Summary
Mitochondrial Ca2+ uptake via the uniporter is central to cell metabolism, signaling and survival. Recent studies identified MCU as the uniporter’s likely pore and MICU1, an EF-hand protein, as its critical regulator. How this complex decodes dynamic cytoplasmic [Ca2+] ([Ca2+]c) signals, to tune out small [Ca2+]c increases yet permit pulse transmission, remains unknown. We report that loss of MICU1 in mouse liver and cultured cells causes mitochondrial Ca2+ accumulation during small [Ca2+]c elevations, yet an attenuated response to agonist-induced [Ca2+]c pulses. The latter reflects loss of positive cooperativity, likely via the EF-hands. MICU1 faces the intermembrane space and responds to [Ca2+]c changes. Prolonged MICU1 loss leads to an adaptive increase in matrix Ca2+ binding, yet cells show impaired oxidative metabolism and sensitization to Ca2+ overload. Collectively, the data indicate that MICU1 senses the [Ca2+]c to establish the uniporter’s threshold and gain, thereby allowing mitochondria to properly decode different inputs.
Keywords: calcium signaling, mitochondria, Ca2+ uniporter, oxidative metabolism, cell death, MCU
Introduction
Measurements of mitochondrial matrix [Ca2+] ([Ca2+]m) in intact cells have revealed that mitochondria respond to hormone and neurotransmitter-induced [Ca2+]c signals by robust increases in [Ca2+]m, which, in turn, effectively stimulate oxidative metabolism (Hajnoczky et al., 1995; Jouaville et al., 1999; Pralong et al., 1994; Rizzuto et al., 1994; Robb-Gaspers et al., 1998). Prolonged stimulation of mitochondrial Ca2+ uptake in combination with other forms of cellular stress induces Ca2+ overload and cell death (Pinton et al., 2001; Szalai et al., 1999). Thus, mitochondrial Ca2+ import must be tightly controlled to avoid continuous uptake while also ensuring a rapid response to [Ca2+]c spikes. Indeed, mitochondrial Ca2+ uptake effectively decodes different signal patterns: it responds to frequency-modulated [Ca2+]c oscillations and tunes out moderate [Ca2+]c increases (Hajnoczky et al., 1995).
One facet of the mitochondria’s ability to decode [Ca2+]c signals is their strategic localization close to ER/SR Ca2+ channels, the source of [Ca2+]c spikes and oscillations, where they are exposed to high [Ca2+]c microdomains in the vicinity of activated IP3 receptors (IP3R) and ryanodine receptors (RyR). Indeed, favorably localized mitochondria seem to respond to spatially confined [Ca2+]c sparks and puffs (Marchant et al., 2002; Pacher et al., 2002). The close associations and tethering between mitochondria and ER/SR were demonstrated in both intact live cells and in ultrastructural studies (Csordas et al., 2006; de Brito and Scorrano, 2008; Rizzuto et al., 1998; Szabadkai et al., 2006; Tinel et al., 1999). Most recently, exposure of mitochondria to a local 10μM [Ca2+]c rise during IP3R-mediated ER Ca2+ release was also documented (Csordas et al., 2010; Giacomello et al., 2010). However, the spatial coupling alone cannot explain the highly cooperative nature of mitochondrial Ca2+ uptake. Indeed, Ca2+-and time-dependent activation of mitochondrial Ca2+ uptake has been observed (Csordas and Hajnoczky, 2003; Kroner, 1986), but the missing molecular identity of the mitochondrial Ca2+ uniporter (mtCU) prevented a better understanding of the non-linear behavior.
A seminal patch clamp study of mitoplasts provided evidence that the mtCU is a highly Ca2+ selective ion channel (IMiCa) (Kirichok et al., 2004). Early candidates for the mtCU were mitochondria-localized RyR1 (Beutner et al., 2005) and UCP2/3 (Trenker et al., 2007), but these proteins are not expressed in some tissues displaying robust mtCU activity. Recently, LETM1 was identified as a protein that imports Ca2+ by Ca2+/H+ exchange when [Ca2+]m is low and is therefore unlikely to contribute to Ca2+ uptake as a Ca2+ channel (Jiang et al., 2009). A recent landmark study identified and proposed MICU1 as a critical regulator of the mtCU (Perocchi et al., 2010), paving the way to the molecular identification of MCU as the putative pore-forming component (Baughman et al., 2011; De Stefani et al., 2011). MCU is a transmembrane protein of the inner mitochondrial membrane (IMM) with two predicted transmembrane domains connected by a loop that seems to contribute to the selectivity filter (Baughman et al., 2011; De Stefani et al., 2011). MCU likely oligomerizes to form a pore. MICU1 resides within a complex with MCU and contains a pair of Ca2+ binding EF-hand motifs (Baughman et al., 2011; Perocchi et al., 2010). The two proteins exhibit striking co-evolution and co-expression (Baughman et al., 2011; Bick et al., 2012) indicating a particularly close functional relationship. Since MICU1 is likely a Ca2+ sensing component of the mtCU, we set out to determine its exact role in the decoding function of mitochondrial Ca2+ uptake and its physiological relevance.
We show that upon MICU1 depletion, mitochondrial Ca2+ uptake is sensitized to low [Ca2+]c levels, and the cooperativity of uptake with rising [Ca2+]c is decreased. We show that MICU1 is associated with the IMM, facing the intermembrane space (IMS) to sense outside Ca2+. Expression of an EF-hand mutant restores the [Ca2+]c threshold but not cooperativity. These results suggest that MICU1 controls both [Ca2+]c threshold and cooperativity of the mtCU, with the latter being dependent on Ca2+ binding to MICU1. Furthermore, MICU-deficient mitochondria fail to effectively respond to short lasting high [Ca2+]c microdomains during calcium oscillations while also failing to ignore submicromolar [Ca2+]c increases. As a result, MICU1-deficient cells show dysregulation of oxidative metabolism and decreased cell tolerance to stress.
Results
Ca2+ handling in MICU1-deficient primary hepatocytes and HeLa cells
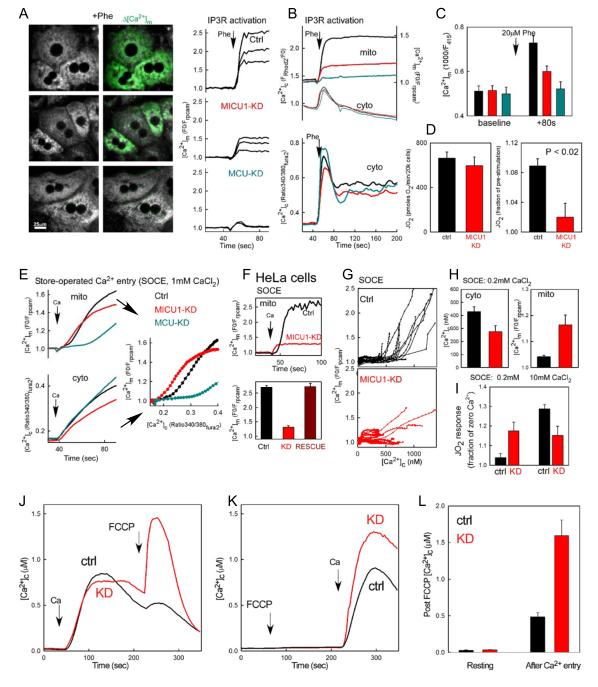
To test the physiological relevance of MICU1 in calcium signaling, we evaluated [Ca2+]c and [Ca2+]m homeostasis in MICU1-deficient hepatocytes. In vivo silencing in mouse liver effectively and specifically decreased MICU1 or MCU mRNA and protein in hepatocytes (SFig1A). Stimulation of hepatocytes with Ca2+ mobilizing agonists elicits [Ca2+]c spikes and oscillations, which are effectively propagated to mitochondria due to local exposure of mitochondria to high [Ca2+]c microdomains by adjacent IP3Rs (Hajnoczky et al., 1995; Robb-Gaspers et al., 1998). The [Ca2+]c rise stimulated by phenylephrine (Fig1ABC) or vasopressin (SFig1D), was unaffected by MICU1 or MCU knockdown, whereas the corresponding [Ca2+]m response was reduced by MICU1 and abolished by MCU silencing (Fig1B). At resting [Ca2+]c (≤100 nM), the [Ca2+]m was unaltered by depletion of MICU1 or MCU, as determined by a genetically-targeted Ca2+ reporter (Fig1C). Furthermore, releasing mitochondrial Ca2+ by elimination of the membrane potential (ΔΨm) with an uncoupler (FCCP 5μM) caused a similar [Ca2+]c rise in control (Ctrl) and MICU1-deficient cells (MICU1-KD) (SFig1B).
Fig1. Ca2+ handling in MICU1-KD hepatocytes and HeLa cells.
