Abstract
Myostatin is involved in an inhibitor of muscular growth and differentiation. Myoblasts derived from double-muscled Japanese shorthorn cattle (DM myoblasts) with absence of functional myostatin had higher abilities to proliferate and differentiate than myoblasts derived from normal-muscled cattle (NM myoblasts). In DM myoblasts, mRNA expressions of fetal myosin heavy chain (MyHC) in growth medium and of fast 2a and 2x MyHC in fusion medium were significantly greater than that in NM myoblasts. No significant difference existed in expressions of embryonic and slow MyHC mRNA between DM and NM myoblasts. The expression of MyoD mRNA was suppressed in myoblasts by administration of myostatin. Two cloned DM myoblast strains (DMc) were established. Addition of myostatin for DMc resulted in less myotube formation and suppression of mRNA expression of fast 2x MyHC. These findings suggest that the endogenous myostatin preferentially down-regulates the expression of the fast 2x MyHC and participates in differentiation of myofiber types during early bovine myogenesis.
Keywords: myostatin, double-muscled cattle, myoblast, myosin heavy chain, skeletal muscle
Introduction
Myostatin, a member of the transforming growth factor β superfamily, has been proposed to be a negative regulator of skeletal muscle mass.1)–3) Myostatin is present during the early development of skeletal muscle and is continuously expressed in adult skeletal muscle.2),3) Myostatin null mice display an increase of muscle mass due to both hypertrophy and hyperplasia of myofibers.1)
In some cattle breeds, mutations of the myostatin gene are responsible for the double-muscled (DM) phenotype.2)–4) The muscle mass of DM cattle is about 20% more than that of normal-muscled cattle.5) DM phenotype in Japanese shorthorn (DM-JS) cattle was found in 1998,6) and coincided with the genetic characterization of Belgian Blue cattle that have an 11 bp deletion in the third exon of the myostatin gene, resulting in a truncated myostatin protein.3),4)
Previous reports have shown that skeletal muscles of DM cattle has a different composition of myofiber types from those of NM cattle in the fetus7) and postnatal periods.8) In DM phenotype after birth of cattle, there is an increase in the size and number of type IIB myofibers.8),9) Myostatin deficiency in its knockout mice leads to an increase in fast glycolytic (type IIB) myofibers.10) Therefore, myostatin seems to influence differentiation and development of myofiber types. However, it still remains unclear how myostatin regulates expression of MyHC isoforms in muscular development of cattle.
The present study is to precisely elucidate how myostatin affects the early myogenesis using established DM myoblast clones derived from DM-JS cattle. This novel approach allowed us to conclude whether myostatin participates in the expression of MyHC isoforms, especially fast type MyHC in bovine myoblasts.
Materials and methods
Animals
Double-muscled (n = 3) and normal-muscled (n = 3) Japanese shorthorn (DM-JS and NM-JS) cattle were used at 8 to 16 months-old. The genetic basis of the DM-JS cattle arises from the mutation of the myostatin gene, which is an 11 bp deletion as similar to the Belgian Blue cattle.6) The cattle were handled in accordance with the Guidelines of the Administrative Panel on Laboratory Animal Care of Tohoku University.
Myoblast primary culture
Induction of muscular regenerating regions of NM-JS and DM-JS cattle were carried out by direct apply of dry ice onto the muscle.11) Myoblasts were obtained from the regenerated M. longissimus thoracis of NM-JS and DM-JS cattle by enzyme dissociation, and were enriched by a differential adhesion method with 94% or more purity.11)–14) Purified myoblasts were suspended in a growth medium: a 1:4 mixture of Dulbecco’s medium (Sigma, St. Louis, MO, USA) and M-199 medium (Sigma) supplemented with 10% fetal bovine serum (FBS: Sigma). All cell culture experiments were conducted in collagen-coated flasks (Sumitomo Bakelite Co. Ltd. Tokyo, Japan) and maintained at 37 °C in a 5% CO2 atmosphere.
Analysis for proliferation and differentiation of myoblasts
Myoblasts were seeded in collagen-coated 6-well plates (Sumitomo Bakelite) at a concentration of 8.75 × 103 cells/cm2 and cultured for 5 days in the growth medium. The cells were removed by 0.04% trypsin in PBS every day after the cultivation and the numbers were counted by the trypan blue dye exclusion method.11) Doubling time (DT) was calculated according the formula: (DT) = (t − t0) log 2/(log N − log N0), where t − t0 is the period of time for the cell growth, and N − N0 is the increased number of cells.
Myoblast differentiation was determined by myotube formation, in which the myotubes were evaluated as the cells with three or more nuclei. After the myoblasts were cultured in the growth medium for 24 h, the medium was replaced with a fusion medium decreased to 2% FBS of growth medium. Thereafter myoblasts were cultured in the fusion medium for 72 h. Myotubes were visualized by Giemsa staining.
