Abstract
OBJECTIVE
The goal was to determine the association between cardiovascular risk factors and microalbuminuria in a nationally representative sample of adolescents and to determine whether being overweight modifies this association.
METHODS
We analyzed cross-sectional data from the National Health and Nutrition Examination Survey(1999–2004) for 2515 adolescents 12 to 19 years of age. Cardiovascular risk factors included abdominal obesity, impaired fasting glucose, diabetes mellitus, insulin resistance, high triglyceride levels, low high-density lipoprotein cholesterol levels, hypertension, smoking, and the metabolic syndrome. Microalbuminuria was defined as a urinary albumin/creatinine ratio of 30 to 299 mg/g in a random morning sample. Overweight was defined as BMI of ≥95th percentile, according to the Centers for Disease Control and Prevention 2000 growth charts.
RESULTS
Microalbuminuria was present in 8.9% of adolescents. The prevalence of microalbuminuria was higher among nonoverweight adolescents than among overweight adolescents. The median albumin/creatinine ratio decreased with increasing BMI z scores. The association of microalbuminuria with cardiovascular risk factors differed according to BMI category. Among nonoverweight adolescents, microalbuminuria was not associated with any cardiovascular disease risk factor except for overt diabetes mellitus. Among overweight adolescents, however, microalbuminuria was associated with impaired fasting glucose, insulin resistance, hypertension, and smoking, as well as diabetes mellitus.
CONCLUSION
For the majority of adolescents, microalbuminuria is not associated with cardiovascular risk factors. Among overweight adolescents, however, microalbuminuria is associated with cardiovascular risk factors. The prognostic importance of microalbuminuria in overweight and nonoverweight adolescents with regard to future cardiovascular and renal disease needs to be defined in prospective studies conducted specifically in children.
Keywords: adolescents, obesity, cardiovascular risk factors, metabolic syndrome, albuminuria, epidemiology, National Health and Nutrition Examination Survey, Centers for Disease Control and Prevention
The prevalence of childhood obesity has tripled since the middle 1980s and continues to increase.1,2 Overweight children and adolescents are thought to be at greater risk for earlier onset of cardiovascular and renal diseases in adulthood, which represents a major public health concern.3
Obesity and its metabolic consequences lead to endothelial dysfunction and cardiovascular disease.4 Increased urinary albumin excretion (microalbuminuria) is a marker of endothelial dysfunction and reflects renal and systemic endovascular damage.5–7 Microalbuminuria, which was originally used to predict the development of overt diabetic nephropathy in patients with diabetes mellitus,8–10 is now also considered to be an early marker of renal damage in nondiabetic subjects11 and an independent predictor of future cardiovascular disease in the general adult population.12–17 Indeed, some authors have even advocated screening for microalbuminuria and using it as a therapeutic target for identification and treatment of patients at increased risk for future cardiovascular disease.18–20
The association between cardiovascular risk factors and microalbuminuria in children is unclear, and previous studies came to seemingly discrepant conclusions. Studies with referral-based populations of overweight adolescents found that cardiovascular risk factors increased the risk for microalbuminuria,21–23 similar to findings observed among adults. However, studies with community-based populations found that overweight adolescents (with presumably more coexisting cardiovascular risk factors) actually had lower prevalence of microalbuminuria.24,25 To clarify this issue, we set out to determine how weight and cardiovascular risk factors were related to microalbuminuria in a large, nationally representative sample of adolescents in the United States. On the basis of the literature reports described above, we hypothesized that the relationship between cardiovascular risk factors and microalbuminuria might differ among overweight and nonoverweight adolescents.
METHODS
Study Design and Population
We used cross-sectional data collected from the 1999 to 2004 National Health and Nutrition Examination Survey (NHANES), a complex-sample survey designed to collect data on health and nutrition from a representative household population in the United States.26 Certain populations (such as adolescents and racial/ethnic minorities) were oversampled, to improve estimates for those groups.
The 1999 to 2004 NHANES data included 2593 adolescents (12–19 years of age) who had fasted for 8 to 24 hours. We excluded 33 adolescents who were pregnant, because waist circumference measurements and BMI values for this group would not reflect adiposity accurately; 22 adolescents who were missing BMI data, because subsequent subgroup analyses were based on BMI values; and 23 adolescents with macroalbuminuria (urinary albumin/creatinine ratio of ≥300 mg/g), because albuminuria in this group may reflect primary renal disease, such as minimal-change disease. Therefore, data for 2515 adolescents were available for our final analysis.
Measures and Data Collection
General Examination
Participants were invited to take part in a standardized, in-home, medical interview. Those who completed the interview were invited to mobile examination centers for the physical examination component of the survey. Age was determined at the time of examination. Race/ethnicity was determined by self-report, and participants were categorized as non-Hispanic white, non-Hispanic black, Hispanic, or other. BMI was calculated as weight (in kilograms) divided by height (in centimeters) squared. Waist circumference was measured above the uppermost lateral border of the right ilium, at the end of normal expiration, to the nearest millimeter. Blood pressure was measured with a mercury sphygmomanometer, by using the American Heart Association guidelines.27 Up to 4 blood pressure measurements were recorded. When >1 blood pressure measurement was available, the average systolic and diastolic blood pressures were calculated.
Laboratory Analyses
During the examination, blood and urine specimens were collected and stored by using a standardized protocol.28 Fasting serum triglyceride levels were measured enzymatically after hydrolysis to glycerol. Fasting plasma glucose levels were measured with the modified hexokinase enzymatic method. The method with which fasting serum insulin levels were measured changed between the NHANES in 1999 to 2002 and the NHANES in 2003 to 2004. Insulin values were adjusted to make the 2 methods comparable by using the linear regression formula published for the NHANES.29 High-density lipoprotein (HDL) cholesterol levels were measured by using the heparin-manganese precipitation method. Serum levels of cotinine (a metabolite of nicotine) were measured with high performance liquid chromatography/atmospheric-pressure chemical ionization tandem mass spectrometry.