(A) Phenylephrine (Phe, 20 μM)-induced [Ca2+]m signals in control (Ctrl), MICU1-KD and MCU-KD mouse hepatocytes. Left: fluorescence images of mitochondria-targeted ratiometric pericam (mtrpcam) obtained with excitation at the pH-insensitive excitation wavelength (415nm) overlaid with a green difference image depicting changes during Phe stimulation. Right: corresponding [Ca2+]m time courses. Rpcam fluorescence is inversely normalized to the baseline (F0/F). (B) Mean [Ca2+]c and [Ca2+]m signals obtained by simultaneous recording of rhod2 and mtrpcam fluorescence (n=43-57 cells, upper) and [Ca2+]c signals recorded separately using fura2 (n=71-121, lower). (C) Fluorescence of mtrpcam in resting and Phestimulated Ctrl, MICU1-KD and MCU-KD primary hepatocytes (n=50-62 from 3 different mice). Fluorescence values are shown in arbitrary units, inverted but without normalization to allow comparison of the resting [Ca2+]m. (D) Resting and agonist-stimulated JO2 in Ctrl and MICU1-KD hepatocytes (n=4). (E) SOCE-associated [Ca2+]c (fura2) and [Ca2+]m (mtrpcam) signals monitored separately in Ctrl, MICU1-KD and MCU-KD hepatocytes pretreated with Tg in a Ca2+-free ECM. To evoke SOCE 1 mM CaCl2 (Ca) was added. Left: mean time courses. Right: [Ca2+]m vs. [Ca2+]c (n=144-172 cells for each). (F) [Ca2+]m responses to SOCE in Ctrl, MICU1-KD and RESCUED stable HeLa cells. The mean traces (upper) and mean peak values (lower) of the single cell recordings are shown. (n=16-52 for each). (G) [Ca2+]m vs. [Ca2+]c curves of individual Ctrl (top) and MICU1-KD cells (bottom) from similar experiments as in (F). (H) Mean peak [Ca2+]c (fura2) and [Ca2+]m (mtipcam) levels recorded during SOCE induced by addition of 0.2mM CaCl2 in stable MICU1-KD and Ctrl HeLa cells (n=16-21). (I) Measurement of the JO2 response during SOCE (n = 4 plates/genotype, 3-4 wells/condition/plate). (J-L) [Ca2+]c rise caused by FCCP after (J) and before SOCE (K).
[Ca2+]m signals stimulate oxidative metabolism that can be assessed by measurements of the O2 consumption rate (JO2). Consistent with an unaltered resting [Ca2+]m, resting JO2 was found similar in both Ctrl and MICU1-KD (Fig1D). However, upon IP3-linked stimulation the increase in JO2 was relatively small in MICU1-KD, reflecting the blunted [Ca2+]m rise (Fig1D).
In contrast to IP3-linked stimulation, wherein mitochondria are exposed to high Ca2+ microdomains, during store operated Ca2+ entry (SOCE) most mitochondria respond to the bulk [Ca2+]c increase (Hajnoczky et al., 1995). The SOCE-mediated [Ca2+]m increase appeared early but the maximum amplitude was blunted in MICU1-KD, while the it was greatly suppressed and delayed in MCU-KD (Fig1E). Plotting [Ca2+]m against [Ca2+]c underscores that MICU1-KD cells show a significant [Ca2+]m increase at lower [Ca2+]c than Ctrl and further reveals the distinct effects of MICU1 and MCU depletion on the [Ca2+]c sensitivity of mitochondria (Fig1E right).
Similar results were obtained in HeLa cells with stable MICU1-KD (see verification of KD in SFig1C), where the specificity of the changes associated with MICU1 silencing was validated by rescuing cells by re-expression of MICU1 (Fig1F). Moreover, simultaneous [Ca2+]c and [Ca2+]m measurements in single cells further supported that relatively low [Ca2+]c was sufficient to evoke a [Ca2+]m increase in MICU1-KD (Fig1G). Ctrl cells showed the onset of the [Ca2+]m signal in the 0.5-1 μM range of [Ca2+]c but in most MICU1-KD cells, a small [Ca2+]m increase also occurred at [Ca2+]c <500 nM (Fig1G). Consistently, when extracellular [Ca2+] was kept low (0.2 mM CaCl2), the SOCE-induced [Ca2+]c rise (300-400 nM) evoked a [Ca2+]m increase in MICU1-KD but not in Ctrl or MCU-KD (Fig1H and not shown). An increase in JO2 was likewise observed only in the MICU1-KD (Fig1I left, resting levels: SFig1E). However, when higher extracellular [Ca2+] was used to support SOCE, the [Ca2+]m increase and JO2 response were larger in the Ctrl (Fig1F&I right). Thus, stimulation of oxidative metabolism closely follows the MICU1-KD-induced changes in the [Ca2+]m signal.
The results obtained in both hepatocytes and HeLa cells using several different approaches show that: (1) when [Ca2+]c is maintained at <100nM by high affinity plasma membrane and ER Ca2+ pumps, the [Ca2+]m and the amount of matrix Ca2+ mobilized by ΔΨm dissipation are not significantly affected by MICU1 depletion, (2) [Ca2+]m signals are attenuated in MICU1-KD, as previously reported (Perocchi et al. 2010), but (3) some [Ca2+]m rise can be evoked by small [Ca2+]c increases in MICU1-KD.
[Ca2+]m reflects the combined contributions of mitochondrial Ca2+ fluxes (mtCU-mediated uptake and exchanger-mediated efflux) and Ca2+ chelation within the matrix. In intact cells, the mitochondrial Ca2+ content can be estimated by the [Ca2+]c increase evoked by a mitochondrial uncoupler. Addition of FCCP after SOCE resulted in a larger [Ca2+]c increase in MICU1-KD than in Ctrl (Fig1J,L). The surplus Ca2+ is likely sequestered by mitochondria, as the ER Ca2+ store was depleted by pre-treatment with thapsigargin (Tg), and washout of extracellular Ca2+ did not prevent the greater [Ca2+]c response to FCCP in MICU1-KD vs. Ctrl (n=3, not shown). Uncoupler-sensitive Ca2+ storage must occur during SOCE, since FCCP added before SOCE caused no [Ca2+]c increase in either MICU1-KD or Ctrl (SFig1B, Fig1K). Notably, the SOCE-induced [Ca2+]c increase after FCCP-pretreatment was larger in MICU1-KD than in Ctrl (Fig1K), indicating an augmented SOCE. The rapid decay of the FCCP-induced Ca2+ mobilization (Fig1J) indicates that the plasma membrane Ca2+ pump remains functional and therefore SOCE seems to be up-regulated as a consequence of MICU1 depletion. Importantly, inhibition of mitochondrial Ca2+ uptake by FCCP pre-treatment enhanced the SOCE-induced [Ca2+]c rise in MICU1-KD but not in Ctrl (Fig1K vs J), further supporting that enhanced Ca2+ uptake by mitochondria effectively buffers [Ca2+]c during SOCE in MICU1-KD. Thus MICU1-KD mitochondria avidly take up Ca2+ during SOCE, which may be in part due to increased SOCE.
MICU1 controls the threshold of mitochondrial Ca2+ uptake
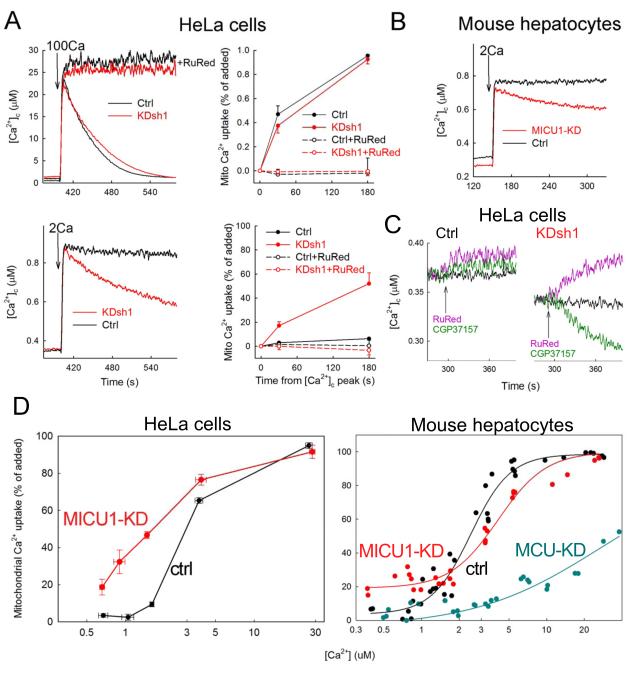
To directly monitor mitochondrial Ca2+ uptake, we measured the ruthenium red (RuRed)-sensitive clearance of Ca2+ in the cytoplasmic buffer in suspensions of permeabilized HeLa cells (Fig2A). When [Ca2+]c was increased to 30 μM, both Ctrl and MICU1-KD showed rapid clearance (Fig2A upper). ER Ca2+ uptake could not participate in the Ca2+ clearance since Tg was present. To confirm the appearance of Ca2+ in mitochondria, we next quantified the total mitochondrial calcium using 45Ca (SFig2A). Upon exposure to ~30μM [Ca2+]c (100μM 45CaCl2 was added), a massive increase in mitochondrial 45Ca in both Ctrl and MICU1-KD (SFig2A) confirmed that both MICU1-KD and Ctrl mitochondria effectively take up Ca2+ when exposed to supra-physiological Ca2+ concentrations.
