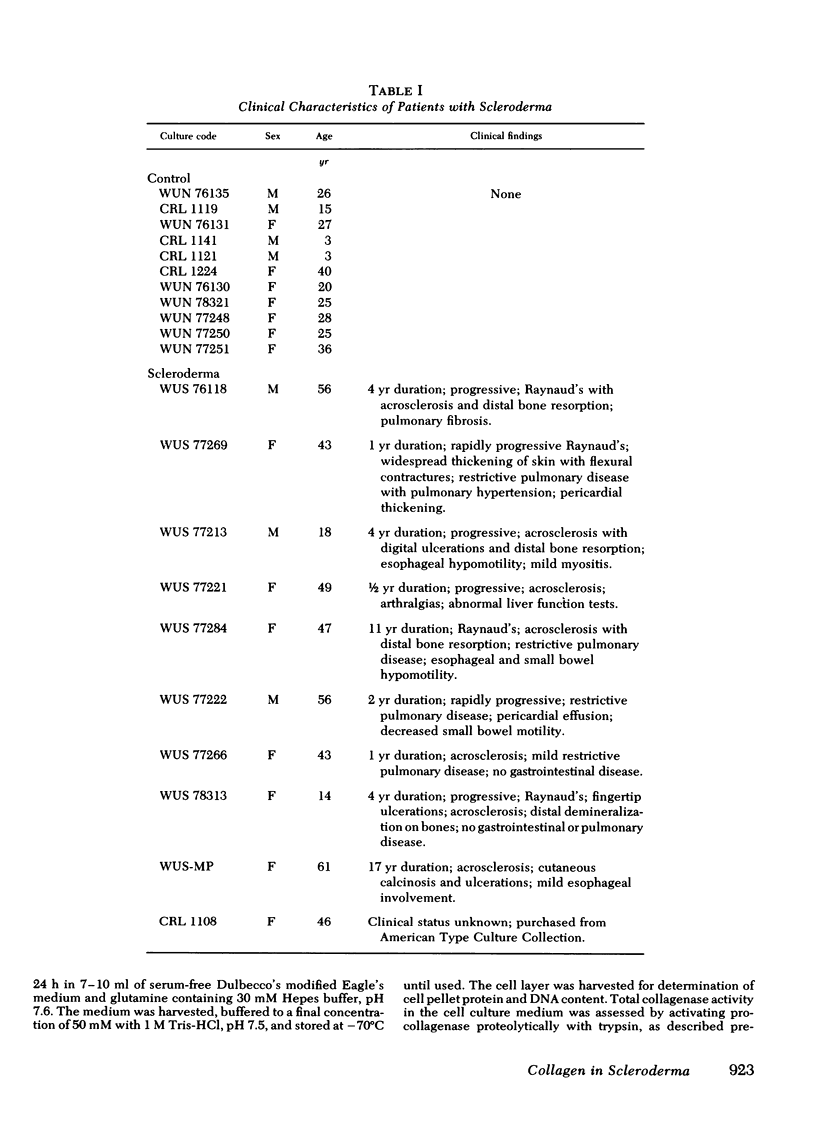
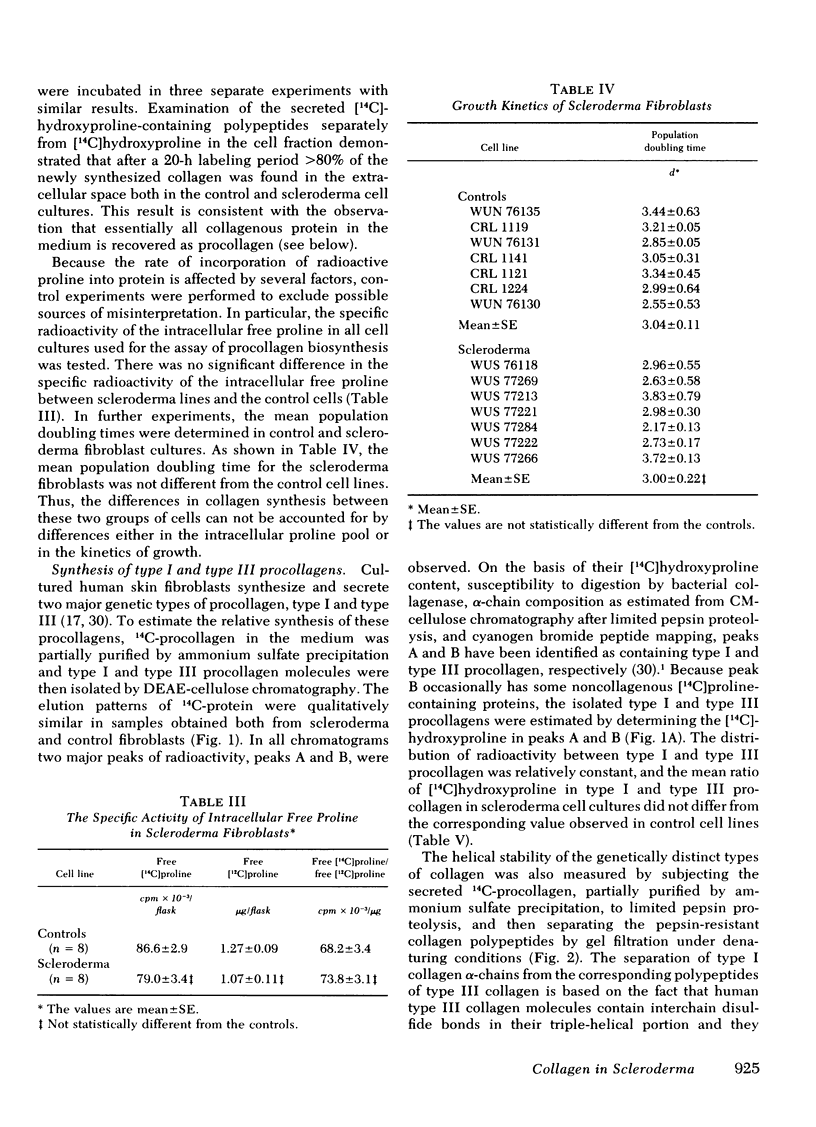
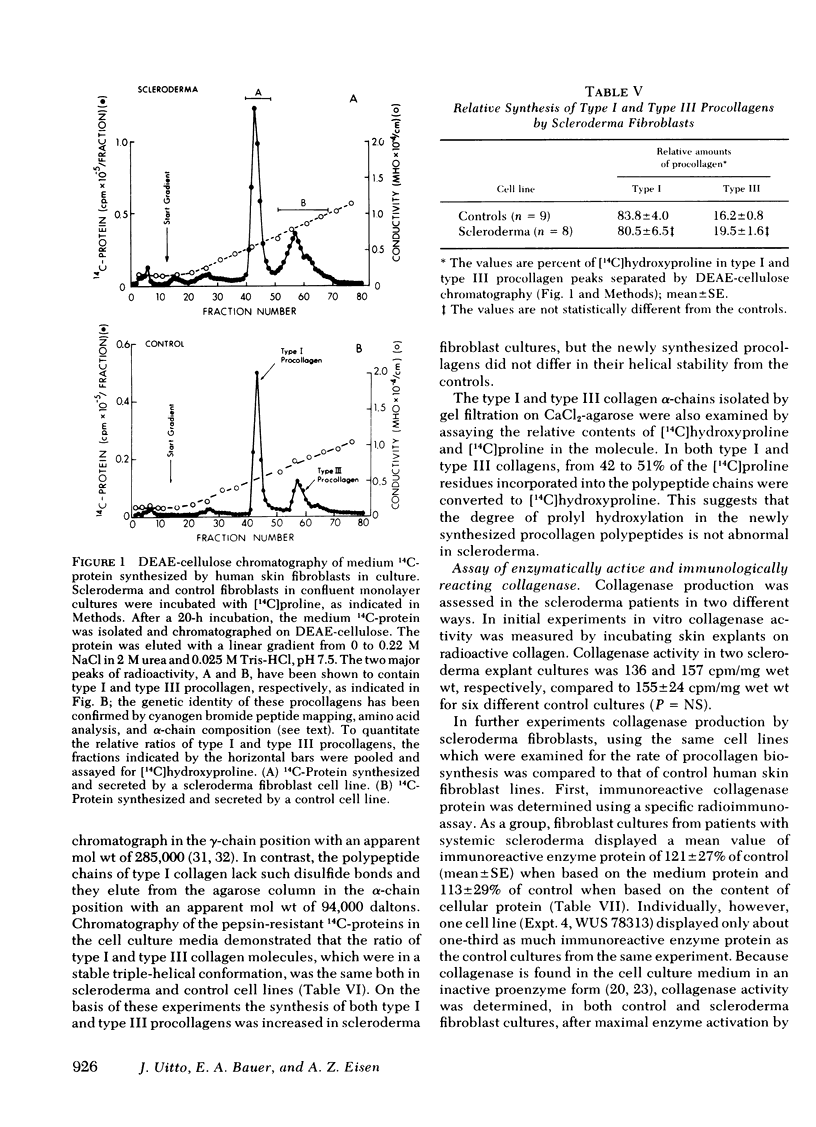
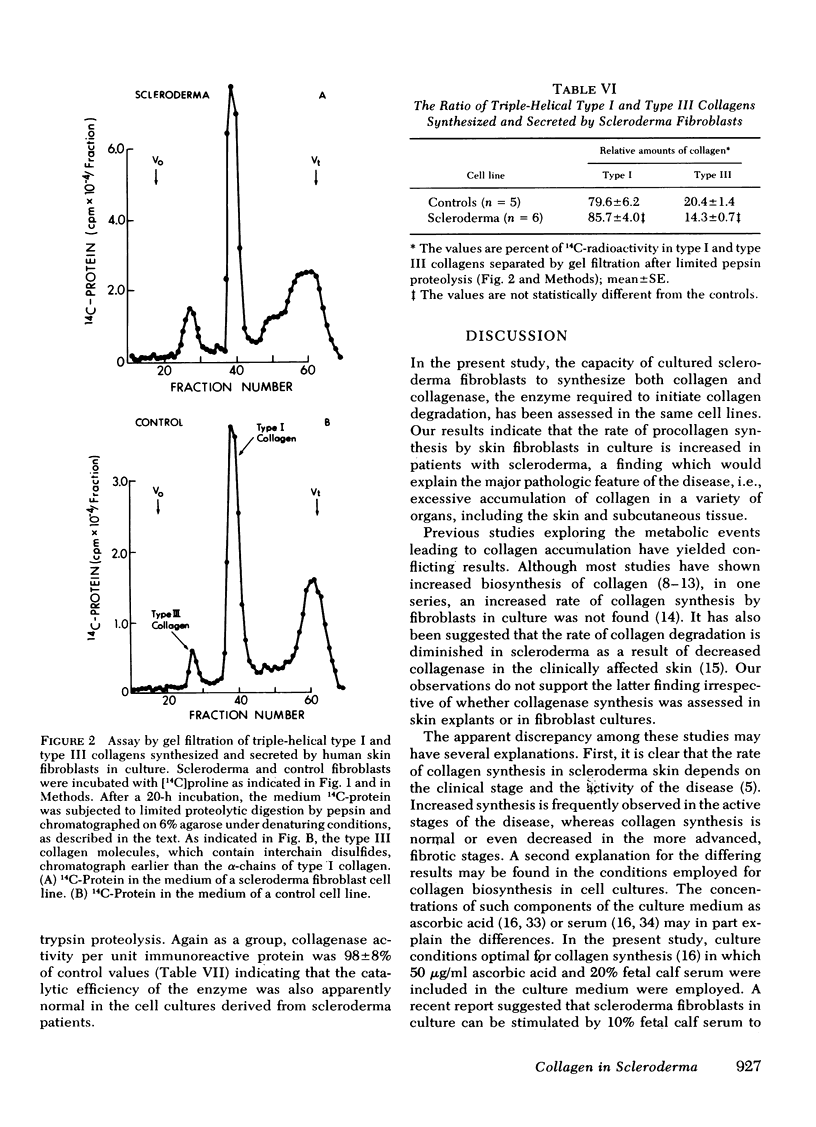
Abstract
To assess potential abnormalities in collagen metabolism in systemic scleroderma, skin fibroblast lines from patients with this disease were established and compared to control cell lines derived from healthy subjects. For studies on the biosynthesis of procollagen, the cells were incubated with [14C]proline in a medium supplemented with ascorbic acid and β-aminopropionitrile, and the synthesis of nondialyzable [14C]hydroxyproline, in relation to DNA or cell protein, was taken as an index of procollagen formation. Five of eight scleroderma fibroblast cell lines demonstrated procollagen biosynthesis rates significantly higher than the controls, and the mean rate of procollagen synthesis by scleroderma fibroblasts was about twice that of the control cells. Control experiments demonstrated that the specific activity of the intracellular free proline was not different in scleroderma and control fibroblasts, and the mean population doubling times of the scleroderma and the control fibroblast cell lines were the same. The relative synthesis of the genetically distinct procollagens was examined by isolating type I and type III procollagens from the cell culture medium using DEAE-cellulose chromatography. The ratios of type I/III procollagens in scleroderma cell lines did not differ from the controls. The helical stability of the collagenous portion of type I and type III procollagens, estimated by the resistance of 14C-collagen to limited proteolytic digestion with pepsin under nondenaturing conditions, was the same in