A random morning urine specimen was collected during the examination. Urinary albumin concentrations were measured with a solid-phase fluorescent immunoassay. Urinary creatinine levels were measured with the Jaffe rate reaction method, by using a CX3 analyzer (Beckman Instruments, Brea, CA). The urinary albumin/creatinine ratio was expressed as milligrams of albumin per gram of creatinine.
Definition of Cardiovascular Risk Factors
The metabolic syndrome was defined by using the recommendations of the National Cholesterol Education Program Adult Treatment Panel III, modified for age. Participants with ≥3 of the following criteria met the definition of the metabolic syndrome: abdominal obesity, hypertension, high triglyceride level, low HDL cholesterol level, impaired fasting glucose, or diabetes mellitus. Abdominal obesity was defined as a waist circumference of ≥90th percentile for age and gender30 or as meeting Adult Treatment Panel III criteria (>88 cm for women and >102 cm for men). The Adult Treatment Panel III criteria were added because some adolescents who met the adult criteria for abdominal obesity were still in <90th percentile for age and gender. Hypertension was defined as an average systolic or diastolic blood pressure of ≥95th percentile for age, gender, and height31; self-reported history of hypertension; or history of antihypertensive medication usage. For adolescents ≥18 years of age, hypertension was defined by using the Adult Treatment Panel III criterion (blood pressure of ≥130/85 mm Hg). A high triglyceride level was defined as a fasting serum triglyceride level of ≥95th percentile for age and gender.32 A low HDL cholesterol level was defined as a HDL cholesterol level of ≤5th percentile for age and gender.32 Impaired fasting glucose was defined as a fasting plasma glucose level between 100 and 125 mg/dL without a history of diabetes mellitus.33 Diabetes mellitus was defined as a fasting glucose level of ≥126 mg/dL, self-reported history of diabetes mellitus, or history of diabetes medication usage.
Insulin resistance was defined as a Homeostatic Model Assessment index of insulin resistance score of ≥4,34 without a history of diabetes mellitus, or diabetes medication usage. The Homeostatic Model Assessment index of insulin resistance was calculated as the product of the fasting plasma insulin level (in microunits per milliliter) and the fasting plasma glucose level (in millimolar), divided by 22.5. Smoking was defined as a serum cotinine (metabolite of nicotine) level of >15 ng/mL or a self-reported history of smoking in the previous 5 days.35
Definition of Albuminuria
Normal urinary albumin excretion was defined as an albumin/creatinine ratio of <30 mg/g. Microalbuminuria was defined as an albumin/creatinine ratio of ≥30 mg/g and <300 mg/g.18,36,37
Data Analyses
All statistical analyses were completed by using Stata 9.0 (Stata, College Station, TX), taking into account samples’ weights, strata, and primary sampling units from the 1999 to 2004 NHANES, to adjust for unequal probabilities of selection and the multistage, stratified, sample design.38 The weighted proportions were extrapolated to the adolescent US population by using 2000 US Census counts.38
Means and proportions for participant characteristics were compared by using Student’s t tests and χ2 tests. The median values for urinary albumin concentration and albumin/creatinine ratio were compared by using regression models after rank transformation, because urinary albumin concentrations and albumin/creatinine ratios are not normally distributed.39 We used logistic regression to calculate the odds ratio (OR) for microalbuminuria, with simultaneous adjustment for multiple covariates.
We evaluated whether being overweight modified the association between cardiovascular risk factors and risk of microalbuminuria, and we found several significant interaction terms (P < .05). Therefore, we report our main analysis stratified according to BMI category, that is, BMI of <95th percentile (nonoverweight) or ≥95th percentile (overweight). Percentiles were calculated according to the Centers for Disease Control and Prevention 2000 growth charts.2
We evaluated the following confounding variables: age, gender, race/ethnicity, total fluid intake, and physical activity. Total fluid intake was self-reported in 24-hour dietary recall records and measured the amount of fluid adolescents drank in the 24-hour period before urine collection. More than 98% of subjects had complete, reliable, food records collected during in-person interviews. Physical activity was measured with a questionnaire, and adolescents were asked to estimate how much physical activity they had in the previous 30 days. Physical activity was reported for 99% of the adolescents in this population. Confounding variables that were statistically significant, that changed the coefficient by >10%, or that had face validity were kept in the final model. The final model adjusted for age, gender, and race/ethnicity simultaneously. P < .05 was considered statistically significant. We also performed several sensitivity analyses by using different published definitions of the metabolic syndrome, changing our exclusion criteria to include subjects with macroalbuminuria or to exclude those with diabetes mellitus, and using urinary albumin concentration alone as the outcome.
RESULTS
Population Characteristics
Demographic features and the prevalence of the cardiovascular risk factors for the overall study population are presented in Table 1. Overall, 10.3% of the study population was overweight. Metabolic syndrome was detected for 3.6% of the study population. Microalbuminuria was detected for 8.9% of the study population.
TABLE 1.
