Abstract
Background
Short-term elevations in fine particulate matter air pollution (PM2.5) are associated with increased risk of acute cerebrovascular events. Evidence from the peripheral circulation suggests that vascular dysfunction may be a central mechanism. However, the effects of PM2.5 on cerebrovascular function and hemodynamics are unknown.
Methods
We used transcranial Doppler ultrasound to measure beat-to-beat blood flow velocity in the middle cerebral artery at rest and in response to changes in end-tidal CO2 (cerebral vasoreactivity) and arterial blood pressure (cerebral autoregulation) in 482 participants from the MOBILIZE Boston Study. We used linear mixed effects models with random subject intercepts to evaluate the association between cerebrovascular hemodynamic parameters and mean PM2.5 levels 1 to 28 days earlier adjusting for age, race, medical history, meteorologic covariates, day of week, temporal trends, and season.
Results
An interquartile range increase (3.0 μg/m3) in mean PM2.5 levels over the previous 28 days was associated with a 8.6% (95% confidence interval [CI]: 3.7%, 13.8%; p<0.001) higher cerebral vascular resistance and a 7.5% (95% CI: 4.2%, 10.6%; p<0.001) lower blood flow velocity at rest. Measures of cerebral vasoreactivity and autoregulation were not associated with PM2.5 levels.
Conclusions
In this cohort of community-dwelling seniors, exposure to PM2.5 was associated with higher resting cerebrovascular resistance and lower cerebral blood flow velocity. If replicated, these findings suggest that alterations in cerebrovascular hemodynamics may underlie the increased risk of particle-related acute cerebrovascular events.
Keywords: air pollution, cerebrovascular, elderly, epidemiology, transcranial Doppler
Existing evidence has convincingly shown that short-term elevations in fine particulate matter air pollution (PM2.5) are associated with increased risk of acute cardiovascular events, including stroke.1 The mechanisms responsible for these effects are not fully understood, but there is substantial evidence suggesting that vascular dysfunction is a central component. Specifically, a number of observational and controlled exposure studies in people have found an association between short-term changes in PM2.5 or its components and increased blood pressure, increased peripheral vascular resistance, decreased brachial artery diameter, and decreased brachial artery flow-mediated dilation.2–8 Further evidence of vascular effects of PM2.5 is provided by animal toxicologic studies.9–11
In healthy individuals, cerebral blood flow is tightly regulated such that it remains relatively constant over a wide range of arterial pressures. Derangements in cerebral vascular function have been associated with stroke incidence and poorer prognosis,12, 13 as well as cognitive impairment, dementia, and depression.14, 15 Given the documented associations between ambient air pollution and both cerebrovascular events and peripheral vascular function, PM2.5 may also affect cerebral vascular function. However, this hypothesis has not been previously investigated. Accordingly, the aim of this study was to evaluate the association between PM2.5 and cerebrovascular hemodynamics at rest and during provocative testing in a cohort of community-dwelling older adults.
Methods
Study Design
We evaluated the association between short-term changes in ambient PM2.5 and measures of cerebral blood flow velocity among 482 participants from the MOBILIZE Boston Study (MBS), a prospective, community-based cohort study of novel risk factors for falls in older adults.16 Briefly, between 2005 and 2008, we recruited 765 non-institutionalized men and women aged ≥65 years, able to communicate in English, residing ≤5 miles (8.0 km) from the study clinic, and able to walk 20 feet (6.1 m) without personal assistance. Individuals with a Mini-Mental State Examination score <18 were not eligible to participate. Upon enrollment, subjects participated in an in-home interview followed within 4 weeks by a clinic examination. Participant characteristics, medical history, medication inventory, smoking history, blood pressure, and height and weight were assessed as previously described.17 A second assessment consisting of an in-home interview and clinic examination was performed a median of 16.5 months after the baseline assessment. All subjects provided written informed consent upon enrollment. This analysis was approved by the Institutional Review Boards at Hebrew SeniorLife and Brown University.