Fig2. MICU1 controls the threshold of mitochondrial Ca2+ uptake.
(A) Clearance of Ca2+ added to the cytoplasmic buffer in suspensions of permeabilized HeLa cells incubated in the presence of Tg (2 μM). Left: time courses of [Ca2+]c upon addition of 100 μM (100Ca, upper) or 2 μM (2Ca, lower) CaCl2 to Ctrl (black) and MICU1-KD (red) cells in the absence and presence of RuRed (3 μM). Right: mitochondrial Ca2+ uptake as the percent recovery of the initial [Ca2+]c rise 30s and 180s after Ca2+ addition (means+S.E., n=11). (B) Mitochondrial clearance of [Ca2+]c elevations evoked by 2μM added CaCl2 in permeabilized Ctrl and MICU1-KD hepatocytes. (C) Mitochondrial maintenance of basal steady-state [Ca2+]c in suspensions of permeabilized Ctrl (left) and MICU1-KD (right) HeLa cells as determined by inhibitors of mitochondrial Ca2+ uptake (RuRed, pink) and Na+-dependent extrusion (CGP37157 20 μM, green). (D) [Ca2+]c dose-response of the mitochondrial Ca2+ uptake in HeLa cells (left) and hepatocytes (right), determined as in Fig2A. The CaCl2 doses added were (in μM) 1, 2, 5, 10, 100 (n=4-9). For the hepatocytes, a sigmoid curve fitted to each data set is shown.
The results shown in Fig1 indicated that MICU1-KD mitochondria might actually sequester more Ca2+ than normal mitochondria during SOCE that raises [Ca2+]c to ≤1 μM. To this end, mitochondrial Ca2+ uptake was also tested in permeabilized cells exposed to a submicromolar [Ca2+]c increase (Fig2A lower). When [Ca2+]c was increased to 750nM, [Ca2+]c clearance was hardly detectable in Ctrl cells, whereas MICU1-KD mitochondria progressively decreased [Ca2+]c (Fig2A lower). This was abolished by RuRed, confirming the role of mtCU (Fig2A). Furthermore, simultaneous [Ca2+]c and ΔΨm measurements showed that mitochondrial depolarization was apparent at lesser [Ca2+]c increases in MICU1-KD (SFig2B). Similar to HeLa cells, in primary hepatocytes, submicromolar [Ca2+]c elevations elicited progressive Ca2+ clearance only in MICU1-KD (Fig2B).
To determine the lower threshold of mitochondrial Ca2+ uptake in MICU1-KD, the steady-state [Ca2+]c was evaluated in permeabilized cell suspensions (Fig2C). In Ctrl, the [Ca2+]c was stabilized at ~400 nM, whereas in MICU1-KD, a lower [Ca2+]c was attained (Fig2C black traces). Addition of RuRed did not affect the steady-state in Ctrl, whereas in MICU1-KD, a gradual [Ca2+]c rise took place until the steady-state level of the Ctrl was attained (Fig2C purple traces). On the other hand, when the exchanger-mediated mitochondrial Ca2+ extrusion was inhibited by CGP37157, [Ca2+]c progressively decreased to <300 nM in MICU1-KD but not in Ctrl (Fig2C green).
These results prompted us to further consider the effect of MICU1-KD on a range of Ca2+ doses (Fig2D). Based on these [Ca2+]c clearance dose-response curves, mitochondria of Ctrl cells showed steep activation of Ca2+ uptake starting at around 1 μM in both HeLa cells and hepatocytes (Fig2D), consistent with a vast amount of literature. However, in MICU1-KD Ca2+ accumulation was detectable well below 1 μM [Ca2+]c. In contrast to MICU1-KD, mitochondria of MCU-KD hepatocytes showed great suppression of Ca2+ uptake over the entire [Ca2+]c range (Fig2D). These results strongly support the unexpected finding that without MICU1, mitochondrial Ca2+ uptake is greatly sensitized to Ca2+.
MICU1 contributes to cooperative activation of the uniporter
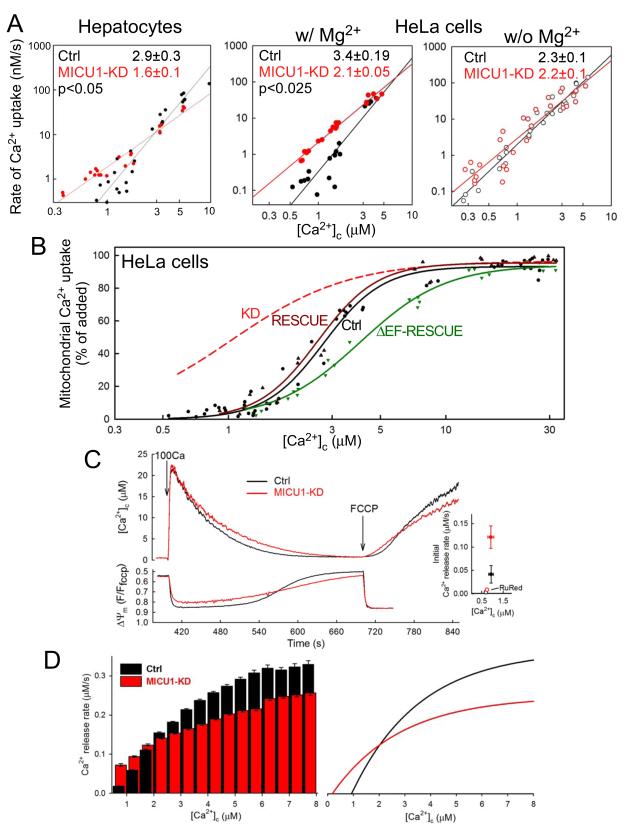
The difference in sigmoidal shape of the [Ca2+]c dose-response for mitochondrial Ca2+ uptake in MICU1-KD vs. Ctrl in both hepatocytes and HeLa cells (Fig2D) prompted us to further explore the the role of MICU1 in mtCU’s cooperativity. To this end, double logarithmic plots of initial Ca2+ uptake rates against [Ca2+]c were created (Fig3A). MICU1-KD in both hepatocytes and HeLa cells showed significantly lesser slope than their respective controls (Fig3A; Hepatocytes; KD: 1.6±0.1 vs. Ctrl: 2.9±0.3, p<0.05 and HeLa cells; KD: 2.1±0.05 vs. Ctrl: 3.4±0.19, p<0.025). Previous literature has proposed that the cooperativity of mtCU’s activation is affected by the presence of Mg2+ (Favaron and Bernardi, 1985; Kroner, 1986). Without Mg2+, the difference in slope between Ctrl and MICU1-KD was no longer detectable and both became similar to MICU1-KD in the presence of Mg2+ (Fig3A right, KD: 2.2±0.1 vs. Ctrl: 2.3±0.1). This may explain why the effect of MICU1 depletion on the mtCU’s cooperativity remained undetected in a recent study performed in Mg2+-free buffer (Mallilankaraman et al., 2012b).
Fig3. MICU1 contributes to cooperative activation of mtCU.
(A) Double logarithmic plots of initial Ca2+ uptake rates vs. [Ca2+]c in Ctrl and MICU1-KD hepatocytes (left) and stable HeLa cells in the presence (middle) and absence of Mg2+ (right). Slope of linear fit for each data set is shown (n=3). (B) [Ca2+]c dose-responses of the fractional mitochondrial Ca2+ uptake in Ctrl, RESCUE and ΔEF-RESCUE HeLa cells. Data points from individual recordings (≥3 for each Ca2+ dose) and a sigmoid (logistic) fit to each data set are shown. The sigmoid fit for MICU1-KD cells from Fig2D is shown as a reference (red). (C) [Ca2+]c (top) and ΔΨm (bottom) time courses showing the mitochondrial clearance of a 100 μM CaCl2 pulse (100Ca) and subsequent FCCP-induced Ca2+ release in suspensions of permeabilized HeLa cells. Inset: mean initial Ca2+ release ([Ca2+]c rise) rates from recordings where by the time of FCCP addition [Ca2+]c dropped below 1.2 μM (Means±S.E., n=3). Rates obtained from recordings in the presence of RuRed (3 μM) added 30 s before FCCP are shown with open symbol. (D) Left: Instantaneous rate of FCCP-induced Ca2+ efflux at each [Ca2+]c (0.5μM binning) obtained via differentiation of the initial period of the [Ca2+]c rises (n=7). Right: Instantaneous Ca2+ release rates extrapolated from compilation of differentiated sigmoidal (logistic) fits. Predicted 0 crossings (the effective thresholds) are 937 and 185 nM for Ctrl and MICU1-KD, respectively.