both scleroderma and control cultures. The capacity of the cells to synthesize enzymatically active and immunologically reacting collagenase was also studied; no marked differences in these parameters could be observed. The results suggest that cultured skin fibroblasts from patients with scleroderma demonstrate a metabolic abnormality expressed as increased synthesis of type I and type III procollagens in a normal ratio. This abnormality may play a role in the excessive accumulation of collagen in the skin and other organs affected in scleroderma.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashey R. I., Jimenez S. A. Increased sensitivity of scleroderma fibroblasts in culture to stimulation of protein and collagen synthesis by serum. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1214–1222. doi: 10.1016/0006-291x(77)90985-8. [DOI] [PubMed] [Google Scholar]
- Bauer E. A., Eisen A. Z., Jeffrey J. J. Radioimmunoassay of human collagenase. Specificity of the assay and quantitative determination of in vivo and in vitro human skin collagenase. J Biol Chem. 1972 Oct 25;247(20):6679–6685. [PubMed] [Google Scholar]
- Bauer E. A., Stricklin G. P., Jeffrey J. J., Eisen A. Z. Collagenase production by human skin fibroblasts. Biochem Biophys Res Commun. 1975 May 5;64(1):232–240. doi: 10.1016/0006-291x(75)90243-0. [DOI] [PubMed] [Google Scholar]
- Brady A. H. Collagenase in scleroderma. J Clin Invest. 1975 Nov;56(5):1175–1180. doi: 10.1172/JCI108194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham R. B., Prince R. K., Rodnan G. P., Taylor F. Increased collagen accumulation in dermal fibroblast cultures from patients with progressive systemic sclerosis (scleroderma). J Lab Clin Med. 1978 Jul;92(1):5–21. [PubMed] [Google Scholar]