Cerebrovascular Hemodynamics
During the clinic visit, we used transcranial Doppler ultrasonography (TCD) to non-invasively evaluate cerebrovascular hemodynamics at rest and during provocative stimulation, as previously described.16 Briefly, we measured cerebral blood flow velocity continuously in the middle cerebral artery (MCA) while subjects sat in a chair. A 2 MHz TCD probe (MultiDop X4, DWL-Transcranial Doppler Systems Inc., Sterling, VA) was placed over the right or left temporal bone with the best signal, and held in place during recordings using a Velcro headband. TCD data could not be obtained in some subjects because of the absence of a suitable acoustic window to insonate the MCA. We obtained continuous measures of arterial blood pressure using a Finometer photoplethysmographic system (Finapres Medical Systems, Arnhem, The Netherlands) placed on a finger and held at heart level with a sling. The envelope of the velocity waveform, derived from a fast-Fourier analysis of the Doppler frequency signal, was digitized at 500 Hz, displayed simultaneously with the blood pressure, electrocardiogram, and end-tidal CO2 signals, and stored for later off-line analysis.
After a 5-min resting period, the hemodynamic responses to CO2 inhalation and posture change were evaluated. We assessed cerebral vasoreactivity by asking participants to breathe room air normally for 2 minutes, inspire a gas mixture of 8% CO2, 21% O2, and balance nitrogen for 2 minutes, and then mildly hyperventilate to an end-tidal CO2 of ~25 mm Hg for 2 minutes. Cerebral vasoreacitivty was calculated as the slope of the linear regression of mean MCA blood flow velocity versus end-tidal CO2 during the maneuver.
We assessed cerebral autoregulation by asking participants to perform a sit-to-stand maneuver, as previously described.18 Briefly, participants sat with their legs elevated at 90 degrees in front of them on a stool for 5 min before standing for 1 min. We calculated cerebrovascular resistance as: CVR=MAP/BFV, where CVR, MAP, and BFV denote cerebrovascular resistance, mean arterial blood pressure, and mean MCA blood flow velocity, respectively. The intraclass correlation coefficients for cerebrovascular resistance, mean arterial pressure, and blood flow velocity ranged from 0.81 to 0.84, indicating excellent within-person reproducibility of these measures over time.
Air pollution and Meteorological data
PM2.5 was measured continuously at the Boston/Harvard ambient monitoring station and daily averages calculated, as previously described.19 This monitoring station is located <10 km from the study clinic site and <20 km from the residential address of any study participant. We obtained hourly meteorological data from the National Weather Service station at Boston’s Logan Airport.
Statistical Methods
For this analysis, we excluded 76 participants reporting a history of stroke. We used linear mixed models with a random subject intercept to evaluate the association between each outcome and PM2.5 levels while accounting for repeated measures within participants. In all analyses, we controlled for age (natural cubic spline with 3 degrees of freedom), sex, race (white versus other), smoking status (never versus ever), hypertension status (normotension, controlled hypertension, uncontrolled hypertension), diabetes, body mass index (natural cubic spline with 3 degrees of freedom), visit number, day of week, mean ambient and mean dew point temperature (natural cubic splines with 3 degrees of freedom each), season (sine and cosine of time with period of 1 year) and long-term temporal trends (time as linear and quadratic functions). We modeled PM2.5 as a continuous variable and the assumption of a linear exposure-response relationship was confirmed by standard techniques.
Previous studies have reported changes in vascular function associated with PM2.5 levels averaged over 1 to 28 days prior to assessment of the outcome. Accordingly, in separate models we considered pollutant levels averaged over the 1, 3, 5, 7, 14, 21 and 28 days prior to cerebral hemodynamic assessment. We evaluated whether the observed associations with PM2.5 differed according to sex, and the presence or absence of hypertension, diabetes, and obesity (BMI ≥ 30 vs < 30), as well as season (warm versus cool with warm defined as April through September) by adding interaction terms to the model. Analyses were performed using SAS (v9.3; SAS Institute Inc., Cary, NC) and R statistical software (R v2.15). A two-sided p value of <0.05 was considered statistically significant.