MICU1 is an EF-hand protein and has been proposed as a Ca2+ sensor for mitochondrial Ca2+ uptake (Perocchi et al., 2010). To test if the Ca2+ sensitivity of mtCU is affected by MICU1’s EF-hands, we compared mitochondrial Ca2+ uptake in Ctrl and MICU1-KD rescued by either wild type MICU1 (RESCUE) or a MICU1 with both EF-hands mutated to prevent Ca2+-binding (ΔEF-RESCUE) (Perocchi et al., 2010). Expression of the rescue constructs was validated by immunoblotting (SFig3A). Both RESCUE and ΔEF-RESCUE mitochondria were able to take up Ca2+ in permeabilized cell assays (SFig3B); however, while the [Ca2+]c dose-response curve was similar in RESCUE and Ctrl, a rightward-shift and less-steep sigmoidal shape was apparent in ΔEF-RESCUE (Fig3B). In double logarithmic plots, expression of the wild type MICU1 construct in MICU1-KD restored the slope observed in the control cells (3.1±0.28) but ΔEF-RESCUE failed to do so (2.5±0.18). Notably, both RESCUE and ΔEF-RESCUE are able to restore the blockade of uptake at low [Ca2+]c. Thus MICU1 is involved in both the closed state and cooperative activation of mtCU, but the EF hands are dispensable for promoting closure at low [Ca2+]c levels and are likely to be relevant in setting the apparent Ca2+ affinity of the mtCU via their Ca2+ binding. However, a limitation of these experiments is that the Ca2+ uptake might be affected by the simultaneous changes in ΔΨm (see SFig2B).
To avoid such complications, we decided to also assess the [Ca2+] dependence of mtCU-mediated Ca2+ efflux in mitochondria of permeabilized cells preloaded with high Ca2+ in the presence of Tg. Once [Ca2+]c returned to near starting levels by mitochondrial uptake, FCCP was added, causing essentially instant dissipation of ΔΨm, followed by an increase in [Ca2+]c as Ca2+ was released from mitochondria (Fig3C). The initial [Ca2+]c rise was greatly suppressed by RuRed, confirming that it was mainly a result of mtCU-mediated Ca2+ efflux (Fig3C inset). When FCCP was added to MICU1-KD cells that had returned [Ca2+]c to ≤1μM, the [Ca2+]c rise started without delay and much faster than in Ctrl cells at similar [Ca2+]c (Fig3C). Hence, mitochondrial Ca2+ uptake and the ensuing increase in [Ca2+]m did not abolish the difference in mtCU activation between MICU1-KD and Ctrl. Additionally, MICU1-KD showed no or less sigmoidal Ca2+ release kinetic and smaller maximum rate of [Ca2+]c rise than the Ctrl (Fig3C). The double logarithmic plot of initial release rates vs. [Ca2+]c also indicated lesser cooperativity for MICU1-KD than for control (SFig3C), further supporting the role of MICU1 in activating mtCU. When the FCCP-induced [Ca2+]c increase traces were combined and the average instantaneous release rates were calculated for a range of [Ca2+]c, the MICU1-KD showed higher rates at <2μM but a steeper increase appeared in the Ctrl with the [Ca2+]c increase leading to higher maximum rates (Fig3D left). MICU1 RESCUE cells likewise showed a steeper increase in the Ca2+ release rate between 2 and 8μM [Ca2+]c than ΔEF-RESCUE cells as well as a higher maximum rate (SFig3D), providing evidence that the EF-hands of MICU1 are central to the cooperative activation of the mtCU. Fitting exponential regressions to the complete data sets generated from the experiments (Fig3D right) highlights the dual role of MICU1 in keeping mtCU’s gate closed at submicromolar [Ca2+]c and maximizing the Ca2+ flux at several μM [Ca2+]c (Fig3D right). The switch between these two effects is likely to be mediated by Ca2+ binding to the EF-hands of MICU1. In technical terms, MICU1 operates as a high-pass filter that provides thresholding and gain functions to improve the signal-to-noise ratio. Modeling the fits of Fig3D as high-pass, ‘Butterworth’ filters we are able to estimate that in the steep part of the response curve a 10-fold increase in [Ca2+]c would result in a ~85-fold increase in the rate of Ca2+ release in Ctrl, compared with only a ~4x increase in MICU1-KD cells.
MICU1 is localized at the outer surface of the IMM to sense [Ca2+]c
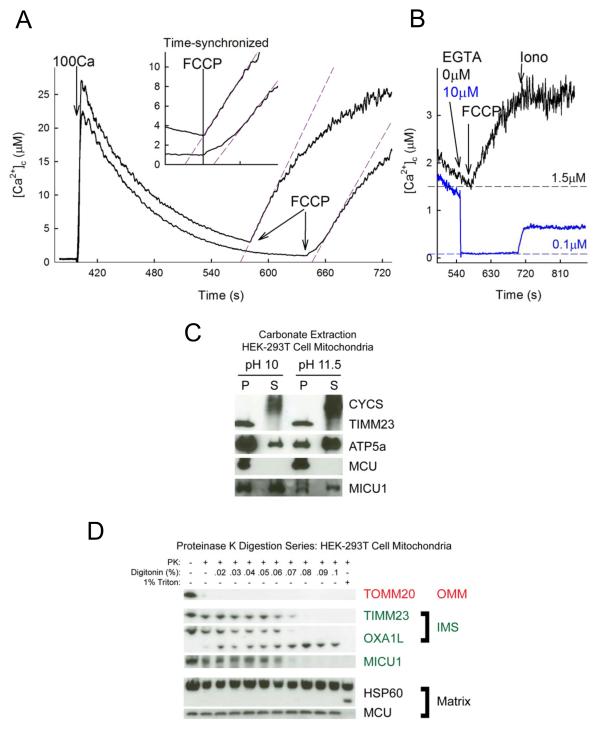
In Ctrl cells, when Ca2+ efflux was initiated at lower [Ca2+]c, a slow-onset and biphasic [Ca2+]c rise occurred (Fig4A). However, when FCCP was added before [Ca2+]c had returned to ≤1uM, the [Ca2+]c rise started steeply, without delay (Fig4A). The initial rate of the [Ca2+]c rise progressively increased with [Ca2+]c (SFig3C). Higher [Ca2+]c at the point of FCCP addition equates to less Ca2+ having been taken up by the mitochondria and therefore the faster release could not be driven by a greater [Ca2+] gradient. The [Ca2+]c requirement for facilitating the mtCU-mediated Ca2+ efflux was further supported by the observation that lowering of [Ca2+]c to 100nM prevented the FCCP-induced Ca2+ efflux (Fig4B). Thus, activation of the Ca2+ flux through mtCU is positively regulated by [Ca2+]c. Considering that neither the activation of mtCU by [Ca2+]c nor the MICU1-dependence (Fig3CD) seems to be eliminated by a large increase in matrix Ca2+, the Ca2+ sensing site(s) of MICU1 is likely present in the IMS rather than the matrix.
Fig4. MICU1 faces the outer surface of the IMM to sense [Ca2+]c.
(A) Representative [Ca2+]c time courses of the mitochondrial clearance of a 100 μM CaCl2 pulse and the subsequent FCCP-induced Ca2+ release (as in Fig3C). FCCP is added at different time points during the [Ca2+]c recovery phase. Linear fits are laid over the fast phase of Ca2+ release (purple dashed lines) to underscore the differences in the initial kinetics. In the inset the two FCCP additions are time-synchronized to further emphasize [Ca2+]c dependence, (90s time-period is shown). (B) 150s after addition of a 10 μM CaCl2 pulse, either 0 (black) or 10 μM (blue) EGTA was added to establish different [Ca2+]c. Without EGTA addition, FCCP fully released the mitochondria-accumulated Ca2+ in 2 minutes (no further [Ca2+]c increase upon addition of Ca2+ ionophore ionomycin). Lowering [Ca2+]c to 0.1 μM by EGTA abolished the [Ca2+]c increase caused by FCCP but subsequent ionomycin addition caused substantial [Ca2+]c increase, indicating the continued accumulation of Ca2+ in the mitochondria. (C-D) Topology of MICU1 was investigated using isolated HEK-293T cell mitochondria. (C) Analysis of supernatant (S) and insoluble pellet (P) fractions following carbonate extraction at pH 10 and pH 11.5 with immunoblotting against MICU1 and the established integral membrane proteins TIMM23 and MCU, the soluble protein CYCS, and the peripheral membrane protein ATP5a. (D) Mitochondria were treated with PK with increasing concentrations of detergent. Proteins with known localization are immunoblotted and labeled according to their topology. Bands representing cleavage products are shown for OXA1L and HSP60 (with Triton).