- Burgeson R. E., El Adli F. A., Kaitila I. I., Hollister D. W. Fetal membrane collagens: identification of two new collagen alpha chains. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2579–2583. doi: 10.1073/pnas.73.8.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E., Miller E. J. Collagen polymorphism: characterization of molecules with the chain composition (alpha 1 (3)03 in human tissues. Science. 1974 Mar;183(130):1200–1201. doi: 10.1126/science.183.4130.1200. [DOI] [PubMed] [Google Scholar]
- Eisen A. Z. Human skin collagenase: localization and distribution in normal human skin. J Invest Dermatol. 1969 May;52(5):442–448. doi: 10.1038/jid.1969.76. [DOI] [PubMed] [Google Scholar]
- Epstein E. H., Jr (Alpha1(3))3 human skin collagen. Release by pepsin digestion and preponderance in fetal life. J Biol Chem. 1974 May 25;249(10):3225–3231. [PubMed] [Google Scholar]
- Fleischmajer R., Gay S., Meigel W. N., Perlish J. S. Collagen in the cellular and fibrotic stages of scleroderma. Arthritis Rheum. 1978 May;21(4):418–428. doi: 10.1002/art.1780210404. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R. The pathophysiology of scleroderma. Int J Dermatol. 1977 Jun;16(5):310–318. doi: 10.1111/j.1365-4362.1977.tb00747.x. [DOI] [PubMed] [Google Scholar]
- Herbert C. M., Lindberg K. A., Jayson M. I., Bailey A. J. Biosynthesis and maturation of skin collagen in scleroderma, and effect of D-penicillamine. Lancet. 1974 Feb 9;1(7850):187–192. doi: 10.1016/s0140-6736(74)92494-5. [DOI] [PubMed] [Google Scholar]
- Jimenez S. A., Bashey R. I. Collagen synthesis by scleroderma fibroblasts in culture. Arthritis Rheum. 1977 Apr;20(3):902–903. doi: 10.1002/art.1780200323. [DOI] [PubMed] [Google Scholar]