Results
Data on cerebral blood flow velocity were available in 482 participants, with 425 completing the cerebral vasoreactivity protocol and 424 completing the cerebral autoregulation protocol. Measures of cerebral blood flow velocity were available at both the baseline and follow-up visit in 72% of participants. Participants included in this analysis were predominantly white and female with a mean age of 77.9 (SD: 5.3) years at baseline (Table 1). In comparison to the 765 participants enrolled in the MBS, the subgroup included in this analysis were slightly younger, more likely to be white and male, and relatively healthier.
Table 1.
Baseline characteristics of 482 participants aged ≥65 years from the MOBILIZE Boston Study with measurements of middle cerebral artery blood flow during either the sit-to-stand or CO2 reactivity protocols, 2005–2009.
| Characteristic | Cerebral Autoregulation Protocol (n=424) | Cerebral Vasoreactivity Protocol (n=425) |
|---|---|---|
| Age, Mean ± SD | 77.7 ± 5.2 | 77.9 ± 5.3 |
| Female, n (%) | 238 (56.1) | 242 (56.9) |
| White, n (%) | 365 (86.1) | 366 (86.1) |
| Hypertension, n (%) | ||
| Normotension | 109 (25.8) | 106 (25.1) |
| Controlled hypertension | 225 (53.3) | 230 (54.5) |
| Uncontrolled hypertension | 89 (21.0) | 86 (20.4) |
| Diabetes Mellitus, n (%) | 65 (15.3) | 65 (15.3) |
| Hyperlipidemia, n (%) | 203 (47.9) | 202 (47.5) |
| Ever Smoker, n (%) | 243 (57.3) | 232 (54.6) |
| Body mass index, Mean ± SD | 26.5 ± 4.7 | 26.8 ± 4.8 |
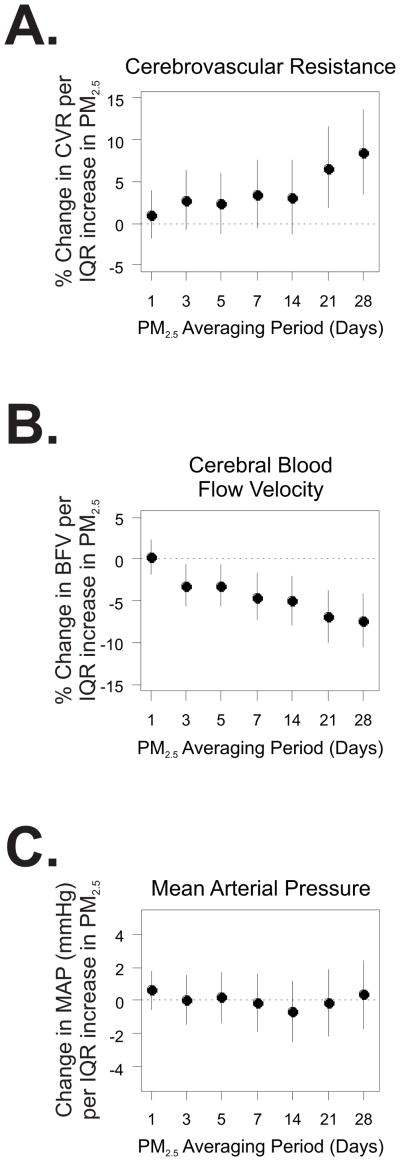
The mean daily PM2.5 level during the study period was 8.6 μg/m3 (SD: 4.9). As the most etiologically relevant exposure window is unknown, we assessed the association between measures of cerebral blood flow and PM2.5 levels averaged over the 1, 3, 5, 7, 14, 21, and 28 days prior to outcome assessment. PM2.5 levels averaged over the prior 3 to 28 days were associated with higher resting cerebrovascular resistance, reaching statistical significance in association with the 21- and 28-day averages of PM2.5 (Fig. 1A). Since cerebrovascular resistance is calculated as the ratio of mean arterial pressure to cerebral blood flow velocity, we assessed whether the observed increase in resistance was due to decreased blood flow velocity, increased mean arterial pressure, or a combination of the two. We found that 21- and 28-day averages of PM2.5 levels were associated with statistically significant decreases in cerebral blood flow velocity (Fig. 1B), with little evidence of change in resting mean arterial pressure (Fig 1C). Specifically, we observed an 8.6% (95% CI: 3.7%, 13.8%) higher cerebrovascular resistance and a 7.5% (95% confidence interval [CI]: 4.2%, 10.6%) lower blood flow velocity comparing the 75th to the 25th percentiles of PM2.5 levels averaged over the past 28 days. These results did not differ significantly according to sex, hypertension status, diabetes, obesity, or season (Table S1).