Establishment of cloned DM-JS myoblast lines
Cell cloning was carried out by a limiting dilution-culture method. The sub-confluent primary cultures of DM-JS myoblasts were removed by trypsin and then the cell number was adjusted to 20 cells/ml in growth medium. An aliquot of 100 μl was dropped into each well of the 96 well plates (Sumitomo Bakelite). On day 5, the wells were microscopically checked for cell growth and the wells that certainly contained a growing single colony were selected for the successive cultures. The passage was carried out when the cell growth reached approximately confluence. Two clonal myoblast lines (DMc myoblasts) were established based on the high myotube formation in fusion medium.
Analysis for gene expressions in skeletal muscle cells
Genomic DNA was isolated from the M. longissimus thoracis and myoblasts of NM-JS and DM-JS cattle using DNAzol regent (Invitrogen, Carlsbad, CA, USA). For identification of DM genotype as an 11 bp deletion (nt821(del11)) in the myostatin gene, two primer sets were designed to allow certain findings for the nt821 mutation from genomic DNA (Table 1). One of the primer set based on Suzuki et al.6) containing the mutation site in DM-JS cattle exhibits the PCR product of 11 bp shorter than that of NM-JS cattle. In the other primer set, the forward primer was designed at the deletion site of myostatin gene.
Table 1.
Primer sequences and cycling conditions for genomic PCR and semi-quantitative RT-PCR
| Primer | Sequence | Fragment Size (bp) | Annealing Temperature (°C) | Cycle number (liniear phase) |
|---|---|---|---|---|
| Genomic Myostatin 1a | 5′-TCTAGGAGAGATTTTGGGCTT 5′-GATGGGTATGAGGATACTTTTGC |
NM cattle: 187 DM cattle: 176 |
60 | 35 |
| Genomic Myostatin 2 | 5′-GTGATGAACACTCCACAG 5′-GGGGAAGACCTTCCATGTTT |
361 | 64 | 25 |
| Embryonic MyHC | 5′-AACCTGGAGCAGACGGTGAAGGAC 5′-AGAGCAAAGACCCGAGACTTCACCT |
370 | 60 | 30 |
| Fetal MyHC | 5′-AACCTGGAGCAGACGGTGAAGGAC 5′-ACACACCTGCCTGGTGCTGCCAAGG |
414 | 60 | 35 |
| Fast2a MyHCb | 5′-CACTTGCTAACAAGGACCTCTGAGTTCA 5′-ATCCAGGCTGCGTAACGCTCTTTGAGGTTGTA |
375 | 60 | 35 |
| Fast2x-MyHCb | 5′-CTTTCCTCATAAAGCTTCAAGTTCTGACC 5′-ATCCAGGCTGCGTAACGCTCTTTGAGGTTGTA |
433 | 57 | 35 |
| Slow-MyHCb | 5′-TGCTGCTCTCAGGCCCCTGCCACCTT 5′-ACACACCTGCCTGGTGCTGCCAAGG |
411 | 56 | 35 |
| MyoD | 5′-TCAAACGCTGCACGTCTAGCAAC 5′-TGTAGTCCATCATGCCGTCGGAA |
246 | 66 | 35 |
| myogenin | 5′-AAGCGCAGACTCAAGAAGGTGAA 5′-GCAGGCGCTCTATGTACTGGATG |
127 | 66 | 35 |
| G3PDHc | 5′-ACCACAGTCCATGCCATCAC 5′-TCCACCACCCTGTTGCTGTA |
450 | 55 | 20 |
For mRNA expression of MyoD and myogenin, the culture medium was replaced to fusion medium containing various doses of recombinant mouse myostatin (rm-MSTN; R & D systems, Minneapolis, MN, USA), when the myoblast cultures were sub-confluent. For mRNA expression of MyHC isoforms, the culture medium was replaced to growth medium containing 100 ng/ml recombinant human HGF (Peprotec EC, London, UK) or fusion medium containing 100 ng/ml rm-MSTN, when the primary myoblasts and DMc myoblasts were sub-confluent. After 72 h cell culture, total RNA was extracted using TRIzol reagent (Invitrogen), and then RT-PCR method was performed using the SuperScript III (Invitrogen) protocol and the specific primers listed in Table 1. Results of RT-PCR were semi-quantitatively analyzed by a computerized image densitometric method.15) Glycerol-3-phosphate dehydrogenase (G3PDH) specific primers were used as internal control.
Statistical analysis
One-way ANOVA and Tukey’s multiple comparison methods were used for statistical analysis. Significant differences were accepted at P < 0.05.