- Johnson R. L., Ziff M. Lymphokine stimulation of collagen accumulation. J Clin Invest. 1976 Jul;58(1):240–252. doi: 10.1172/JCI108455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- Keiser H. R., Sjoerdsma A. Direct measurement of the rate of collagen synthesis in skin. Clin Chim Acta. 1969 Feb;23(2):341–346. doi: 10.1016/0009-8981(69)90050-3. [DOI] [PubMed] [Google Scholar]
- Keiser H. R., Stein H. D., Sjoerdsma A. Increased protocollagen proline hydroxylase activity in sclerodermatous skin. Arch Dermatol. 1971 Jul;104(1):57–60. [PubMed] [Google Scholar]
- Kondo H., Rabin B. S., Rodnan G. P. Cutaneous antigen-stimulating lymphokine production by lymphocytes of patients with progressive systemic sclerosis (scleroderma). J Clin Invest. 1976 Dec;58(6):1388–1394. doi: 10.1172/JCI108594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LeRoy E. C. Increased collagen synthesis by scleroderma skin fibroblasts in vitro: a possible defect in the regulation or activation of the scleroderma fibroblast. J Clin Invest. 1974 Oct;54(4):880–889. doi: 10.1172/JCI107827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy E. C. Connective tissue synthesis by scleroderma skin fibroblasts in cell culture. J Exp Med. 1972 Jun 1;135(6):1351–1362. doi: 10.1084/jem.135.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein J. R., Byers P. H., Smith B. D., Martin G. R. Identification of the collagenous proteins synthesized by cultured cells from human skin. Biochemistry. 1975 Apr 22;14(8):1589–1594. doi: 10.1021/bi00679a007. [DOI] [PubMed] [Google Scholar]