Figure 1.
Association between ambient fine particles (PM2.5) and resting cerebrovascular resistance (A.), blood flow velocity (B.) and mean arterial pressure (C.). The y-axis denotes the change in each outcome per interquartile range (IQR) increase in PM2.5 averaged over the 1 to 28 days prior to assessment. The x-axis denotes the length of the PM2.5 averaging period in days.
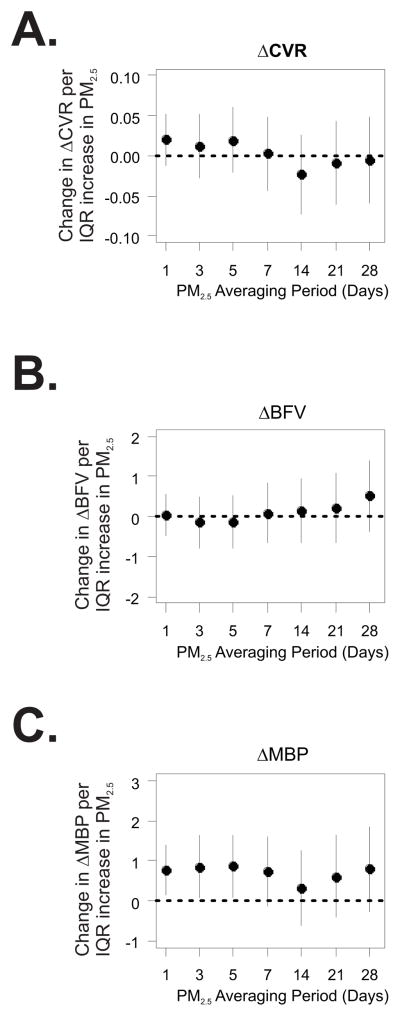
Cerebral vasoreactivity was not associated with PM2.5 at any averaging period (Table S2). Measures of cerebral autoregulation were also not related to PM2.5. Upon standing from the sitting position, mean arterial pressure decreased by an average of 18.9 ± 6.4 mmHg, and was accompanied by a 0.16 ± 0.31 mmHg/(cm/s) decrease in cerebrovascular resistance. The magnitude of the changes in cerebrovascular resistance and blood flow velocity upon standing were not associated with PM2.5 (Fig. 2). However, the decrease in mean arterial pressure observed upon standing was smaller following 1 to 5 days with higher average PM2.5.
Figure 2.
Association between PM2.5 and ΔCVR (A.), ΔBFV (B.), and ΔMAP (C.) assessed during the cerebral autoregulation protocol. ΔCVR, ΔBFV, and ΔMAP denote standing minus sitting values for cerebrovascular resistance (in units of (cm/s)/mmHg), blood flow velocity (cm/s), and mean arterial pressure (mmHg), respectively. The y-axis denotes the change in each outcome per interquartile range (IQR) increase in PM2.5 averaged over the 1 to 28 days prior to assessment. The x-axis denotes the length of the PM2.5 averaging period in days.
Discussion
The results of this study suggest that exposure to fine particulate matter air pollution may be associated with adverse changes in the cerebral vasculature. Specifically, in this population-based cohort of community-dwelling older adults we found that PM2.5 levels within the past month were associated with higher cerebrovascular resistance and lower MCA blood flow velocity at rest, in the absence of significant changes in resting mean arterial pressure. Our results did not differ significantly among participants with diabetes, hypertension, smoking, or by sex or season.