Population Characteristics of Study Sample and Projected US Population of Adolescents 12 to 19 Years of Age (NHANES, 1999–2004)
| Study Sample | Projected US Populationa | |
|---|---|---|
| Total, n | 2515 | 28.0 × 106 |
| Age, mean ± SEM, y | 15.4 ± 0.04 | 15.5 ± 0.07 |
| Gender, n (%) | ||
| Boys | 1323 | 14.0 × 106 (51.4) |
| Girls | 1192 | 13.0 × 106 (48.7) |
| Race/ethnicity, n (%) | ||
| Non-Hispanic white | 672 | 17.0 × 106 (63.3) |
| Non-Hispanic black | 790 | 3.9 × 106 (14.3) |
| Hispanic | 977 | 4.6 × 106 (16.9) |
| Other race | 76 | 1.5 × 106 (5.6) |
| BMI, n (%) | ||
| <95th percentile | 2205 | 25.0 × 106 (89.7) |
| ≥95th percentile | 310 | 2.8 × 106 (10.3) |
| Abdominal obesity, n (%) | 505 | 5.0 × 106 (18.2) |
| Glucose level, n (%) | ||
| Normal fasting glucose levelb | 2125 | 25.0 × 106 (89.4) |
| Impaired fasting glucose level | 249 | 2.7 × 106 (9.9) |
| Diabetes mellitus | 10 | 0.2 × 106 (0.8) |
| Insulin resistance, n (%) | 429 | 4.0 × 106 (15.0) |
| Hypertension, n (%) | 72 | 0.8 × 106 (2.9) |
| High triglyceride levels, n (%) | 244 | 3.1 × 106 (11.3) |
| Low HDL cholesterol levels, n (%) | 191 | 2.5 × 106 (9.1) |
| Smoking, n (%) | 291 | 4.0 × 106 (14.8) |
| Metabolic syndrome, n (%) | 82 | 1.0 × 106 (3.6) |
| Microalbuminuria, n (%) | 226 | 2.4 × 106 (8.9) |
Adjusted for survey design and based on 2000 US Census data.
Fasting glucose level of <100 mg/dL without a history of diabetes mellitus or use of diabetes medication.
The median albumin/creatinine ratio for girls was significantly greater than that for boys (7.9 vs 4.9 mg/g; P < .0001). The median albumin/creatinine ratio for boys 12 to 15 years of age did not differ significantly from that for boys 16 to 19 years of age (5.0 vs 4.6 mg/g; P = .09). The median albumin/creatinine ratio for girls 12 to 15 years of age did not differ significantly from that for girls 16 to 19 years of age (8.2 mg/g vs 7.6 mg/g; P = .15).
The median urinary albumin concentration for girls was significantly higher than that for boys (10.3 vs 7.7 μg/mL; P < .0001). Boys 16 to 19 years of age had a higher median urinary albumin concentration than did boys 12 to 15 years of age (8.8 vs 6.9 μg/mL; P = .03). The median urinary albumin concentration for girls 12 to 15 years of age did not differ significantly from that for girls 16 to 19 years of age (9.8 vs 10.9 μg/mL; P = .16).
Nonoverweight and overweight adolescents did not differ with respect to age (P = .31) or gender (P = .22). The prevalence of overweight among all non-Hispanic white adolescents was 8.78%, whereas the prevalence of overweight was 14.8% for non-Hispanic black adolescents, 13.14% for Hispanic adolescents, and 8.24% for adolescents of other races.
Cardiovascular risk factors stratified according to BMI category are described in Fig 1. As anticipated, the prevalence of adverse cardiovascular risk factors was greater among overweight adolescents, compared with nonover-weight adolescents. Specifically, overweight adolescents had greater prevalence rates of abdominal obesity (96.6% vs 9.2%; P < .0001), impaired fasting glucose (16.5% vs 9.1%; P = .04), insulin resistance (62.5% vs 9.4%; P < .0001), hypertension (10.7% vs 2%; P < .0001), high triglyceride levels (26.4% vs 9.5%; P < .0001), low HDL cholesterol levels (24.7% vs 7.3%; P < .0001), and metabolic syndrome (22.0% vs 1.5%; P < .0001), compared with nonoverweight adolescents. Diabetes mellitus was more prevalent among overweight adolescents than among nonoverweight adolescents, but the difference was not statistically significant (1.7% vs 0.7%; P = .29). Fewer overweight adolescents smoked, compared with nonover-weight adolescents, but the difference was not statistically significant (11.8% vs 15.2%; P = .31).
FIGURE 1.
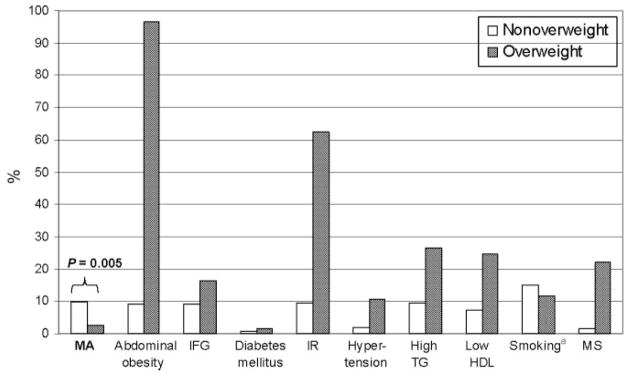
Prevalence of microalbuminuria and cardiovascular risk factors in adolescents according to BMI category. MA indicates microalbuminuria; TG, triglyceride level; LDL, low-density lipoprotein level; IFG, impaired fasting glucose level; IR, insulin resistance; MS, metabolic syndrome. Except where indicated, P < .05 for all comparisons between nonoverweight and overweight adolescents. a P = .3.
Factors Associated With Microalbuminuria
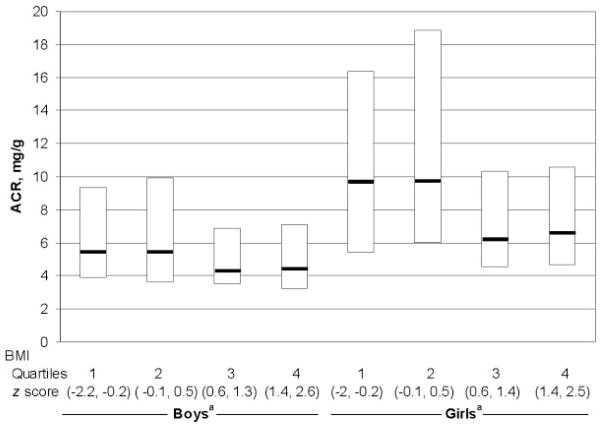
Despite the clustering of adverse cardiovascular risk factors among overweight adolescents, microalbuminuria was more prevalent among nonoverweight adolescents than among overweight adolescents (8.7% vs 0.3%; P = .005) (Fig 1). The median albumin/creatinine ratio decreased with increasing BMI z scores for boys (overall test of trend, P = .03) and girls (overall test of trend, P < .0001) (Fig 2).