- Lovell C. R., Nicholls A. C., Duance V. C., Bailey A. J. Characterization of dermal collagen in systemic sclerosis. Br J Dermatol. 1979 Apr;100(4):359–369. doi: 10.1111/j.1365-2133.1979.tb01635.x. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Lapiere C. M., Gross J. Tadpole collagenase. Preparation and purification. Biochemistry. 1966 Oct;5(10):3123–3130. doi: 10.1021/bi00874a007. [DOI] [PubMed] [Google Scholar]
- Perlish J. S., Bashey R. I., Stephens R. E., Fleischmajer R. Connective tissue synthesis by cultured scleroderma fibroblasts. I. In vitro collagen synthesis by normal and scleroderma dermal fibroblasts. Arthritis Rheum. 1976 Sep-Oct;19(5):891–901. doi: 10.1002/art.1780190510. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Snyderman R., Kang A. H. The chemotactic attraction of human fibroblasts to a lymphocyte-derived factor. J Exp Med. 1976 Nov 2;144(5):1188–1203. doi: 10.1084/jem.144.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes R. K., Miller E. J. Physicochemical characterization and molecular organization of the collagen A and B chains. Biochemistry. 1978 Aug 22;17(17):3442–3448. doi: 10.1021/bi00610a003. [DOI] [PubMed] [Google Scholar]
- Stricklin G. P., Bauer E. A., Jeffrey J. J., Eisen A. Z. Human skin collagenase: isolation of precursor and active forms from both fibroblast and organ cultures. Biochemistry. 1977 Apr 19;16(8):1607–1615. doi: 10.1021/bi00627a013. [DOI] [PubMed] [Google Scholar]
- Stricklin G. P., Eisen A. Z., Bauer E. A., Jeffrey J. J. Human skin fibroblast collagenase: chemical properties of precursor and active forms. Biochemistry. 1978 Jun 13;17(12):2331–2337. doi: 10.1021/bi00605a012. [DOI] [PubMed] [Google Scholar]