After finding functional evidence to support an IMS localization of the calcium sensing site(s) of MICU1, we sought to corroborate with biochemical evidence. To this end, we examined the topology of MICU1, including both the nature of membrane association and the sub-mitochondrial localization. An alkaline carbonate extraction of isolated HEK-293T cell mitochondria reveals that MICU1 is likely strongly associated with the IMM (Fig4C): MICU1 is observed in both supernatant and pellet fractions at both pH 10 and pH 11.5 following carbonate extraction. Integral membrane proteins such as TIMM23 and MCU, on the other hand, remain only in the pellet under either condition. In order to determine the submitochondrial localization of MICU1, we further used a proteinase K (PK) digestion with a series of detergent concentrations to differentially permeabilize the IMM and OMM in HEK-293T cell mitochondria (Fig4D). MICU1 is protected from proteolysis similarly to the IMS proteins TIMM23 and OXA1L, whereas the matrix proteins HSP60 and MCU do not get digested until the end of the series. All proteins shown, however, are substrates for PK as evidenced by digestion in 1% Triton X-100. Collectively, these results provide both biochemical and functional evidence that MICU1 and its Ca2+ sensing site are exposed to [Ca2+]c at the outer surface of the IMM.
Enhanced mitochondrial matrix Ca2+ chelation as an adaptive response to MICU1 depletion
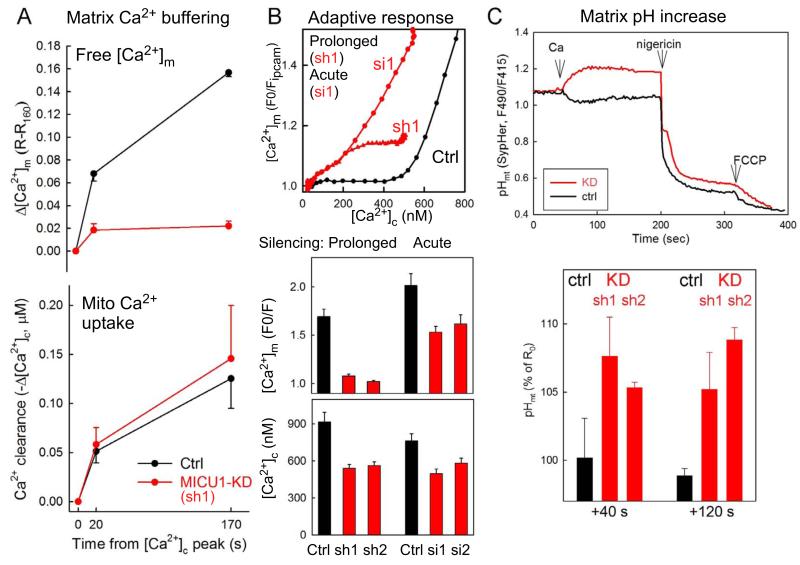
We next explored the blunted [Ca2+]m rise in MICU1-KD cells. A decrease in the cooperativity of mtCU’s activation might be a factor in the blunted [Ca2+]m increases elicited by short lasting [Ca2+]c microdomains during IP3-linked stimulation. However, it is unlikely to fully account for the suppression of the [Ca2+]m signal during the sustained SOCE (Fig1EFG). Also, Fig1J-L showed that the attenuated [Ca2+]m increase was associated with enhanced Ca2+ sequestration. The combination of enhanced Ca2+ storage and an attenuated [Ca2+]m rise indicates that an increased fraction of Ca2+ was in bound form in the mitochondrial matrix of stable MICU1-KD. To directly test this possibility, simultaneous quantification of mitochondrial Ca2+ accumulation and the corresponding [Ca2+]m increase was set up in permeabilized cells (Fig5A). During continuous mitochondrial Ca2+ uptake, the [Ca2+]m showed a progressive increase in Ctrl but only a small [Ca2+]m increase in MICU1-KD (Fig5A). This result indicates that much of the accumulated Ca2+ is in bound form, suggesting increased matrix Ca2+ buffering capacity.
Fig5. Enhanced mitochondrial matrix Ca2+ chelation as an adaptive response to MICU1 depletion.
(A) Mitochondrial Ca2+ uptake (lower) and the corresponding increase in [Ca2+]m (upper) were monitored simultaneously in permeabilized HeLa cells in the presence of Tg. To obtain comparable Ca2+ uptake, 5μM and 2μM CaCl2 were added to Ctrl and stable MICU1-KD cells, respectively (n=4). (B) Top: [Ca2+]m vs. [Ca2+]c relationships during SOCE in acute (48hr, si1) and stable MICU1-KD (sh1,2) cells. Middle and lower graphs show the peak [Ca2+]m and [Ca2+]c increases evoked by SOCE (1mM CaCl2) (n=24-62). (C) Mitochondrial matrix alkalinization in stable MICU1-KD (sh1 & sh2) cells during SOCE (1mM CaCl2) recorded by mtSypHer. Ionophore (Nigericin, 5μM) and FCCP (5μM) were added to induce matrix acidification. Representative traces (upper) and mean normalized post-SOCE (40 and 120 s) values (lower n=3-4, p<0.05).
To test if the change in matrix Ca2+ buffering is directly linked to MICU1 depletion, Ca2+ transport was also monitored during short term (48 hr) silencing (si1 in Fig5B). MICU1 depletion upon short term silencing was similar to that in the stable cell lines (not shown). Both short and long term depletion of MICU1 caused a similar decrease in the [Ca2+]c threshold for the [Ca2+]m rise, but suppression of the amplitude of the [Ca2+]m response was not induced by acute MICU1 silencing (Fig5B). Thus, upregulation of the matrix Ca2+ buffering may be an adaptive response to prolonged MICU1 depletion.
Mitochondrial matrix pH (pHm) is central to matrix Ca2+ buffering (Chalmers and Nicholls, 2003). Measurement of pHm by a genetically targeted reporter, mtSypHer (Poburko et al., 2011), revealed alkalinization in stable MICU1-KD cells during mitochondrial Ca2+ uptake (Fig5C), whereas no change or a slight acidification was observed in the Ctrl, in agreement with a previous study (Poburko et al., 2011).
Collectively, these results suggest that prolonged MICU1 depletion may lead to an adaptive increase in mitochondrial matrix Ca2+ binding, which may be linked to pHm. Upregulation of matrix Ca2+ buffering provides an explanation for the blunted [Ca2+]m increases during [Ca2+]c signaling events in long-term silenced HeLa cells.
Increased sensitivity of MICU1-KD cells to delayed mitochondrial Ca2+ dysregulation: role of ROS
An increase in mitochondrial Ca2+ storage has been linked to delayed mitochondrial dysregulation, membrane permeabilization and cell injury (Chalmers and Nicholls, 2003). Continuous mitochondrial Ca2+ loading in conjunction with other stress factors triggers loss of IMM integrity, which manifests as dissipation of ΔΨm and release of Ca2+ (Csordas et al., 2006; Pacher and Hajnoczky, 2001). When prolonged SOCE was stimulated in HeLa cells (SFig4A) or hepatocytes (SFig4C), MICU1-KD cells showed a greatly enhanced vulnerability to ΔΨm loss. The impairment of mitochondria was followed by an increase in cell death (SFig4B).
Enhanced production of reactive oxygen species (ROS) might contribute to the mitochondrial injury since Ca2+ stimulates ROS production and ROS is known to synergize with Ca2+ to induce mitochondrial membrane permeabilization. Using mtHyPer, an H2O2 reporter genetically targeted to the mitochondrial matrix (Belousov et al., 2006), no change in resting H2O2 was observed in association with MICU1 depletion (SFig4D left panel). However, during prolonged SOCE a gradual increase in matrix H2O2 was detected in MICU1-KD (SFig4D right panel), which might make some contribution in mitochondrial membrane permeabilization.
These results show that loss of MICU1 causes vulnerability of mitochondria to stress leading to impaired cellular function. The impairments involve stress-related enhanced ROS generation. Thus, the physiological roles of MICU1 might be to prevent mitochondrial Ca2+ overload at moderate [Ca2+]c elevations and to allow optimal [Ca2+]m and metabolic responses to [Ca2+]c spikes.