Results
Confirmation of DM phenotype in the myoblasts from DM-JS cattle
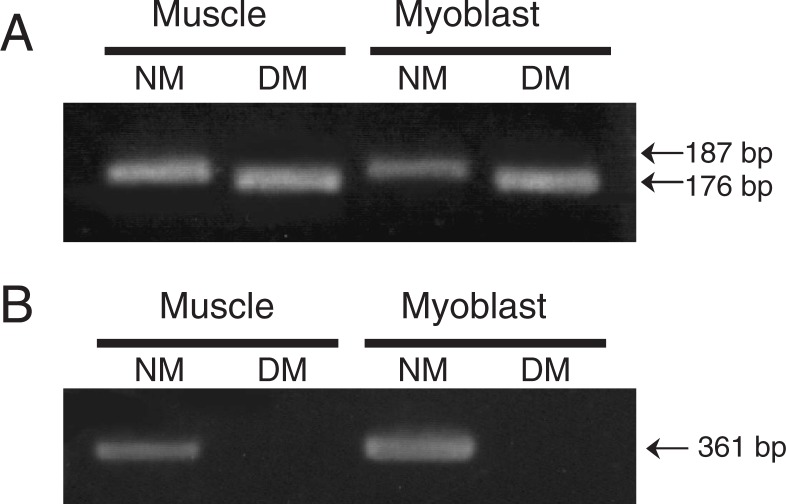
By the primer set responsible for the 11 bp deletion of myostatin gene, the PCR products of the M. longissimus thoracis and myoblasts in DM-JS cattle were predictably 11 bp less than those in the NM breed (Fig. 1A). Furthermore, the primer set that designed forward primer at the 11 bp deletion sites of myostatin gene did not amplify any myostatin PCR products in the DM-JS cattle (Fig. 1B). The 11 bp deletion of the myostatin gene in DMc myoblasts was also confirmed (data not shown).
Fig. 1.
Detection of DM genotype of cattle.
Genomic DNA was obtained from the M. longissimus thoracis and myoblasts of DM- and NM-JS cattle. The PCR products of myoblasts and muscle from DM-JS cattle are 11 bp shorter than those of NM cattle (A), or absent (B).
Myoblast proliferation and myotube formation of DM and NM myoblasts
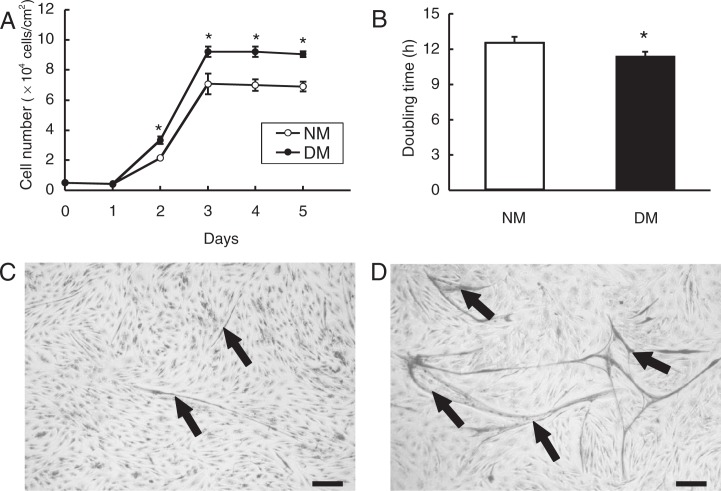
In growth medium, the number of DM myoblasts was significantly larger than that of NM myoblasts during 2 to 5 days after seeding (Fig. 2A). The doubling time of DM myoblasts (11.4 h ± 0.44) was significantly shorter than that of NM myoblasts (12.6 h ± 0.32) (Fig. 2B). When growth medium was replaced to fusion medium, myotube formation from myoblasts was induced, in which DM myoblasts formed more and larger myotubes than NM myoblasts did (Fig. 2C, D). Thus, myostatin deficiency in DM myoblast cultures substantially enhanced the proliferation of myoblasts and myotube formation.
Fig. 2.
Increased proliferation and myotube formation of DM myoblasts.
The cell number of DM and NM myoblasts was measured by the trypan blue exclusion method at indicated days (A). The data are shown as mean ± S.D. (n = 3) and the doubling time of myoblasts was assessed during exponential cell growth (B). Myotube formation in NM (C) and DM (D) cultures were visualized by Giemsa staining. *: P < 0.05. Arrows: myotubes. Bar = 100 μm.
Expression of MyHC isoform mRNA in DM myoblasts
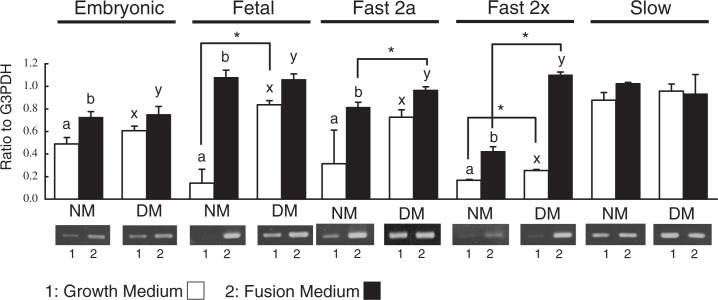
The mRNA expressions of MyHC isoforms in the primary cultures of DM and NM myoblasts were showed in Fig. 3. Except for slow type, the mRNA expressions of MyHC isoforms were significantly higher in fusion medium than in growth medium. DM myoblasts exhibited significantly higher expressions of fetal and fast 2x MyHC mRNA than NM myoblasts did in growth medium. Significantly larger expressions of fast 2a and 2x MyHC mRNA were existed in DM myoblasts cultured in fusion medium. In contrast, there was no difference in the mRNA expression of slow MyHC in either NM or DM cultures of both growth and fusion medium.