FIGURE 2.
Box plot of ACR of adolescents according to gender and BMI z-score quartiles. Shown are the 25th, 50th, and 75th percentiles of ACR distribution for boys and girls without microalbuminuria according to age group. The boxes enclose the 25th to 75th percentile, and the middle horizontal lines represent the median values. a P < .05 for trend.
Similarly, microalbuminuria was more prevalent among adolescents without abdominal obesity, compared with adolescents with abdominal obesity (7.9% vs 1.0%; P = .03), and among adolescents without insulin resistance, compared with adolescents with insulin resistance (7.8% vs 0.7%; P = .02). Similar results were seen when urinary albumin concentration alone (urinary albumin concentration of ≥30 μg/mL) was used to define microalbuminuria (data not shown).
In classification of all adolescents according to fasting glucose levels and albuminuria status, 81.4% had normal fasting glucose and normal albuminuria, 7.9% had normal fasting glucose and microalbuminuria, 9.3% had impaired fasting glucose and normal albuminuria, 0.5% had impaired fasting glucose and microalbuminuria, 0.3% had diabetes mellitus and normal albuminuria, and 0.5% had diabetes mellitus and microalbuminuria. In analyses of the entire sample, hypertension, high triglyceride levels, low HDL cholesterol levels, smoking, and the metabolic syndrome were not significantly associated with microalbuminuria. Significantly more girls than boys had microalbuminuria (5.8% vs 3.1%; P = .0002). The prevalence of microalbuminuria did not differ with respect to race/ethnicity (P = .85) or age (P = .15).
Modification of Associations Between Cardiovascular Risk Factors and Microalbuminuria by Being Overweight
Our main results were then stratified according to the presence or absence of overweight (BMI of ≥95th percentile) and were adjusted for age, gender, and race/ethnicity. Among nonoverweight adolescents, microalbuminuria was not associated with any cardiovascular disease risk factor except for overt diabetes mellitus (Table 2).
TABLE 2.
Unadjusted and Adjusted ORs for Microalbuminuria in Adolescents, Stratified According to BMI Category
| Unadjusted OR (95% CI) | Adjusted OR (95% CI)a | |
|---|---|---|
| Nonoverweight | ||
| Abdominal obesity | 0.88 (0.44–1.76) | 0.77 (0.37–1.59) |
| Fasting glucose level | ||
| Normal | Reference | Reference |
| Impaired | 0.53 (0.22–1.28) | 0.61 (0.24–1.54) |
| Diabetes mellitus | 14 (1.34–1426)b | 14 (1.64–125)b |
| Insulin resistance | 0.55 (0.26–1.19) | 0.49 (0.23–1.05) |
| Hypertension | —c | —c |
| Elevated triglyceride level | 1.31 (0.68–2.55) | 1.35 (0.70–2.63) |
| Low HDL cholesterol level | 0.69 (0.24–1.94) | 0.65 (0.22–1.90) |
| Smoker | 0.51 (0.26–1.00) | 0.61 (0.32–1.16) |
| Metabolic syndrome | 1.06 (0.15–7.49) | 1.25 (0.17–8.95) |
| Overweight | ||
| Abdominal obesity | —c | —c |
| Fasting glucose level | ||
| Normal | Reference | Reference |
| Impaired | 6.25 (0.71–54.95) | 13 (1.51–118)b |
| Diabetes mellitus | 148 (11–1957)b | 1373 (15–123757)b |
| Insulin resistance | 19 (1.89–189)b | 75 (1.58–3573)b |
| Hypertension | 7.12 (0.78–65) | 11 (2.47–53)b |
| Elevated triglyceride level | 2.22 (0.24–21) | 4.79 (0.92–25) |
| Low HDL cholesterol level | 2.46 (0.27–22) | 3.83 (0.82–18) |
| Smoker | 4.01 (0.38–42) | 25 (1.29–484)b |
| Metabolic syndrome | 2.71 (0.29–26) | 7.60 (0.90–64) |
Adjusted for age, gender, and race/ethnicity.
P < .05.
Point estimate could not be calculated because of small sample size for one comparison group in these cells.
A different picture emerged among overweight adolescents. Among overweight adolescents, in addition to diabetes mellitus (adjusted OR: 1373; 95% confidence interval [CI]: 15–123 757), cardiovascular risk factors such as impaired fasting glucose (adjusted OR: 13; 95% CI: 1.51–118), insulin resistance (adjusted OR: 75; 95% CI: 1.58–3573), hypertension (adjusted OR: 11; 95% CI: 2.47–53), and smoking (adjusted OR: 25; 95% CI: 1.29–484) were associated with microalbuminuria (Table 2). Furthermore, other cardiovascular risk factors examined (high triglyceride levels, low HDL cholesterol levels, and the metabolic syndrome) were also associated with an increased risk of microalbuminuria, although these associations did not reach conventional levels of statistical significance (Table 2).
Among nonoverweight adolescents, girls were more likely than boys to have microalbuminuria (OR: 2.07; 95% CI: 1.45–2.96). However, among overweight adolescents, this association was attenuated and no longer statistically significantly (OR: 1.71; 95% CI: 0.19–15.34). Neither race/ethnicity nor age was significantly associated with microalbuminuria among nonoverweight or overweight adolescents.
Sensitivity Analyses
We observed similar associations between the cardiovascular risk factors and microalbuminuria when we used alternative definitions for the metabolic syndrome and its individual criteria, as published by Cook et al,40 de Ferranti et al,41 and Invitti et al23 (which changed the prevalence of the metabolic syndrome to 6.3%, 12.9%, and 6.7%, respectively). Similar results were also observed when we included adolescents with macroalbuminuria and when we excluded adolescents with diabetes mellitus (except that hypertension could not be assessed, because all adolescents with hypertension and microalbuminuria also had diabetes mellitus).