Role of MICU1 in decoding of cytoplasmic calcium signals by the mitochondria
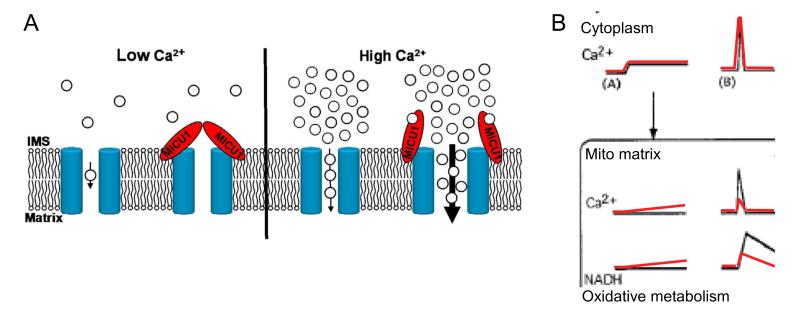
Taken together our data lead us to construct the model of mtCU regulation by MICU1 illustrated in Fig6A. MICU1 localizes to the outer surface of the IMM and presents a site for regulation of mtCU by [Ca2+]c. In this model, MICU1 contributes to keeping mtCU closed at low [Ca2+]c. At high [Ca2+]c, MICU1 promotes activation of the channel. Therefore, MICU1 can be envisioned as a Ca2+-sensitive switch supporting effective transitions of mtCU between closed and open states.
Fig6. MICU1 supports decoding of [Ca2+]c signals by stabilizing the closed state and supporting Ca2+-dependent activation of mtCU.
(A) Graphical representation of the modulation of mtCU by MICU1. (B) The model depicts the two major flaws in mitochondrial [Ca2+]c signal detection and the coupled Ca2+ control of oxidative metabolism in MICU1 deficient cells. Small [Ca2+]c elevations (A) are tuned out by Ctrl (black) mitochondria owing to MICU1-dependent thresholding; but are able to propagate to the MICU1-deficient (red) mitochondria causing stimulation and long-term exhaustion of oxidative metabolism. Large [Ca2+]c transients (B) such as the rapid short-lasting IP3R-derived high [Ca2+]c microdomains are effectively transferred to the mitochondrial matrix due to MICU1-dependent cooperative activation of mtCU. In the absence of MICU1 the efficacy of signal propagation decreases. Please note that oxidative metabolism was assessed by NAD(P)H imaging in (Hajnoczky et al., 1995) and by cellular JO2 measurement in the present study.
How do the effects of MICU1 on mtCU activity contribute to the decoding of calcium spikes and oscillations? Fig1E,G&H have already shown that submicromolar [Ca2+]c increases can reach the mitochondrial matrix only in MICU1-KD cells. Thus, MICU1 helps mitochondria to tune out [Ca2+]c baseline fluctuations. Fig1BC and SFig1D also showed suppression of the IP3-linked [Ca2+]m transients in hepatocytes. This could be because mitochondrial Ca2+ uptake was less efficient in MICU1-KD but local [Ca2+]c transients sensed by mitochondria or Ca2+ binding in the mitochondrial matrix could also be affected by MICU1 KD. To discriminate among these possibilities, first, local [Ca2+] at the ER-mitochondrial interface was directly monitored (Csordas et al., 2010) and no difference was found between MICU1-KD and Ctrl (SFig5A). Then, the effects on [Ca2+]m of IP3-linked stimulation and SOCE were tested in side-by-side measurements. The IP3R-mediated local high [Ca2+] could evoke only a slowly developing [Ca2+]m increase in MICU1-KD compared to Ctrl (SFig5B). Furthermore, when [Ca2+]m was recorded simultaneously with and plotted against the local [Ca2+]c at the mitochondrial surface, unlike the Ctrl (SFig5C left), the MICU1-KD failed to show steeper correlation upon histamine stimulation (continuous line) than during SOCE (dashed, right (SFig5C right). Differential suppression of the histamine-induced [Ca2+]m increase in MICU1-KD cannot be explained by an increase in mitochondrial matrix Ca2+ chelation. These results further support that MICU1 is specifically required for the steep activation of the uniporter and mitochondrial Ca2+ uptake during short lasting IP3R-mediated [Ca2+]c signals. Thus MICU1 provides a Ca2+-dependent molecular switch that allows mitochondrial decoding of the [Ca2+]c signal, specifically both the exclusion of slow and small [Ca2+]c increases and the positive selection of IP3R-mediated [Ca2+]c spikes to propagate to the mitochondrial matrix via mtCU. To highlight the function of MICU1, the scheme in Fig6B shows an adaptation of the descriptive model linking cytoplasmic and mitochondrial Ca2+ signals to metabolism put forward previously (Hajnoczky et al., 1995). The original figure, representing the control condition, is shown in black with the alterations occurring in the absence of MICU1 shown in red.
Discussion
Our findings show that MICU1 has two distinct functions in the regulation of the mtCU: (1) it sets a [Ca2+]c threshold (≥500 nM); and (2) it provides cooperative activation as [Ca2+]c rises. As a result, MICU1 enables mitochondria to tune out small and slow [Ca2+]c increases, avoiding chronic, superfluous stimulation, while responding effectively to large and rapid [Ca2+]c spikes and oscillations during periods of activity (see schematic in Fig6B). Because MICU1 lacking functional Ca2+-binding EF-hands is able to threshold Ca2+ uniport at low [Ca2+]c as wild type MICU1, but cannot confer cooperative activation as [Ca2+]c rises, the steep transition between fully closed and open states of MCU must be mediated by binding of Ca2+ to MICU1’s EF hands. As MICU1 and MCU reside within the same macromolecular complex, we propose that Ca2+ binding to MICU1 induces a conformational change that is relayed to the pore, positively regulating the uniporter activity (Fig6A).
In a recent paper, Mallilankaraman et al. also described the thresholding function of MICU1, however, they did not observe a role of MICU1 in controlling mtCU cooperativity. Thus, both studies agree that a key role for MICU1 is to keep mtCU closed. In the high [Ca2+]c range, the role of MICU1 in the allosteric control of mtCU may have been missed previously because the transport assay was performed in the absence of physiological [Mg2+] (Mallilankaraman et al., 2012b). Previous studies have shown that Mg2+ controls the allosteric activation of the uniporter (Bragadin et al., 1979; Favaron and Bernardi, 1985; Kroner, 1986) and we demonstrate here that Mg2+ is needed to support the effect of MICU1 on mtCU cooperativity (SFig3).
Our results show that resting [Ca2+]m is unaltered in MICU1-KD cells, but the IP3R-mediated increase of [Ca2+]m is suppressed (Fig1). Previously, Perocchi et al described suppression of both resting and stimulated [Ca2+]m levels in HeLa cells (Perocchi et al., 2010) and Mallilankaraman et al. reported an elevation of the resting [Ca2+]m but no change in the IP3R-mediated increase (Mallilankaraman et al., 2012b). The reasons for the differences appear to be at least in part technical. Mallilankaraman et al used a non-ratiometric dye-based assay (rhod2) to record [Ca2+]m without calibration or confirmation of dye distribution. This way, fluorescence intensity values may not equate to Ca2+ concentrations. MCU or MICU1 silencing may also change rhod2 accumulation to the mitochondria and in other organelles. To avoid these problems, we used genetically targeted Ca2+ sensors, as did Perocchi et al. However, these probes can be influenced by pH and we have documented alkalinization in the mitochondrial matrix during SOCE in MICU1-KD (Fig5). Therefore, we used multiple probes, including ratiometric pericam that is pH insensitive when excited at 415nm (Jiang et al., 2009)(Fig1). Moreover, we estimated the resting mitochondrial Ca2+ by monitoring [Ca2+]c increase caused by uncoupling in intact cells (SFig1). All these results support that in MICU1-KD cells, mitochondria do not take up Ca2+ at the resting [Ca2+]c which is set by high affinity plasma membrane and ER Ca2+ pumps (≤100 nM). However, when Ca2+ pumps are disabled, mitochondria establish a steady state of [Ca2+]c < 300 nM in MICU1-KD (Fig2C). Therefore a possible explanation for any elevated basal [Ca2+]m by Mallilankaraman et al can be that the resting [Ca2+]c was somewhat higher or the Ca2+ pumps were less active in their model.
The attenuated IP3R-mediated [Ca2+]m increase likely reflects decreased uptake in response to the high [Ca2+]c microdomains due to lessened cooperativity in mtCU activation. Furthermore, we have shown that depletion of MICU1 induces adaptive responses that include an increase in the bound fraction of total mitochondrial calcium (Fig5). This adaptation is more apparent in stable (long term) than in short term silencing (Fig5B). Notably, we also observed some increase in the exchanger-mediated mitochondrial Ca2+ efflux in stable MICU1-KD (not shown), which may contribute to lowering the mitochondrial Ca2+ load. Thus, model specific differences in the compensatory responses might also contribute to the variability in suppression of the [Ca2+]m signal shown in the three studies.