- Stuart J. M., Postlethwaite A. E., Kang A. H. Evidence for cell-mediated immunity to collagen in progressive systemic sclerosis. J Lab Clin Med. 1976 Oct;88(4):601–607. [PubMed] [Google Scholar]
- TROLL W., LINDSLEY J. A photometric method for the determination of proline. J Biol Chem. 1955 Aug;215(2):655–660. [PubMed] [Google Scholar]
- Uitto J. Collagen biosynthesis in human skin. A review with emphasis on scleroderma. Ann Clin Res. 1971 Oct;3(5):250–258. [PubMed] [Google Scholar]
- Uitto J. Collagen polymorphism: isolation and partial characterization of alpha 1(I)-trimer molecules in normal human skin. Arch Biochem Biophys. 1979 Feb;192(2):371–379. doi: 10.1016/0003-9861(79)90105-x. [DOI] [PubMed] [Google Scholar]
- Uitto J., Halme J., Hannuksela M., Peltokallio P., Kivirikko K. I. Protocollagen proline hydroxylase activity in the skin of normal human subjects and of patients with scleroderma. Scand J Clin Lab Invest. 1969 May;23(3):241–247. doi: 10.3109/00365516909077656. [DOI] [PubMed] [Google Scholar]
- Uitto J., Helin P., Rasmussen O., Lorenzen I. Skin collagen in patients with scleroderma: biosynthesis and maturation in vitro, and the effect of D-penicillamine. Ann Clin Res. 1970 Sep;2(3):228–234. [PubMed] [Google Scholar]
- Uitto J., Lichtenstein J. R., Bauer E. A. Characterization of procollagen synthesized by matrix-free cells isolated from chick embryo tendons. Biochemistry. 1976 Nov 2;15(22):4935–4942. doi: 10.1021/bi00667a029. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B. Lymphocyte-mediated activation of fibroblast proliferation and collagen production. J Immunol. 1978 Sep;121(3):942–946. [PubMed] [Google Scholar]
- Winkelmann R. K. Pathogenesis and staging of scleroderma. Acta Derm Venereol. 1976;56(2):83–92. [PubMed] [Google Scholar]