Although we are not aware of any prior studies examining this hypothesis, short-term exposure to ambient air pollution has been linked with changes in the peripheral vasculature including increased arterial blood pressure, increased peripheral vascular resistance, decreased brachial artery diameter, decreased brachial artery flow-mediated dilation, and decreased small-vessel elasticity.2–8 Additionally, studies in humans and animals have shown that air pollution exposure can attenuate the vascular response to the endothelium-dependent vasodilators acetylcholine and bradykinin, and potentiate the vasoconstriction induced by phenylepherine.9, 20 These findings support the notion that short-term exposure to ambient air pollution can alter endothelial function in the peripheral vasculature.
The results of the current study extend this prior work and suggest that ambient air pollution may also be detrimental to cerebrovascular endothelial function. Basal resting cerebral blood flow is determined, at least in part, by release of nitric oxide (NO) from vascular endothelial cells.21, 22 Moreover, resting cerebral blood flow velocity declines with age, is lower in obese individuals and diabetics with microvascular complications, and is correlated with indices of systemic arterial stiffness.23–25 These observations suggest an important role of endothelium in the maintenance of resting cerebral blood flow, although multiple other factors are also clearly involved.
In healthy participants, cerebral blood flow is altered in response to fluctuations in arterial CO2 concentrations via a mechanism that also depends on endothelium-derived NO.26 Thus, cerebral vasoreactivity has been proposed as a marker of cerebrovascular endothelial function analogous to brachial artery flow-mediated dilation.26 Contrary to our expectations, we did not find an association between ambient pollution and cerebral vasoreactivity. In this regard, our results seem closest to those of Brook et al.2 who observed a reduction in brachial artery diameter, but no change in flow-mediated dilation following controlled exposure to PM2.5 and ozone. Like flow-mediated dilation, cerebral vasoreactivity represents a dynamic response to an acute stimulus while basal cerebral blood flow represents an assessment of flow under steady-state conditions. While NO is thought to be involved in both static and dynamic responses, the physiologic mechanisms and pathophysiological influences are likely quite different.27, 28
We also did not observe an association between PM2.5 and cerebral autoregulation as assessed by the change in either MCA blood flow velocity or cerebrovascular resistance upon standing from a seated position. This finding is not surprising given that the maintenance of cerebral blood flow in response to acute changes in arterial pressure is largely attributable to a myogenic response (i.e.: mechanoregulation) without key involvement of either neurogenic or endothelial factors.21, 26
Short-term changes in ambient PM2.5 levels have been associated with increased risk of ischemic stroke onset29 and long-term exposure to higher levels of PM2.5 has been associated with higher risk of stroke30 and faster cognitive decline.31 Indeed, within the MOBILIZE Boston Study cohort we have found an association between long-term exposure to traffic pollution and lower cognitive function.32 If causal, the results of the current study suggest a potential common mechanism for these observations mediated through alterations in endothelial function potentially leading to cerebral hypoperfusion.
A limitation of our study is that we were not able to obtain TCD measurements on 37% of participants, and those participants with TCD measurements tended to be healthier than those without measurements. While a TCD acoustic window is expected to be absent in about one third of elderly participants,33 the observation that participants with versus without TCD acoustic windows tended to be healthier may limit the generalizability of our results. In addition, because it is likely that the effects of PM2.5 vary depending on pollution sources, particle constituents, and age or other participant characteristics, our results are not necessarily generalizable to other geographic locations or study populations. Identifying the sources of PM2.5 responsible for observed health effects remains a top research priority and additional analyses using detailed PM2.5 speciation data are currently underway. Third, TCD provides a measure of cerebral blood flow velocity rather than absolute flow. However, studies using a variety of techniques have confirmed that MCA blood flow velocity is correlated with regional cerebral blood flow and relative changes in cerebral blood flow velocity are representative of changes in cerebral blood flow.34–36 Fourth, use of PM2.5 measurements from a single monitoring site is expected to lead to some exposure misclassification, decreasing the precision of our estimates but not otherwise biasing our results.37 However, all participants lived <20 km from the PM2.5 monitoring site, limiting the potential for misclassification. Finally, we did not have data on the amount of time participants spent indoors. However, studies suggest that ambient PM2.5 is a relatively good surrogate of personal exposure to PM2.5 of ambient origin,38, 39 the metric on which current environmental regulations are based.