Fig. 3.
mRNA expression of MyHC isoforms in DM myoblasts.
DM and NM myoblasts were cultured in growth medium containing 10% FBS and in fusion medium containing 2% FBS for 72 h. The mRNA expressions of MyHC isoforms, embryonic, fetal, fast 2a, fast 2x and slow MyHC isoforms, were analyzed by semi-quantified densitometric method. Significant differences (P < 0.05) are indicated with asterisks between genotypes and different letters within genotypes. The data are shown as mean ± S.D. (n = 3).
Myostatin effects on myotube formation and mRNA expression of MyoD, myogenin in DM myoblasts
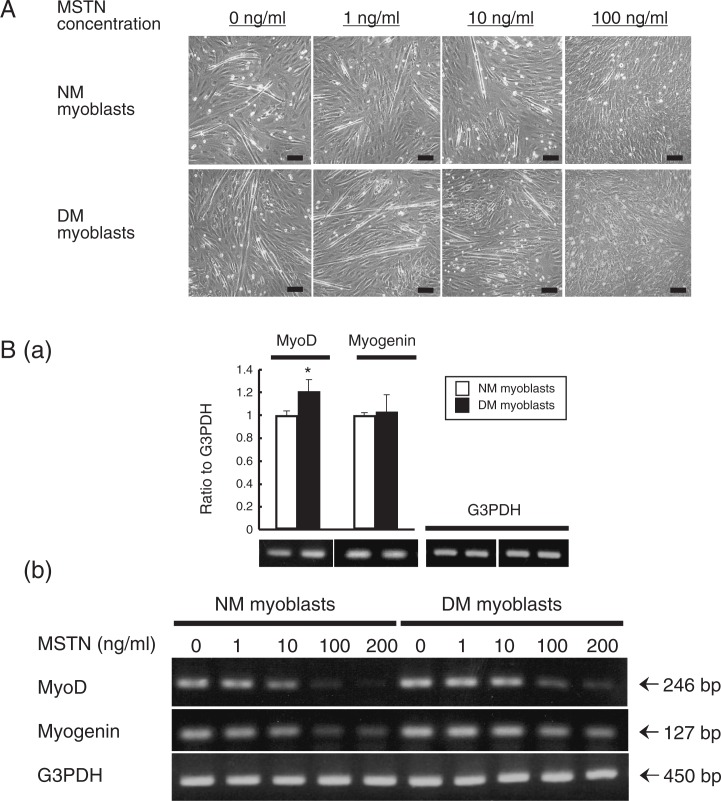
Myostatin reduced the myotube formation of NM and DM myoblasts in a dose-dependent manner, and the myotube formation was completely suppressed by treatment with 100 ng/ml rm-MSTN (Fig. 4A). In DM myoblasts, the mRNA expression of MyoD was significantly higher than that in NM myoblasts (Fig. 4B-a). The expression of MyoD was extremely suppressed in both NM and DM myoblast cultures when 100 to 200 ng/ml rm-MSTN were added to the fusion medium (Fig. 4B-b). On the other hand, the suppression for myogenin mRNA expression by myostatin treatment was greater in the NM myoblast cultures than that in the DM myoblast cultures (Fig. 4B-b).
Fig. 4.
Suppression of myotube formation and mRNA expressions of MyoD and myogenin by myostatin in DM and NM myoblasts.
DM and NM myoblasts after 72 h culture with fusion medium containing 1, 10 and 100 ng/ml myostatin are shown (A). Expression of MyoD mRNA of DM myoblasts in fusion medium is significantly higher than that of NM myoblasts (B-a), and the expression is suppressed by 100 ng/ml myostatin (B-b). Expressions of mRNA were analyzeded by a semi-quantified densitometric method in triplicate for each cattle. The data are shown as mean ± S.D. (n = 3). *: P < 0.05. Bar = 100 μm.