Mitochondria in most tissues and cell types effectively respond to [Ca2+]c spikes and oscillations that involve elevation of the global [Ca2+]c to ~1 μM. This has been attributed to strategic localization of mitochondria close to Ca2+ release channels of the ER/SR (Csordas et al., 1999; Hajnoczky et al., 1995; Lawrie et al., 1996; Rizzuto et al., 1993; Rizzuto et al., 1998), because these mitochondria are exposed to local [Ca2+] rises attaining 5-10 μM (Csordas et al., 2010; Giacomello et al., 2010). However, these local [Ca2+] increases are usually short-lasting. Therefore, it is central to the mitochondrial sensing of the [Ca2+]c spikes that mitochondrial Ca2+ uptake is rapidly and steeply activated in the micromolar range of [Ca2+]c. Indeed, a regulatory Ca2+-binding site has already been proposed (Csordas and Hajnoczky, 2003; Gunter and Pfeiffer, 1990; Kroner, 1986). However, the Ca2+ sensor remained elusive. Here, we demonstrated that the cooperative activation of mtCU is suppressed in cells lacking MICU1 or expressing MICU1 mutated at both Ca2+ binding EF-hand domains. Thus, MICU1 represents a molecular switch that binds Ca2+ to sharply enhance mtCU-mediated Ca2+ influx in the micromolar [Ca2+]c range.
Previously, patch clamp studies demonstrated that IMiCa amplitude was not noticeably altered when pipette (intramitoplast) [Ca2+] was varied from <10 nM to about 10 μM (Kirichok et al., 2004). Furthermore, our results demonstrated that preloading of mitochondria with Ca2+ (1) did not affect the biphasic kinetic of mtCU-mediated Ca2+ flux (Fig4A) and (2) did not attenuate the MICU1-dependent difference in the Ca2+ flux if submicromolar [Ca2+]c was restored (Fig3). We further show biochemical evidence that MICU1 faces the IMS, and not the matrix as previously reported (Mallilankaraman et al., 2012b), and probably its EF hands form the proposed allosteric Ca2+ regulatory site of mtCU in the IMS (Igbavboa and Pfeiffer, 1988).
Mitochondria isolated from various tissues and in a wide range of cell lines display minimal Ca2+ uptake in the physiological range of global [Ca2+]c (Gunter et al., 1994), though higher affinity mitochondrial Ca2+ uptake has been documented in some hormone producing cell types and cardiac muscle (Sparagna et al., 1995; Spat et al., 2008). Similar to most tissues and cell types, in primary hepatocytes and HeLa cells, mitochondrial Ca2+ uptake is noticeable only in the micromolar [Ca2+]c range and a [Ca2+]m increase requires ≥500 nM [Ca2+]c in practically every cell (Fig1). By contrast, in MICU1-deficient cells mitochondrial Ca2+ uptake is detectable below 300 nM of [Ca2+]c (Figs1&2). This difference does not seem to have much effect on mitochondrial Ca2+ handling when [Ca2+]c is maintained at the resting level (<100 nM) by the activity of the high affinity plasma membrane and ER Ca2+ pumps. However, submicromolar [Ca2+]c fluctuations that are normally ignored by the mitochondria in plain cells are routed to the mitochondria if MICU1 expression is decreased. Thus physiological changes in global [Ca2+]c that are handled mostly by the plasma membrane and ER Ca2+ pumps in normal cells are shifted towards mitochondria if MICU1 is not expressed at normal levels.
MICU1 resides in a complex with MCU, which is believed to be the pore-forming component of mtCU since it is sufficient by itself to provide a current similar to IMiCa (Baughman et al., 2011; De Stefani et al., 2011; Kirichok et al., 2004). At present we do not know whether MICU1 impacts the ion selectivity of mtCU. Our data indicate that MICU1 without bound Ca2+ helps to keep the gate closed at submicromolar [Ca2+]c At higher [Ca2+]c, mtCU activity increases even without MICU1 or if the EF-hands are mutated but a much steeper activation is seen with native MICU1. These results are in line with earlier observations that the open probability for IMiCa in mitoplasts, where MICU1 is expected to be present, is much higher than that of the reconstituted MCU channel in lipid bilayer under comparable electrical (-160 mV) and [Ca2+] (symmetrical ~100 mM) conditions (De Stefani et al., 2011; Kirichok et al., 2004). Our prediction, then, is that binding of Ca2+ to MICU1 results in a conformational change sensed by the MCU and translated to positive cooperative gating of the channel by Ca2+. Many ion channels of the plasma membrane or ER/SR are regulated by Ca2+ binding. In most cases the pore-forming component of the channel contains Ca2+ binding site(s) (e.g. L-type voltage gated Ca2+ channels, BK channels, TRPV5/6, IP3R, RyR). However, in some cases a separate Ca2+ sensing protein is employed to control the gate, such as calmodulin for the SK potassium channels and STIM1 for ORAI. Perhaps the closest analogue to our model of mtCU is the SK channel, where calmodulin is permanently associated with the pore and upon binding of Ca2+, triggers the opening of the gate (Fakler and Adelman, 2008).
The current study proposes a simple model that combines the Ca2+ sensing activity of MICU1 and the pore-forming subunit MCU (Fig6), however, the mechanisms controlling mtCU dynamics are likely to be more complicated. MICU2, a paralog of MICU1, resides within the MICU1/MCU complex (Plovanich et al., 2013). MCU itself has a paralog, CCDC109b, whose function is still not clear. Several other regulators, including MCUR1 (Mallilankaraman et al., 2012a) have been identified, that may interact more indirectly. We anticipate that the relationship between MICU1/2 and MCU is particularly intimate, given the striking co-evolution of these proteins (Baughman et al., 2011; Bick et al., 2012), their strong co-expression (Baughman et al., 2011), and their genomic adjacency (Bick et al., 2012).
Decoding of [Ca2+]c spikes and oscillations by mtCU was shown to be central to cytoplasmic Ca2+ signaling (Hajnoczky et al., 1999; Jouaville et al., 1995; Tinel et al., 1999), control of oxidative metabolism (Hajnoczky et al., 1995; Jouaville et al., 1999; Robb-Gaspers et al., 1998) and even to induction of apoptosis under conditions of cellular stress (Szalai et al., 1999). All these functions of mtCU are severely disturbed when MICU1 is depleted. Sensitization of mtCU to [Ca2+]c results in mitochondrial suppression of both Ca2+ entry and IP3R-dependent fluctuations in global [Ca2+]c. Attenuation of the agonist-induced [Ca2+]m elevations leads to weakened activation of the matrix dehydrogenases (Perocchi et al., 2010). The observed dysregulation of respiration in the MICU1-depleted cells is also likely caused by the altered mitochondrial Ca2+ handling. Finally, the impairment of bioenergetics makes the cells vulnerable to various stress factors, increasing the risk for cell death. Thus, the MICU1-dependent control over the [Ca2+]c thresholding and cooperativity of mtCU is critical for effective cytoplasmic and mitochondrial calcium signaling and in turn, for cell survival.
Experimental Procedures
Detailed protocols are listed at the Supplemental Experimental Procedures.
DNA constructs and transient expression: See the Supplemental Information.
Silencing of MICU1 or MCU: In vivo silencing in mouse liver was applied for 4 weeks as described previously (Baughman et al., 2011). Stable expression of shRNAs and wild-type and mutant proteins has been described (Baughman et al., 2011; Perocchi et al., 2010). For short-term silencing, HeLa cells were transfected with the siRNA version of the same sh1 and sh2 hairpins used for stable cells (Ambion) or with scrambled siRNA for 48hr using Oligofectamine (Invitrogen).
Live cell imaging and fluorometric measurements of [Ca2+]c, [Ca2+]m and ΔΨm, respectively were performed as described earlier (Csordas and Hajnoczky, 2003; Csordas et al., 2010). pHm was assureded with mtSypHer (Poburko et al., 2011). For SOCE experiments ER stores were depleted by 10 min pre-treatment with 2μM Tg in Ca2+-free buffer.
MICU1 Topology Analysis: Localization analysis and alkaline carbonate extraction were performed using HEK-293T mitochondria according to (Sato and Mihara, 2010) and (Ryan et al., 2001), respectively.
Measurement of cellular oxygen consumption: JO2 was measured using an XF24 Extracellular Flux analyzer (Seahorse Biosciences), at 37°C.
Statistics: Experiments were carried out with ≥3 different cell preparations and with each at least in triplicate. Data are presented as mean ± S.E. Significance of differences from the relevant controls was calculated by Student’s t test or Tukey post hoc test following 2-way ANOVA. Modeling is described in the Supplemental Information.
Supplementary Material
Highlights.
MICU1 regulates the Ca2+ threshold and cooperativity of the mitochondrial uniporter
MICU1 faces the intermembrane space to sense cytoplasmic Ca2+
Loss of MICU1 initiates an adaptive increase in mitochondrial matrix Ca2+ binding
Yet decoding of Ca2+ signals is compromised, impairing ATP output and cell survival.