On the other hand, important strengths of our study include detailed assessment of hemodynamic responses in a large, prospective cohort of community-dwelling elderly subjects evaluated repeatedly. Because MOBILIZE Boston Study participants are representative of seniors in the Boston area in terms of age, sex, race, and ethnicity,16 our results are broadly relevant to elderly individuals rather than a selected patient population. Additionally, these associations were observed at PM2.5 levels that are common in urban environments and below the current standards set by the US government.
In conclusion, the current study found that short-term exposure to ambient PM2.5 was associated with lower resting cerebrovascular flow velocity and higher resting cerebrovascular resistance in community-dwelling elderly participants. If confirmed in future studies, these findings suggest that ambient air pollution may be detrimental to endothelial function in the cerebral vasculature and suggest a novel mechanism for pollution-related cerebrovascular events.
Supplementary Material
Acknowledgments
Sources of Funding:
The project described was supported by grants ES015774, AG004390, AG25037, AG030967, ES009825, and ES000002 from the National Institutes of Aging (NIA) and Environmental Health Sciences (NIEHS), NIH, and grants R832416 and RD83479801 from the US Environmental Protection Agency (US EPA). Dr. Lipsitz holds the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine at Hebrew SeniorLife. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the sponsoring institutions.
Footnotes
Disclosures: None
References
- 1.Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the american heart association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 2.Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105:1534–1536. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- 3.Zanobetti A, Canner MJ, Stone PH, Schwartz J, Sher D, Eagan-Bengston E, et al. Ambient pollution and blood pressure in cardiac rehabilitation patients. Circulation. 2004;110:2184–2189. doi: 10.1161/01.CIR.0000143831.33243.D8. [DOI] [PubMed] [Google Scholar]
- 4.O’Neill MS, Veves A, Zanobetti A, Sarnat JA, Gold DR, Economides PA, et al. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111:2913–2920. doi: 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
- 5.Briet M, Collin C, Laurent S, Tan A, Azizi M, Agharazii M, et al. Endothelial function and chronic exposure to air pollution in normal male subjects. Hypertension. 2007;50:970–976. doi: 10.1161/HYPERTENSIONAHA.107.095844. [DOI] [PubMed] [Google Scholar]
- 6.Schneider A, Neas L, Herbst MC, Case M, Williams RW, Cascio W, et al. Endothelial dysfunction: Associations with exposure to ambient fine particles in diabetic individuals. Environ Health Perspect. 2008;116:1666–1674. doi: 10.1289/ehp.11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auchincloss AH, Roux AV, Dvonch JT, Brown PL, Barr RG, Daviglus ML, et al. Associations between recent exposure to ambient fine particulate matter and blood pressure in the multi-ethnic study of atherosclerosis (mesa) Environ Health Perspect. 2008;116:486–491. doi: 10.1289/ehp.10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54:659–667. doi: 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Q, Yue P, Ying Z, Cardounel AJ, Brook RD, Devlin R, et al. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of rho/rock. Arterioscler Thromb Vasc Biol. 2008;28:1760–1766. doi: 10.1161/ATVBAHA.108.166967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartoli CR, Wellenius GA, Diaz EA, Lawrence J, Coull BA, Akiyama I, et al. Mechanisms of inhaled fine particulate air pollution-induced arterial blood pressure changes. Environ Health Perspect. 2009;117:361–366. doi: 10.1289/ehp.11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ying Z, Yue P, Xu X, Zhong M, Sun Q, Mikolaj M, et al. Air pollution and cardiac remodeling: A role for rhoa/rho-kinase. Am J Physiol Heart Circ Physiol. 2009;296:H1540–1550. doi: 10.1152/ajpheart.01270.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silvestrini M, Vernieri F, Pasqualetti P, Matteis M, Passarelli F, Troisi E, et al. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. Jama. 2000;283:2122–2127. doi: 10.1001/jama.283.16.2122. [DOI] [PubMed] [Google Scholar]