Myostatin effects on MyHC isoform expression in DMc myoblast cultures
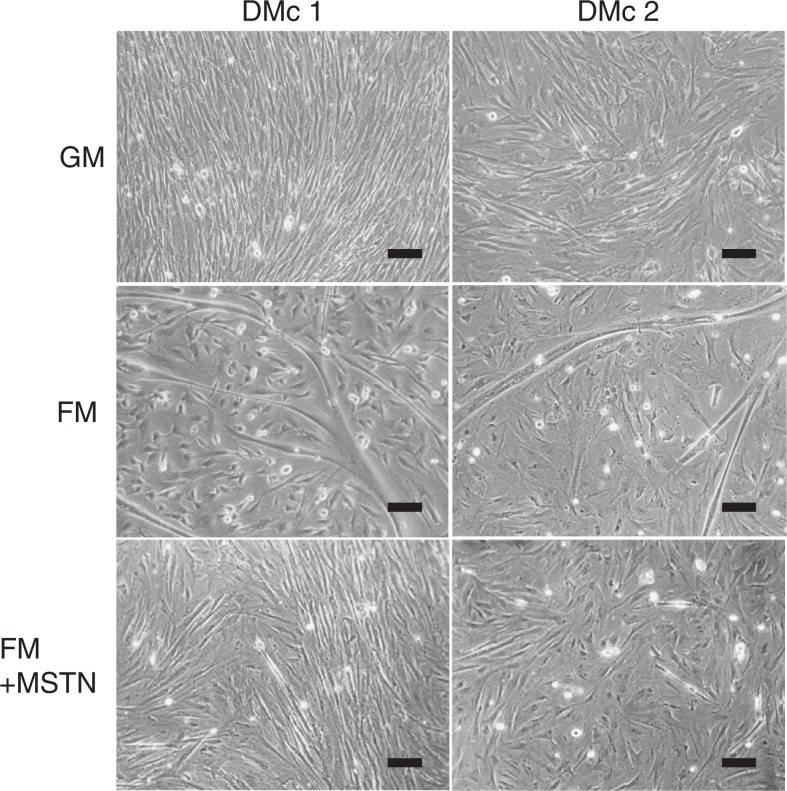
Two strains of DM myoblast clones (DMc1 and DMc2) without active form of myostatin were established from DM myoblast primary cultures. Both clones exhibited high proliferation in growth medium containing 100 ng/ml recombinant human HGF (Fig. 5; top row) and large myotube formation in fusion medium (Fig. 5; middle row). Myotube formation of DMc myoblast was suppressed effectively by an addition of 100 ng/ml rm-MSTN for fusion medium (Fig. 5; bottom row).
Fig. 5.
Inhibition of myotube formation in DMc myoblast cultures by myostatin.
Two myoblasts clones (DMc1 and 2) from the DM-JS muscle were cultured in growth medium for 24 h. Then the DMc cultures were replaced with three experiments for 72 h in growth medium containing 100 ng/ml recombinant human HGF (GM), fusion medium (FM), and FM with 100 ng/ml rm-MSTN. Bar = 100 μm.
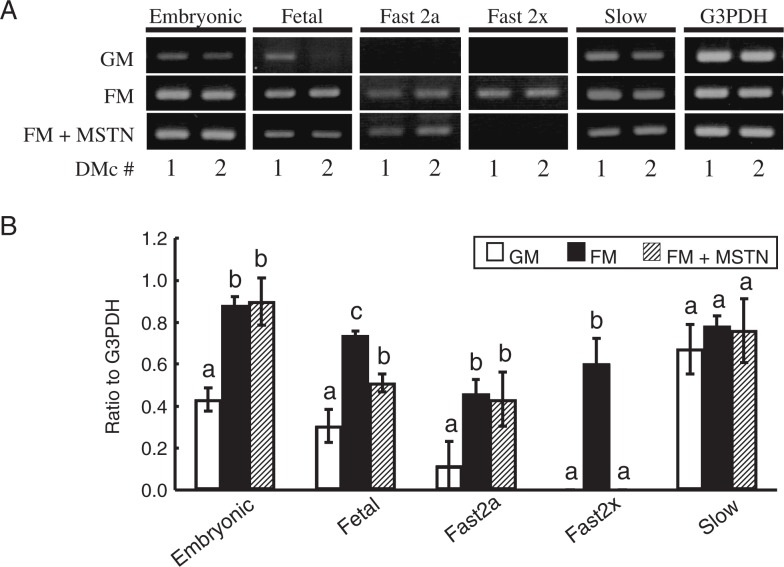
Figure 6A shows the differential MyHC mRNA expressions in DMcs. The results of RT-PCR from triplicate cultures of DMc1 were semi-quantitatively analyzed using a densitometric method (Fig. 6B). The data were confirmed by the repeated experiments. No mRNA expression of fast 2x MyHC was detected in growth medium in both clones. In fusion medium, mRNA expressions of embryonic, fetal and fast 2a MyHC were augmented and expression of fast 2x MyHC mRNA was newly expressed. When 100 ng/ml of rm-MSTN was put into the fusion medium, expression of fetal MyHC mRNA was significantly suppressed and the expression of fast 2x MyHC was almost disappeared.
Fig. 6.
Myostatin effects on mRNA expression of MyHC isoforms in DMc myoblasts.