We also used urinary albumin concentrations of ≥30 μg/mL as the outcome, to avoid any potential confounding attributable to differences in generation of creatinine among overweight and nonoverweight adolescents; 15.1% of adolescents had urinary albumin concentrations of ≥30 μg/mL. Urinary albumin concentrations of ≥30 μg/mL were more prevalent among nonoverweight adolescents than among overweight adolescents (14.6% vs 0.5%; P = .0001). We observed associations between the cardiovascular risk factors and urinary albumin concentrations of ≥30 μg/mL similar to those our original analysis (data not shown).
DISCUSSION
Findings From Previous Studies
Because of the burgeoning epidemic of pediatric obesity, increasing attention is being paid to the cardiovascular health of children and adolescents. There is much interest in determining whether microalbuminuria is an early marker of renal and systemic endovascular damage.
Pediatric studies examining the association of cardiovascular risk factors with albuminuria have led to conflicting conclusions. Previous pediatric studies from community-based populations showed that overweight children (with presumably more coexisting cardiovascular risk factors) had less albuminuria than leaner children.24,25 In contrast, studies from referral-based obesity clinics found that, among overweight children, various cardiovascular risk factors were associated with more albuminuria.21–23
Findings From This Study
In the current study, which is based on a large and nationally representative sample of adolescents, we confirmed the unexpected finding that being overweight is associated with lower prevalence of microalbuminuria. Furthermore, among nonoverweight adolescents, all cardiovascular disease risk factors examined were not risk factors for microalbuminuria (with the exception of overt diabetes mellitus). In contrast, within the subgroup of overweight adolescents, cardiovascular risk factors such as impaired fasting glucose, insulin resistance, hypertension, and smoking were associated strongly with microalbuminuria. The novel contribution of this study is to demonstrate that the association between cardiovascular risk factors and microalbuminuria is strongly modified by overweight. This interaction explains the seemingly discrepant reports in the literature.
The observation that cardiovascular risk factors are associated with microalbuminuria among overweight adolescents is consistent with previous observations in the adult population.42–49 Excess weight is thought to increase intraglomerular capillary pressure, resulting in glomerular hyperfiltration, a permissive environment or condition for end-organ damage. In this setting, hypertension, impaired fasting glucose, diabetes mellitus, or smoking may provide a second “hit,” causing endothelial dysfunction that leads to microalbuminuria.50
The basis for the observation, now made in 3 independent studies,24,25 that leaner adolescents are more likely to have microalbuminuria is unknown. We speculate that orthostatic proteinuria may be an important confounding variable. Changes in posture (from supine to upright) can increase urinary protein excretion and is commonly seen in adolescents.51 A preliminary report by Hornberger et al52 indicated that orthostatic proteinuria is more prevalent among nonoverweight adolescents. This observation might explain why nonoverweight adolescents have more microalbuminuria than overweight adolescents.
In this context, it is notable that we and others observed that girls are more likely to have microalbuminuria than boys. Davies et al53 showed that girls have significantly higher daytime but not nighttime urinary albumin excretion, compared with boys, which suggests that girls have more orthostatic albuminuria. Interestingly, we found that this gender difference was attenuated among overweight adolescents. This would also be consistent with the hypothesis that orthostatic proteinuria is less prevalent among overweight adolescents.
Implications of Our Findings
Our results show that overweight adolescents with impaired glucose tolerance and insulin resistance have microalbuminuria. Prolonged exposure to hyperglycemia or unrecognized diabetes mellitus contributes to the early development of microvascular disease in patients with type 2 diabetes mellitus.54–56 Recent data showed that youth-onset type 2 diabetes leads to higher rates of end-stage renal disease and death, compared with adult-onset type 2 diabetes or nondiabetic status.57 This implies that overweight adolescents with impaired glucose tolerance and insulin resistance may benefit from early intervention with angiotensin-converting enzyme inhibitors, in addition to behavioral and lifestyle modifications. Because we also showed that hypertension is associated with microalbuminuria in overweight adolescents, angiotensin-converting enzyme inhibitors may be indicated to treat obesity-related hypertension that is refractory to behavioral and lifestyle modifications.
Unlike in adults, where microalbuminuria is related to excess weight,58,59 our study shows that the relationship between microalbuminuria and excess weight is more complicated in adolescents. Considerations unique to children and adolescents, such as orthostatic proteinuria, need to be taken into account, and studies conducted specifically in children should be undertaken. Our data suggest that more study is required before microalbuminuria is included as a qualifying trait to define the metabolic syndrome, as proposed by the World Health Organization.60 If random urinary albumin/creatinine ratios were used to define microalbuminuria, then a large number of adolescents might be mislabeled as having the metabolic syndrome. Using microalbuminuria to screen the general adolescent population for those at high risk for future renal and cardiovascular disease, as has been proposed for the general adult population, also would not be prudent. However, microalbuminuria can be used to identify high-risk patients within certain pediatric subpopulations, such as overweight and diabetic adolescents. Our studies should spur additional studies to explain why lean adolescents have more microalbuminuria than do overweight adolescents.
Strengths and Limitations
The strengths of this study include the high-quality survey data from a nationally representative sample. The study population is racially and ethnically diverse, which is especially important because renal and cardiovascular complications attributable to obesity disproportionately affect black individuals, Hispanic individuals, and individuals of other racial minorities.61–63
Limitations include the cross-sectional design, which cannot demonstrate temporal relationships among overweight, cardiovascular risk factors, and microalbuminuria. However, previous prospective studies in adults showed that impaired fasting glucose levels preceded the development of microalbuminuria.44 Also, we do not have follow-up data to determine whether overweight adolescents with microalbuminuria indeed had higher rates of adverse cardiovascular and renal disease events, compared with overweight adolescents without microalbuminuria, and whether microalbuminuria is indeed benign in nonoverweight adolescents, as the risk factor profiles suggest. These questions could be investigated in a prospective study of pediatric subjects. Although our study is one of the largest pediatric studies to examine the association of cardiovascular risk factors and microalbuminuria in children, relatively large CIs were obtained. However, we were able to define associations that were statistically and clinically significant.