- 13.Troisi E, Matteis M, Silvestrini M, Paolucci S, Grasso MG, Pasqualetti P, et al. Altered cerebral vasoregulation predicts the outcome of patients with partial anterior circulation stroke. European neurology. 2012;67:200–205. doi: 10.1159/000334851. [DOI] [PubMed] [Google Scholar]
- 14.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Direk N, Koudstaal PJ, Hofman A, Ikram MA, Hoogendijk WJ, Tiemeier H. Cerebral hemodynamics and incident depression: The rotterdam study. Biological psychiatry. 2012 doi: 10.1016/j.biopsych.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Leveille SG, Kiel DP, Jones RN, Roman A, Hannan MT, Sorond FA, et al. The mobilize boston study: Design and methods of a prospective cohort study of novel risk factors for falls in an older population. BMC geriatrics. 2008;8:16. doi: 10.1186/1471-2318-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wellenius GA, Wilhelm-Benartzi CS, Wilker EH, Coull BA, Suh HH, Koutrakis P, et al. Ambient particulate matter and the response to orthostatic challenge in the elderly: The maintenance of balance, independent living, intellect, and zest in the elderly (mobilize) of boston study. Hypertension. 2012;59:558–563. doi: 10.1161/HYPERTENSIONAHA.111.180778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorond FA, Serrador JM, Jones RN, Shaffer ML, Lipsitz LA. The sit-to-stand technique for the measurement of dynamic cerebral autoregulation. Ultrasound in medicine & biology. 2009;35:21–29. doi: 10.1016/j.ultrasmedbio.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang CM, Koutrakis P, Suh HH. Hourly measurements of fine particulate sulfate and carbon aerosols at the harvard-u.S. Environmental protection agency supersite in boston. Journal of the Air & Waste Management Association (1995) 2010;60:1327–1334. doi: 10.3155/1047-3289.60.11.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tornqvist H, Mills NL, Gonzalez M, Miller MR, Robinson SD, Megson IL, et al. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. American journal of respiratory and critical care medicine. 2007;176:395–400. doi: 10.1164/rccm.200606-872OC. [DOI] [PubMed] [Google Scholar]
- 21.Lavi S, Egbarya R, Lavi R, Jacob G. Role of nitric oxide in the regulation of cerebral blood flow in humans: Chemoregulation versus mechanoregulation. Circulation. 2003;107:1901–1905. doi: 10.1161/01.CIR.0000057973.99140.5A. [DOI] [PubMed] [Google Scholar]
- 22.White RP, Hindley C, Bloomfield PM, Cunningham VJ, Vallance P, Brooks DJ, et al. The effect of the nitric oxide synthase inhibitor l-nmma on basal cbf and vasoneuronal coupling in man: A pet study. J Cereb Blood Flow Metab. 1999;19:673–678. doi: 10.1097/00004647-199906000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Xu TY, Staessen JA, Wei FF, Xu J, Li FH, Fan WX, et al. Blood flow pattern in the middle cerebral artery in relation to indices of arterial stiffness in the systemic circulation. Am J Hypertens. 2012;25:319–324. doi: 10.1038/ajh.2011.223. [DOI] [PubMed] [Google Scholar]
- 24.Selim M, Jones R, Novak P, Zhao P, Novak V. The effects of body mass index on cerebral blood flow velocity. Clin Auton Res. 2008;18:331–338. doi: 10.1007/s10286-008-0490-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim YS, Davis SC, Truijen J, Stok WJ, Secher NH, van Lieshout JJ. Intensive blood pressure control affects cerebral blood flow in type 2 diabetes mellitus patients. Hypertension. 2011;57:738–745. doi: 10.1161/HYPERTENSIONAHA.110.160523. [DOI] [PubMed] [Google Scholar]