After 72 h culture in growth (GM), fusion (FM) or FM+myostatin (FM+MSTN) medium, mRNA expressions of embryonic, fetal, fast 2a, fast 2x and slow MyHC in DMc myoblasts were indicated by RT-PCR (A). In DMc1, mRNA expressions were analyzed by a densitometric method in triplicate (B). The data are shown as mean ± S.D. (n = 3). a–c: P < 0.05.
Discussion
Previous studies have reported the negative influence of myostatin on myoblast proliferation16)–18) and myoblast differentiation.19),20) The present study confirmed the 11 bp deletion of the myostatin gene in the DM-JS cattle, and detailed the effects of myostatin on myoblast proliferation, differentiation and expression of the MyHC isoform using DM and DMc myoblasts. Since DM and DMc myoblasts produce no active-form of myostatin, these cells allow practical investigation of the direct effect of myostatin on myogenesis without the influence of endogenous myostatin. The results first demonstrate that myostatin negatively regulates MyHC isoform expression in myoblasts derived from the M. longissimus thoracis of cattle.
DM myoblasts showed significantly higher proliferation than NM myoblasts. Thomas et al.18) indicated that double-muscled myoblasts derived from Belgian blue cattle had higher cell growth than normal-muscled myoblasts. Also, myoblasts derived from myostatin KO mice proliferate faster than wild-type myoblasts do.21) Under inhibition of myogenic differentiation by BrdU treatment, proliferating myoblasts upregulated expression of myostatin.22) Chicken satellite cells faintly expressed myostatin at start of culture but the expression became to be strong after 48 h culture.23) Joulia et al.16) have reported that the over-expression of myostatin inhibits the proliferation of C2C12 cells through myogenin, and p21 and Rb related systems. Our findings confirmed that myostatin indeed inhibited the proliferation of myoblasts. On the other hand, myostatin over-expression of C2C12 cells inhibits their myotube formation.20) Exogenous myostatin represses the levels of MyoD, Myf5 and myogenin and thereby induce the inhibition of myogenic differentiation.19) In the present study, DM myoblasts constantly formed the increased and large-sized myotubes. These data suggest that endogenous myostatin may induce a substantial blocking effect on myoblast differentiation in cattle.
During myogenic differentiation in fusion medium, mRNA expressions of fast 2a and 2x MyHC in DM myoblasts were higher than that in NM myoblasts, whereas there was no difference in mRNA expression of slow MyHC. The exposure of cultures to myostatin inhibited myotube formation, and the mRNA expression of MyoD and myogenin. These findings suggest that myostatin may down-regulate MyHC isoforms preferentially during early differentiation of myogenesis in cattle. In our cloned DM-JS myoblast cell lines, mRNA expressions of fetal and fast 2x MyHC were significantly down-regulated by exogenous myostatin. These data clearly demonstrate that myostatin negatively regulates expression of MyHC isoforms, especially fast 2x MyHC, during early myogenesis.
Myofibers are histochemically classified into type I (slow oxidative), type IIA (fast-twitch oxidative glycolytic) and type IIB (fast-twitch glycolytic) myofibers, which are based on the ATPase reactivity and activity of SDH or NADH dehydrogenase.24)–26) Myofiber types mainly correspond with content of MyHC isoforms. In porcine muscles, it has been shown that type I and IIA myofibers contain the mRNA of slow and fast 2a MyHC isoforms, respectively, whereas type IIB myofibers were heterogeneous myofibers composed of the mRNA of fast 2x, hybrid fast 2x/2b, and fast 2b MyHC isoforms.27) Recently, Chikuni et al.28) have reported that no expression of fast 2b MyHC in adult bovine skeletal muscles. In Belgian blue cattle, myofiber types consisted in slow, fast 2a and 2x MyHC isoforms.29)
A higher proliferation of secondary myoblasts has been observed in bovine DM fetal muscle.30) Myostatin in vivo has been reported to be associated exclusively with type IIB myofiber in the skeletal muscle of homozygouse Belgian blue bulls, because the population of type IIB myofibers is increased in them.9) Also, by differential proteomic analysis, it has been reported that homozygote Belgian blue bulls show an increased fast muscle phenotype in comparison with wild-type bulls.31) Other investigators have reported that the soleus muscle of myostatin knockout mice displays a larger proportion of fast type myofibers compared with wild-type littermates.10) These experiments suggest that myostatin acts as a regulator in the development of type IIB (fast-twitch glycolytic) myofibers that mainly consist of fast 2x MyHC.
Seward et al.32) and Wheeler et al.33) have reported that MyoD positively regulates the expression of fast MyHC isoforms. In the present study, mRNA expression of MyoD in DM myoblast cultures was strongly inhibited by myostatin. However, the mRNA level of myogenin was less affected by myostatin. MyoD expression is increased in DM cattle fetuses, whereas no up-regulation of myogenin is found in this case.34) These findings suggest that MyoD is probably a major target of myostatin and myostatin regulates MyHC isoforms thorough the depressed MyoD gene that is induced by myostatin.