There were several limitations regarding conditions under which urine was collected. Urine was not collected in the supine position, and some of the proteinuria detected may reflect orthostatic proteinuria. This may be one explanation for why we found a higher prevalence of microalbuminuria in our population than did a Japanese study that examined first morning urine samples.64 In fact, we hypothesize that orthostatic proteinuria may explain some of our paradoxical findings, as elaborated above. A single urine sample was used to estimate the population prevalence of (persistent) microalbuminuria, which might have lead to overestimation, because albuminuria can be transient. Some authors have advocated that multiple first morning urine samples be collected to minimize measurement error.65 However, the effect of measurement error would bias our results to finding no association between cardiovascular risk factors and microalbuminuria. Therefore, the true strength of association may be stronger than we reported. Despite these limitations, we still found a large significant association between cardiovascular risk factors and microalbuminuria.
Other factors that can affect albumin excretion, such as hydration status and exercise, could be taken into account only indirectly. The use of the urinary albumin/creatinine ratio helps adjust for hydration status; urinary albumin concentration alone is greatly affected by urine volume. However, because urinary creatinine levels also vary according to age and gender, the albumin/creatinine ratio may overestimate albuminuria for subjects with lower levels of creatinine excretion (younger children and girls). This study did not measure urine specific gravity or other measurements of urine concentration; however, we measured fluid intake from dietary recall records for the 24-hour period before urine collection. When total fluid intake was included as a confounding variable, the associations of cardiovascular risk factors and microalbuminuria did not change. All urine samples were collected in the morning for this study population, which makes exercise shortly before examination less likely. However, because subjects were not asked to limit physical activity in the 24 hours before urine collection, we could not exclude the possibility that leaner adolescents have more microalbuminuria because they are more likely to exercise. We were able to use a measure of chronic physical activity, which, when included as a confounding variable, had little effect on the associations of cardiovascular risk factors and microalbuminuria.
CONCLUSIONS
There is much interest in determining the consequences of childhood obesity. Albuminuria has emerged as a powerful risk factor for future renal and cardiovascular disease in various adult populations. However, our results indicate that, for adolescents who are not overweight and do not have diabetes mellitus, that is, the majority of adolescents, microalbuminuria is not associated with cardiovascular risk factors. For the subset of adolescents who are overweight, microalbuminuria is a good marker to identify individuals who may need aggressive intervention because of evidence of endovascular damage. Whether our results can be explained by the phenomenon of orthostatic proteinuria should be the subject of future investigations. Our study highlights the need for more outcome-based research in pediatrics, because findings in adults cannot be simply extrapolated to children.
Acknowledgments
This study was supported in part by the National Institutes of Health (grants DK07219 and DK61520) and the Department of Pediatrics, University of California, San Francisco.
Abbreviations
- HDL
high-density lipoprotein
- NHANES
National Health and Nutrition Examination Survey
- OR
odds ratio
- CI
confidence interval
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
This trial has been registered at www.clinicaltrials.gov (identifier 2006-3594).
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 3.Reilly JJ, Methven E, McDowell ZC, et al. Health consequences of obesity. Arch Dis Child. 2003;88:748–752. doi: 10.1136/adc.88.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caballero AE. Endothelial dysfunction in obesity and insulin resistance: a road to diabetes and heart disease. Obes Res. 2003;11:1278–1289. doi: 10.1038/oby.2003.174. [DOI] [PubMed] [Google Scholar]
- 5.Ritz E. Albuminuria and vascular damage: the vicious twins. N Engl J Med. 2003;348:2349–2352. doi: 10.1056/NEJMe030066. [DOI] [PubMed] [Google Scholar]
- 6.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage: the Steno hypothesis. Diabetologia. 1989;32:219–226. doi: 10.1007/BF00285287. [DOI] [PubMed] [Google Scholar]
- 7.Stehouwer CD, Nauta JJ, Zeldenrust GC, Hackeng WH, Donker AJ, den Ottolander GJ. Urinary albumin excretion, cardiovascular disease, and endothelial dysfunction in non-insulin-dependent diabetes mellitus. Lancet. 1992;340:319–323. doi: 10.1016/0140-6736(92)91401-s. [DOI] [PubMed] [Google Scholar]
- 8.Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet. 1982;1:1430–1432. doi: 10.1016/s0140-6736(82)92450-3. [DOI] [PubMed] [Google Scholar]
- 9.Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med. 1984;310:356–360. doi: 10.1056/NEJM198402093100605. [DOI] [PubMed] [Google Scholar]
- 10.Mogensen CE, Christensen CK. Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med. 1984;311:89–93. doi: 10.1056/NEJM198407123110204. [DOI] [PubMed] [Google Scholar]
- 11.Verhave JC, Gansevoort RT, Hillege HL, Bakker SJ, De Zeeuw D, de Jong PE. An elevated urinary albumin excretion predicts de novo development of renal function impairment in the general population. Kidney Int Suppl. 2004;(92):S18–S21. doi: 10.1111/j.1523-1755.2004.09205.x. [DOI] [PubMed] [Google Scholar]