Acknowledgements
We thank Olga Goldberger for technical assistance, and B. R. Bettencourt, K. Charisse, S. Kuchimanchi and A. Akinc of Alnylam Pharmaceuticals for siRNA design, synthesis, and formulation. This work was supported by NIH grants DK080261 to VKM and DK051526 to GH. Victor Koteliansky was formerly an employee at Alnylam Pharmaceutical and received compensation from it.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011 doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nature methods. 2006;3:281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- Beutner G, Sharma VK, Lin L, Ryu SY, Dirksen RT, Sheu SS. Type 1 ryanodine receptor in cardiac mitochondria: Transducer of excitation-metabolism coupling. Biochimica et biophysica acta. 2005 doi: 10.1016/j.bbamem.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Bick AG, Calvo SE, Mootha VK. Evolutionary diversity of the mitochondrial calcium uniporter. Science. 2012;336:886. doi: 10.1126/science.1214977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragadin M, Pozzan T, Azzone GF. Kinetics of Ca2+ carrier in rat liver mitochondria. Biochemistry. 1979;18:5972–5978. doi: 10.1021/bi00593a033. [DOI] [PubMed] [Google Scholar]
- Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. The Journal of biological chemistry. 2003;278:19062–19070. doi: 10.1074/jbc.M212661200. [DOI] [PubMed] [Google Scholar]
- Csordas G, Hajnoczky G. Plasticity of mitochondrial calcium signaling. The Journal of biological chemistry. 2003;278:42273–42282. doi: 10.1074/jbc.M305248200. [DOI] [PubMed] [Google Scholar]
- Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. The Journal of cell biology. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Thomas AP, Hajnoczky G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. The EMBO journal. 1999;18:96–108. doi: 10.1093/emboj/18.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Varnai P, Golenar T, Roy S, Purkins G, Schneider TG, Balla T, Hajnoczky G. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Molecular cell. 2010;39:121–132. doi: 10.1016/j.molcel.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011 doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakler B, Adelman JP. Control of K(Ca) channels by calcium nano/microdomains. Neuron. 2008;59:873–881. doi: 10.1016/j.neuron.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Favaron M, Bernardi P. Tissue-specific modulation of the mitochondrial calcium uniporter by magnesium ions. FEBS letters. 1985;183:260–264. doi: 10.1016/0014-5793(85)80789-4. [DOI] [PubMed] [Google Scholar]
- Giacomello M, Drago I, Bortolozzi M, Scorzeto M, Gianelle A, Pizzo P, Pozzan T. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Molecular cell. 2010;38:280–290. doi: 10.1016/j.molcel.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Gunter KK, Sheu SS, Gavin CE. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol. 1994;267:C313–339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258:C755–786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Hager R, Thomas AP. Mitochondria suppress local feedback activation of inositol 1,4, 5-trisphosphate receptors by Ca2+ The Journal of biological chemistry. 1999;274:14157–14162. doi: 10.1074/jbc.274.20.14157. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- Igbavboa U, Pfeiffer DR. EGTA inhibits reverse uniport-dependent Ca2+ release from uncoupled mitochondria. Possible regulation of the Ca2+ uniporter by a Ca2+ binding site on the cytoplasmic side of the inner membrane. The Journal of biological chemistry. 1988;263:1405–1412. [PubMed] [Google Scholar]
- Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326:144–147. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouaville LS, Ichas F, Holmuhamedov EL, Camacho P, Lechleiter JD. Synchronization of calcium waves by mitochondrial substrates in Xenopus laevis oocytes. Nature. 1995;377:438–441. doi: 10.1038/377438a0. [DOI] [PubMed] [Google Scholar]
- Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- Kroner H. “Allosteric regulation” of calcium-uptake in rat liver mitochondria. Biol Chem Hoppe Seyler. 1986;367:483–493. doi: 10.1515/bchm3.1986.367.1.483. [DOI] [PubMed] [Google Scholar]
- Lawrie AM, Rizzuto R, Pozzan T, Simpson AW. A role for calcium influx in the regulation of mitochondrial calcium in endothelial cells. The Journal of biological chemistry. 1996;271:10753–10759. doi: 10.1074/jbc.271.18.10753. [DOI] [PubMed] [Google Scholar]
- Mallilankaraman K, Cardenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenar T, Csordas G, Madireddi P, Yang J, Muller M, Miller R, Kolesar JE, Molgo J, Kaufman B, Hajnoczky G, Foskett JK, Madesh M. MCUR1 is an essential component of mitochondrial Ca(2+) uptake that regulates cellular metabolism. Nature cell biology. 2012a;14:1336–1343. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallilankaraman K, Doonan P, Cardenas C, Chandramoorthy HC, Muller M, Miller R, Hoffman NE, Gandhirajan RK, Molgo J, Birnbaum MJ, Rothberg BS, Mak DO, Foskett JK, Madesh M. MICU1 Is an Essential Gatekeeper for MCU-Mediated Mitochondrial Ca(2+) Uptake that Regulates Cell Survival. Cell. 2012b;151:630–644. doi: 10.1016/j.cell.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant JS, Ramos V, Parker I. Structural and functional relationships between Ca2+ puffs and mitochondria in Xenopus oocytes. Am J Physiol Cell Physiol. 2002;282:C1374–1386. doi: 10.1152/ajpcell.00446.2001. [DOI] [PubMed] [Google Scholar]
- Pacher P, Hajnoczky G. Propagation of the apoptotic signal by mitochondrial waves. The EMBO journal. 2001;20:4107–4121. doi: 10.1093/emboj/20.15.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Thomas AP, Hajnoczky G. Ca2+ marks: miniature calcium signals in single mitochondria driven by ryanodine receptors. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:2380–2385. doi: 10.1073/pnas.032423699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Rapizzi E, Di Virgilio F, Pozzan T, Rizzuto R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. The EMBO journal. 2001;20:2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plovanich M, Bogorad RL, Sancak Y, Kamer KJ, Strittmatter L, Li AA, Girgis HS, Kuchimanchi S, De Groot J, Speciner L, Taneja N, Oshea J, Koteliansky V, Mootha VK. MICU2, a Paralog of MICU1, Resides within the Mitochondrial Uniporter Complex to Regulate Calcium Handling. PloS one. 2013;8:e55785. doi: 10.1371/journal.pone.0055785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poburko D, Santo-Domingo J, Demaurex N. Dynamic regulation of the mitochondrial proton gradient during cytosolic calcium elevations. The Journal of biological chemistry. 2011;286:11672–11684. doi: 10.1074/jbc.M110.159962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pralong WF, Spat A, Wollheim CB. Dynamic pacing of cell metabolism by intracellular Ca2+ transients. The Journal of biological chemistry. 1994;269:27310–27314. [PubMed] [Google Scholar]
- Rizzuto R, Bastianutto C, Brini M, Murgia M, Pozzan T. Mitochondrial Ca2+ homeostasis in intact cells. The Journal of cell biology. 1994;126:1183–1194. doi: 10.1083/jcb.126.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Robb-Gaspers LD, Burnett P, Rutter GA, Denton RM, Rizzuto R, Thomas AP. Integrating cytosolic calcium signals into mitochondrial metabolic responses. The EMBO journal. 1998;17:4987–5000. doi: 10.1093/emboj/17.17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MT, Voos W, Pfanner N. Assaying protein import into mitochondria. Methods in cell biology. 2001;65:189–215. doi: 10.1016/s0091-679x(01)65012-x. [DOI] [PubMed] [Google Scholar]
- Sato T, Mihara K. Mammalian Oxa1 protein is useful for assessment of submitochondrial protein localization and mitochondrial membrane integrity. Analytical biochemistry. 2010;397:250–252. doi: 10.1016/j.ab.2009.10.035. [DOI] [PubMed] [Google Scholar]
- Sparagna GC, Gunter KK, Sheu SS, Gunter TE. Mitochondrial calcium uptake from physiological-type pulses of calcium. A description of the rapid uptake mode. The Journal of biological chemistry. 1995;270:27510–27515. doi: 10.1074/jbc.270.46.27510. [DOI] [PubMed] [Google Scholar]
- Spat A, Szanda G, Csordas G, Hajnoczky G. High- and low-calcium-dependent mechanisms of mitochondrial calcium signalling. Cell calcium. 2008;44:51–63. doi: 10.1016/j.ceca.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadkai G, Bianchi K, Varnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. The Journal of cell biology. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalai G, Krishnamurthy R, Hajnoczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. The EMBO journal. 1999;18:6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinel H, Cancela JM, Mogami H, Gerasimenko JV, Gerasimenko OV, Tepikin AV, Petersen OH. Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca(2+) signals. The EMBO journal. 1999;18:4999–5008. doi: 10.1093/emboj/18.18.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenker M, Malli R, Fertschai I, Levak-Frank S, Graier WF. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nature cell biology. 2007;9:445–452. doi: 10.1038/ncb1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.