Taken together, our results suggest that myostatin preferentially down-regulates the expression of fast 2x MyHC isoform in cattle, although the intimate mechanisms accounting for the hypothesis remain to be further investigated.
Acknowledgments
We thank Ms. Yu Miura for technical assistance. This study was supported by a Grant-in-Aid for Scientific Research (A) (No. 17208024) from the Ministry of Education, Culture, Sports, Science and Technology and a Research project for utilizing advanced technologies in agriculture, forestry and fisheries (No. 1523) from the Ministry of Agriculture, Forestry and Fisheries, Japan.
References
- 1).McPherron, A.C., Lawler, A.M. and Lee, S.J. (1997) Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 387, 83–90 [DOI] [PubMed] [Google Scholar]
- 2).McPherron, A.C. and Lee, S.J. (1997) Double muscling in cattle due to mutations in the myostatin gene. Proc. Natl. Acad. Sci. USA 94, 12457–12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Kambadur, R., Sharma, M., Smith, T.P. and Bass, J.J. (1997) Mutations in myostatin (GDF8) in double-muscled Belgian blue and Piedmontese cattle. Genome Res. 7, 910–916 [DOI] [PubMed] [Google Scholar]
- 4).Grobet, L., Martin, L.J., Poncelet, D., Pirottin, D., Brouwers, B., Riquet, J., Schoeberlein, A., Dunner, S.,Ménissier, F., Massabandaet al. (1997) A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat. Genet. 17, 71–74 [DOI] [PubMed] [Google Scholar]
- 5).Charlier, C., Coppieters, W., Farnir, F., Grobet, L., Leroy, P.L., Michaux, C., Mni, M., Schwers, A., Vanmanshoven, P., Hanset, R.et al. (1995) The mh gene causing double-muscling in cattle maps to bovine chromosome 2. Mamm. Genome 6, 788–792 [DOI] [PubMed] [Google Scholar]
- 6).Suzuki, T., Ohtawara, K., Noguchi, T., Sugimoto, Y., Tanaka, S., Komatsu, S. and Yoshikawa, Y. (2002) Identification of deletion mutation in myostatin gene associated with double muscling in Japanese Shorthorn cattle breed and its effect on meat productive traits. Tohoku J. Anim. Sci. Technol. 52, 11–17(in Japanese with English abstract). [Google Scholar]
- 7).Ashmore, C.R., Parker, W., Stokes, H. and Doerr, L. (1974) Comparative aspects of muscle fiber types in fetuses of the normal and “double-muscled” cattle. Growth 38, 501–506 [PubMed] [Google Scholar]
- 8).Holmes, J.H. and Ashmore, C.R. (1972) A histochemical study of development of muscle fiber type and size in normal and “double muscled” cattle. Growth 36, 351–372 [PubMed] [Google Scholar]
- 9).Wegner, J., Albrecht, E., Fiedler, I., Teuscher, F., Papstein, H.J. and Ender, K. (2000) Growth- and breed-related changes of muscle fiber characteristics in cattle. J. Anim. Sci. 78, 1485–1496 [DOI] [PubMed] [Google Scholar]
- 10).Girgenrath, S., Song, K. and Whittemore, L.A. (2005) Loss of myostatin expression alters fiber-type distribution and expression of myosin heavy chain isoforms in slow- and fast-type skeletal muscle. Muscle Nerve 31, 34–40 [DOI] [PubMed] [Google Scholar]
- 11).Hayashi, S., Aso, H., Watanabe, K., Nara, H., Rose, M.T., Ohwada, S. and Yamaguchi, T. (2004) Sequence of IGF-I, IGF-II, and HGF expression in regenerating skeletal muscle. Histochem. Cell Biol. 122, 427–434 [DOI] [PubMed] [Google Scholar]
- 12).Yaffe, D. (1968) Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc. Natl. Acad. Sci. USA 61, 477–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Yamaguchi, T., Kitazawa, H. and Hoshino, T. (1991) Anim. Sci. Technol. (Jpn.) 62, 620–627 [Google Scholar]
- 14).Nara, H., Imanaka, T. and Yamaguchi, T. (2000) Enhanced expression of acetylcholinesterase activity in bovine satellite cells treated with insulin-like growth factor. Anim. Sci. J. 71, 63–70 [Google Scholar]
- 15).Muroya, S., Nakajima, I. and Chikuni, K. (2002) Related expression of MyoD and Myf5 with myosin heavy chain isoform types in bovine adult skeletal muscles. Zool. Sci. 19, 755–761 [DOI] [PubMed] [Google Scholar]
- 16).Joulia, D., Bernardi, H., Garandel, V., Rabenoelina, F., Vernus, B. and Cabello, G. (2003) Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin. Exp. Cell Res. 286, 263–275 [DOI] [PubMed] [Google Scholar]