- 12.Hillege HL, Fidler V, Diercks GF, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 13.Romundstad S, Holmen J, Kvenild K, Hallan H, Ellekjaer H. Microalbuminuria and all-cause mortality in 2 089 apparently healthy individuals: a 4.4-year follow-up study: the Nord-Trondelag Health Study (HUNT), Norway. Am J Kidney Dis. 2003;42:466–473. doi: 10.1016/s0272-6386(03)00742-x. [DOI] [PubMed] [Google Scholar]
- 14.Arnlov J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112:969–975. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- 15.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 16.Schrader J, Luders S, Kulschewski A, et al. Microalbuminuria and tubular proteinuria as risk predictors of cardiovascular morbidity and mortality in essential hypertension: final results of a prospective long-term study (MARPLE Study) J Hypertens. 2006;24:541–548. doi: 10.1097/01.hjh.0000209991.48928.c4. [DOI] [PubMed] [Google Scholar]
- 17.Yuyun MF, Khaw K-T, Luben R, et al. Microalbuminuria independently predicts all-cause and cardiovascular mortality in a British population: the European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) population study. Int J Epidemiol. 2004;33:189–198. doi: 10.1093/ije/dyh008. [DOI] [PubMed] [Google Scholar]
- 18.Basi S, Lewis JB. Microalbuminuria as a target to improve cardiovascular and renal outcomes. Am J Kidney Dis. 2006;47:927–946. doi: 10.1053/j.ajkd.2006.02.182. [DOI] [PubMed] [Google Scholar]
- 19.de Zeeuw D, Remuzzi G, Parving HH, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation. 2004;110:921–937. doi: 10.1161/01.CIR.0000139860.33974.28. [DOI] [PubMed] [Google Scholar]
- 20.Ibsen H, Olsen MH, Wachtell K, et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension. 2005;45:198–202. doi: 10.1161/01.HYP.0000154082.72286.2a. [DOI] [PubMed] [Google Scholar]
- 21.Burgert TS, Dziura J, Yeckel C, et al. Microalbuminuria in pediatric obesity: prevalence and relation to other cardiovascular risk factors. Int J Obes (Lond) 2005;30:273–280. doi: 10.1038/sj.ijo.0803136. [DOI] [PubMed] [Google Scholar]
- 22.Csernus K, Lanyi E, Erhardt E, Molnar D. Effect of childhood obesity and obesity-related cardiovascular risk factors on glomerular and tubular protein excretion. Eur J Pediatr. 2005;164:44–49. doi: 10.1007/s00431-004-1546-2. [DOI] [PubMed] [Google Scholar]
- 23.Invitti C, Maffeis C, Gilardini L, et al. Metabolic syndrome in obese Caucasian children: prevalence using WHO-derived criteria and association with nontraditional cardiovascular risk factors. Int J Obes (Lond) 2006;30:627–633. doi: 10.1038/sj.ijo.0803151. [DOI] [PubMed] [Google Scholar]
- 24.Bangstad HJ, Dahl-Jorgensen K, Kjaersgaard P, Mevold K, Hanssen KF. Urinary albumin excretion rate and puberty in non-diabetic children and adolescents. Acta Paediatr. 1993;82:857–862. doi: 10.1111/j.1651-2227.1993.tb17628.x. [DOI] [PubMed] [Google Scholar]
- 25.Mueller PW, Caudill SP. Urinary albumin excretion in children: factors related to elevated excretion in the United States population. Ren Fail. 1999;21:293–302. doi: 10.3109/08860229909085091. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Examination Survey Data. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention; 1999–2004. Available at www.cdc.gov/nchs/nhanes.htm. [Google Scholar]
- 27.Perloff D, Grim C, Flack J, et al. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Laboratory Procedure Manual. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2001. [Accessed November 26, 2007]. Available at: www.cdc.gov/nchs/data/nhanes/lab1-6.pdf; www.cdc.gov/nchs/data/nhanes/lab7-11.pdf. [Google Scholar]
- 29.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS) MEC Laboratory Component: Plasma Glucose, Serum C-peptide, and Insulin. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2006. [Accessed November 26, 2007]. National Health and Nutrition Examination Survey 2003–2004. Available at: www.cdc.gov/nchs/data/nhanes/nhanes_03_04/110am_c.pdf. [Google Scholar]
- 30.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145:439–444. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 31.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(suppl 4th Report):555–576. [PubMed] [Google Scholar]
- 32.American Academy of Pediatrics. National Cholesterol Education Program: Report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics. 1992;89:525–584. [PubMed] [Google Scholar]
- 33.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(suppl 1):S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 34.Reinehr T, Andler W. Changes in the atherogenic risk factor profile according to degree of weight loss. Arch Dis Child. 2004;89:419–422. doi: 10.1136/adc.2003.028803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weitzman M, Cook S, Auinger P, et al. Tobacco smoke exposure is associated with the metabolic syndrome in adolescents. Circulation. 2005;112:862–869. doi: 10.1161/CIRCULATIONAHA.104.520650. [DOI] [PubMed] [Google Scholar]
- 36.Hogg RJ, Furth S, Lemley KV, et al. National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease in children and adolescents: evaluation, classification, and stratification. Pediatrics. 2003;111:1416–1421. doi: 10.1542/peds.111.6.1416. [DOI] [PubMed] [Google Scholar]
- 37.Eknoyan G, Hostetter T, Bakris GL, et al. Proteinuria and other markers of chronic kidney disease: a position statement of the National Kidney Foundation (NKF) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Am J Kidney Dis. 2003;42:617–622. doi: 10.1016/s0272-6386(03)00826-6. [DOI] [PubMed] [Google Scholar]
- 38.National Center for Health Statistics. National Health and Nutrition Examination Survey Analytic and Reporting Guidelines. Hyattsville, MD: National Center for Health Statistics; 2006. [Google Scholar]
- 39.Conover WJ, Iman RL. Rank transformations as a bridge between parametric and nonparametric statistics. Am Stat. 1981;35:124–129. [Google Scholar]