- 26.Lavi S, Gaitini D, Milloul V, Jacob G. Impaired cerebral co2 vasoreactivity: Association with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2006;291:H1856–1861. doi: 10.1152/ajpheart.00014.2006. [DOI] [PubMed] [Google Scholar]
- 27.Tiecks FP, Lam AM, Aaslid R, Newell DW. Comparison of static and dynamic cerebral autoregulation measurements. Stroke; a journal of cerebral circulation. 1995;26:1014–1019. doi: 10.1161/01.str.26.6.1014. [DOI] [PubMed] [Google Scholar]
- 28.Stoner L, Erickson ML, Young JM, Fryer S, Sabatier MJ, Faulkner J, et al. There’s more to flow-mediated dilation than nitric oxide. Journal of atherosclerosis and thrombosis. 2012;19:589–600. doi: 10.5551/jat.11973. [DOI] [PubMed] [Google Scholar]
- 29.Wellenius GA, Burger MR, Coull BA, Schwartz J, Suh HH, Koutrakis P, et al. Ambient air pollution and the risk of acute ischemic stroke. Archives of internal medicine. 2012;172:229–234. doi: 10.1001/archinternmed.2011.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. The New England journal of medicine. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 31.Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, Grodstein F. Exposure to particulate air pollution and cognitive decline in older women. Archives of internal medicine. 2012;172:219–227. doi: 10.1001/archinternmed.2011.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wellenius GA, Boyle LD, Coull BA, Milberg WP, Gryparis A, Schwartz J, et al. Residential proximity to nearest major roadway and cognitive function in community-dwelling seniors: Results from the mobilize boston study. J Am Geriatr Soc. 2012;60:2075–2080. doi: 10.1111/j.1532-5415.2012.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Transcranial doppler hemodynamic parameters and risk of stroke: The rotterdam study. Stroke; a journal of cerebral circulation. 2007;38:2453–2458. doi: 10.1161/STROKEAHA.107.483073. [DOI] [PubMed] [Google Scholar]
- 34.Dahl A, Russell D, Nyberg-Hansen R, Rootwelt K. A comparison of regional cerebral blood flow and middle cerebral artery blood flow velocities: Simultaneous measurements in healthy subjects. J Cereb Blood Flow Metab. 1992;12:1049–1054. doi: 10.1038/jcbfm.1992.142. [DOI] [PubMed] [Google Scholar]
- 35.Larsen FS, Olsen KS, Hansen BA, Paulson OB, Knudsen GM. Transcranial doppler is valid for determination of the lower limit of cerebral blood flow autoregulation. Stroke; a journal of cerebral circulation. 1994;25:1985–1988. doi: 10.1161/01.str.25.10.1985. [DOI] [PubMed] [Google Scholar]
- 36.Clark JM, Skolnick BE, Gelfand R, Farber RE, Stierheim M, Stevens WC, et al. Relationship of 133xe cerebral blood flow to middle cerebral arterial flow velocity in men at rest. J Cereb Blood Flow Metab. 1996;16:1255–1262. doi: 10.1097/00004647-199611000-00021. [DOI] [PubMed] [Google Scholar]
- 37.Zeger SL, Thomas D, Dominici F, Samet JM, Schwartz J, Dockery D, et al. Exposure measurement error in time-series studies of air pollution: Concepts and consequences. Environ Health Perspect. 2000;108:419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarnat JA, Koutrakis P, Suh HH. Assessing the relationship between personal particulate and gaseous exposures of senior citizens living in baltimore, md. Journal of the Air & Waste Management Association (1995) 2000;50:1184–1198. doi: 10.1080/10473289.2000.10464165. [DOI] [PubMed] [Google Scholar]
- 39.Avery CL, Mills KT, Williams R, McGraw KA, Poole C, Smith RL, et al. Estimating error in using residential outdoor pm2.5 concentrations as proxies for personal exposures: A meta-analysis. Environ Health Perspect. 2010;118:673–678. doi: 10.1289/ehp.0901158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.