- 17).Taylor, W.E., Bhasin, S., Artaza, J., Byhower, F., Azam, M., Willard, D.H.Jr., Kull, F.C.Jr. and Gonzalez-Cadavid, N. (2001) Myostatin inhibits cell proliferation and protein synthesis in C2C12 muscle cells. Am. J. Physiol. Endocrinol. Metab. 280, E221–228 [DOI] [PubMed] [Google Scholar]
- 18).Thomas, M., Langley, B., Berry, C., Sharma, M., Kirk, S., Bass, J. and Kambadur, R. (2000) Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J. Biol. Chem. 275, 40235–40243 [DOI] [PubMed] [Google Scholar]
- 19).Langley, B., Thomas, M., Bishop, A., Sharma, M., Gilmour, S. and Kambadur, R. (2002) Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J. Biol. Chem. 277, 49831–49840 [DOI] [PubMed] [Google Scholar]
- 20).Ríos, R., Carneiro, I., Arce, V.M. and Devesa, J. (2002) Myostatin is an inhibitor of myogenic differentiation. Am. J. Physiol. Cell Physiol. 282, C993–999 [DOI] [PubMed] [Google Scholar]
- 21).McCroskery, S., Thomas, M., Maxwell, L., Sharma, M. and Kambadur, R. (2003) Myostatin negatively regulates satellite cell activation and self-renewal. J. Cell Biol. 162, 1135–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Deveaux, V., Picard, B., Bouley, J. and Cassar-Malek, I. (2003) Location of myostatin expression during bovine myogenesis in vivo and in vitro. Reprod. Nutr. Dev. 43, 527–542 [DOI] [PubMed] [Google Scholar]
- 23).Kocamis, H., McFarland, D.C. and Killefer, J. (2001) Temporal expression of growth factor genes during myogenesis of satellite cells derived from the biceps femoris and pectoralis major muscles of the chicken. J. Cell Physiol. 186, 146–152 [DOI] [PubMed] [Google Scholar]
- 24).Peter, J.B., Barnard, R.J., Edgerton, V.R., Gillespie, C.A. and Stempel, K.E. (1972) Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochem. 11, 2627–2633 [DOI] [PubMed] [Google Scholar]
- 25).Suzuki, A. and Tamate, H. (1988) Distribution of myofiber types in the hip and thigh musculature of sheep. Anat. Rec. 221, 494–502 [DOI] [PubMed] [Google Scholar]
- 26).Picard, B., Lefaucheur, L., Berri, C. and Duclos, M.J. (2002) Muscle fibre ontogenesis in farm animal species. Reprod. Nutr. Dev. 42, 415–431 [DOI] [PubMed] [Google Scholar]
- 27).Lefaucheur, L., Hoffman, R.K., Gerrard, D.E., Okamura, C.S., Rubinstein, N. and Kelly, A. (1998) Evidence for three adult fast myosin heavy chain isoforms in type II skeletal muscle fibers in pigs. J. Anim. Sci. 76, 1584–1593 [DOI] [PubMed] [Google Scholar]
- 28).Chikuni, K., Muroya, S. and Nakajima, I. (2004) Absence of the functional myosin heavy chain 2b isoform in equine skeletal muscles. Zool. Sci. 21, 589–596 [DOI] [PubMed] [Google Scholar]
- 29).Tanabe, R., Muroya, S. and Chikuni, K. (1998) Sequencing of the 2a, 2x, and slow isoforms of the bovine myosin heavy chain and the different expression among muscles. Mamm. Genome 9, 1056–1058 [DOI] [PubMed] [Google Scholar]
- 30).Deveaux, V., Cassar-Malek, I. and Picard, B. (2001) Comparison of contractile characteristics of muscle from Holstein and double-muscled Belgian blue foetuses. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 131, 21–29 [DOI] [PubMed] [Google Scholar]
- 31).Bouley, J., Meunier, B., Chambon, C., De Smet, S., Hocquette. J.F. and Picard, B. (2005) Proteomic analysis of bovine skeletal muscle hypertrophy. Proteomics 5, 490–500 [DOI] [PubMed] [Google Scholar]
- 32).Seward, D.J., Haney, J.C., Rudnicki, M.A. and Swoap, S.J. (2001) bHLH transcription factor MyoD affects myosin heavy chain expression pattern in a muscle-specific fashion. Am. J. Physiol. Cell Physiol. 280, C408–413 [DOI] [PubMed] [Google Scholar]
- 33).Wheeler, M.T., Snyder, E.C., Patterson, M.N. and Swoap, S.J. (1999) An e-box within the MHC IIB gene is bound by MyoD and is required for gene expression in fast muscle. Am. J. Physiol. Cell Physiol. 276, C1069–1078 [DOI] [PubMed] [Google Scholar]
- 34).Oldham, J.M., Martyn, J.A., Sharma, M., Jeanplong, F., Kambadur, R. and Bass, J.J. (2001) Molecular expression of myostatin and MyoD is greater in double-muscled than normal-muscled cattle fetuses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R1488–1493 [DOI] [PubMed] [Google Scholar]