- 40.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 41.de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, New-burger JW, Rifai N. Prevalence of the metabolic syndrome in American adolescents: findings from the Third National Health and Nutrition Examination Survey. Circulation. 2004;110:2494–2497. doi: 10.1161/01.CIR.0000145117.40114.C7. [DOI] [PubMed] [Google Scholar]
- 42.Cirillo M, Senigalliesi L, Laurenzi M, et al. Microalbuminuria in nondiabetic adults: relation of blood pressure, body mass index, plasma cholesterol levels, and smoking: the Gubbio Population Study. Arch Intern Med. 1998;158:1933–1939. doi: 10.1001/archinte.158.17.1933. [DOI] [PubMed] [Google Scholar]
- 43.Ejerblad E, Fored CM, Lindblad P, et al. Association between smoking and chronic renal failure in a nationwide population-based case-control study. J Am Soc Nephrol. 2004;15:2178–2185. doi: 10.1097/01.ASN.0000135048.35659.10. [DOI] [PubMed] [Google Scholar]
- 44.Fujikawa R, Okubo M, Egusa G, Kohno N. Insulin resistance precedes the appearance of albuminuria in non-diabetic subjects: 6 years follow up study. Diabetes Res Clin Pract. 2001;53:99–106. doi: 10.1016/s0168-8227(01)00241-8. [DOI] [PubMed] [Google Scholar]
- 45.Hoehner CM, Greenlund KJ, Rith-Najarian S, Casper ML, McClellan WM. Association of the insulin resistance syndrome and microalbuminuria among nondiabetic native Americans: the Inter-Tribal Heart Project. J Am Soc Nephrol. 2002;13:1626–1634. doi: 10.1097/01.asn.0000015762.92814.85. [DOI] [PubMed] [Google Scholar]
- 46.Horner D, Fliser D, Klimm HP, Ritz E. Albuminuria in normotensive and hypertensive individuals attending offices of general practitioners. J Hypertens. 1996;14:655–660. doi: 10.1097/00004872-199605000-00016. [DOI] [PubMed] [Google Scholar]
- 47.Palaniappan L, Carnethon M, Fortmann SP. Association between microalbuminuria and the metabolic syndrome: NHANES III. Am J Hypertens. 2003;16:952–958. doi: 10.1016/s0895-7061(03)01009-4. [DOI] [PubMed] [Google Scholar]
- 48.Pinto-Sietsma SJ, Mulder J, Janssen WM, Hillege HL, de Zeeuw D, de Jong PE. Smoking is related to albuminuria and abnormal renal function in nondiabetic persons. Ann Intern Med. 2000;133:585–591. doi: 10.7326/0003-4819-133-8-200010170-00008. [DOI] [PubMed] [Google Scholar]
- 49.Tozawa M, Iseki K, Iseki C, Oshiro S, Ikemiya Y, Takishita S. Influence of smoking and obesity on the development of proteinuria. Kidney Int. 2002;62:956–962. doi: 10.1046/j.1523-1755.2002.00506.x. [DOI] [PubMed] [Google Scholar]
- 50.Nenov VD, Taal MW, Sakharova OV, Brenner BM. Multi-hit nature of chronic renal disease. Curr Opin Nephrol Hypertens. 2000;9:85–97. doi: 10.1097/00041552-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Hogg RJ. Adolescents with proteinuria and/or the nephrotic syndrome. Adolesc Med Clin. 2005;16:163–172. doi: 10.1016/j.admecli.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 52.Hornberger L, Simon S, Hampl S, Brewer L, Alon US. Prevalence of Proteinuria and Its Relationship to Body Mass in Adolescents. San Francisco, CA: Pediatric Academic Society; 2006. [Google Scholar]
- 53.Davies AG, Postlethwaite RJ, Price DA, Burn JL, Houlton CA, Fielding BA. Urinary albumin excretion in school children. Arch Dis Child. 1984;59:625–630. doi: 10.1136/adc.59.7.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haffner SM, Gonzales C, Valdez RA, et al. Is microalbuminuria part of the prediabetic state? The Mexico City Diabetes Study. Diabetologia. 1993;36:1002–1006. doi: 10.1007/BF02374491. [DOI] [PubMed] [Google Scholar]
- 55.Mykkanen L, Haffner SM, Kuusisto J, Pyorala K, Laakso M. Microalbuminuria precedes the development of NIDDM. Diabetes. 1994;43:552–557. doi: 10.2337/diab.43.4.552. [DOI] [PubMed] [Google Scholar]
- 56.Eppens MC, Craig ME, Cusumano J, et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care. 2006;29:1300–1306. doi: 10.2337/dc05-2470. [DOI] [PubMed] [Google Scholar]
- 57.Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA. 2006;296:421–426. doi: 10.1001/jama.296.4.421. [DOI] [PubMed] [Google Scholar]
- 58.de Jong PE, Verhave JC, Pinto-Sietsma SJ, Hillege HL. Obesity and target organ damage: the kidney. Int J Obes Relat Metab Disord. 2002;26(suppl 4):S21–S24. doi: 10.1038/sj.ijo.0802213. [DOI] [PubMed] [Google Scholar]
- 59.Liese AD, Hense HW, Doring A, Stieber J, Keil U. Microalbuminuria, central adiposity and hypertension in the non-diabetic urban population of the MONICA Augsburg survey 1994/95. J Hum Hypertens. 2001;15:799–804. doi: 10.1038/sj.jhh.1001266. [DOI] [PubMed] [Google Scholar]
- 60.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications, part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 61.Krop JS, Coresh J, Chambless LE, et al. A community-based study of explanatory factors for the excess risk for early renal function decline in blacks vs whites with diabetes: the Atherosclerosis Risk in Communities study. Arch Intern Med. 1999;159:1777–1783. doi: 10.1001/archinte.159.15.1777. [DOI] [PubMed] [Google Scholar]
- 62.Tareen N, Zadshir A, Martins D, Pan D, Nicholas S, Norris K. Chronic kidney disease in African American and Mexican American populations. Kidney Int Suppl. 2005;(97):S137–S140. doi: 10.1111/j.1523-1755.2005.09723.x. [DOI] [PubMed] [Google Scholar]
- 63.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 64.Pugia MJ, Lott JA, Kajima J, et al. Screening school children for albuminuria, proteinuria and occult blood with dipsticks. Clin Chem Lab Med. 1999;37:149–157. doi: 10.1515/CCLM.1999.027. [DOI] [PubMed] [Google Scholar]
- 65.Gibb DM, Shah V, Preece M, Barratt TM. Variability of urine albumin excretion in normal and diabetic children. Pediatr Nephrol. 1989;3:414–419. doi: 10.1007/BF00850218. [DOI] [PubMed] [Google Scholar]